Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 15
Adipose-Derived Stem Cells (ASCs) for Regeneration of Intervertebral Disc Degeneration: Review Article
Authors Romaniyanto F , Mahyudin F , Prakoeswa CRS, Notobroto HB , Tinduh D, Ausrin R , Rantam FA , Suroto H, Utomo DN , Rhatomy S
Received 23 June 2022
Accepted for publication 8 September 2022
Published 4 November 2022 Volume 2022:15 Pages 67—76
DOI https://doi.org/10.2147/SCCAA.S379714
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Bernard Binetruy
FNU Romaniyanto,1– 3 Ferdiansyah Mahyudin,4,5 Cita Rosita Sigit Prakoeswa,5,6 Hari Basuki Notobroto,7 Damayanti Tinduh,5,8 Ryan Ausrin,2,3 Fedik Abdul Rantam,9,10 Heri Suroto,4,5 Dwikora Novembri Utomo,4,5 Sholahuddin Rhatomy11
1Doctoral Program, Faculty of Medicine, Airlangga University, Surabaya, Indonesia; 2Department of Orthopedic and Traumatology, Prof. Dr. R. Soerharso Orthopedic Hospital, Surakarta, Indonesia; 3Faculty of Medicine, Sebelas Maret University, Surakarta, Indonesia; 4Department of Orthopedic and Traumatology, Dr. Soetomo General Hospital, Surabaya, Indonesia; 5Faculty of Medicine, Airlangga University, Surabaya, Indonesia; 6Department of Dermatology and Venereology, Dr. Soetomo General Hospital, Surabaya, Indonesia; 7Faculty of Public Health, Airlangga University, Surabaya, Indonesia; 8Department of Physical Medicine and Medical Rehabilitation, Dr. Soetomo General Hospital, Surabaya, Indonesia; 9Virology and Immunology Laboratory, Microbiology Department, Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia; 10Stem Cell Research and Development Center, Airlangga University, Surabaya, Indonesia; 11Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia
Correspondence: Sholahuddin Rhatomy, Department of Orthopaedics and Traumatology, Dr. Soeradji Tirtonegoro General Hospital, Klaten, Indonesia, Tel +62 272-321163, Fax +62 272-321104, Email [email protected]; [email protected]
Abstract: The intervertebral disc (IVD) is an important structure in the human body because it functions as a weight-bearing. This structure undergoes a process of degeneration like the rest of the body and this process is known as intervertebral disc degeneration (IDD) which is the most common cause of low back pain (LBP). The current common management, either conservative or surgical, is pain-relieving and has not been able to restore degenerated disc optimally. Changes in the IVD microenvironment in IDD conditions make it difficult for the regeneration process to occur. Research to reverse the degeneration process continues to develop, one of them is the use of adipose-derived stem cells (ASCs). ASCs is superior due to the ability to differentiate into several other cells such as adipocytes, chondrocytes, and osteoblasts, it also has ability to act as immunomodulators by stimulating the migration of immune cells to damaged tissues. ASCs becomes a good choice because it is easy to obtain, low donor site morbidity, high proliferation rate, and excellent differentiation abilities. Research on the optimal preparation process for ASCs and their application to various disorders continues to advanced. This study aims to review the potential use of ASCs for regeneration of intervertebral disc degeneration.
Keywords: adipose-derived stem cells, stem cell, intervertebral disc, intervertebral disc degeneration, low back pain
Introduction
One of the health problems that affect a person’s quality of life and impact the world’s socio-economics is low back pain (LBP).1,2 Intervertebral disc degeneration (IDD) is the most common cause of LBP.3 As a degenerative disease, this disorder is part of the aging process and it is estimated that more than 90% of people have this condition, but they mostly are asymptomatic.4 This disorder begins to appear in the second decade of life.5,6 This abnormality interferes with the function of the IVD as a weight-bearing structure.7,8
Intervertebral disc (IVD) has limitations to recover from damage, including degenerative processes.9 The current IDD treatment used has not been able to regenerate damaged IVD.10 Conservative or surgery management has not been able to optimally restore IDD and only for symptomatic relief and muscular stabilization.10,11
Recent progress in understanding IVD physiopathology and management led to IVD regenerative medicine. However, owing to the restrictive regulatory framework and the difficult clinical translatability of cell-based therapies, the use of biological factors targeting IVD degenerative processes was also contemplated.12 In this case, the main purpose of regenerative medicine is to overcome the limit of the low regenerative power of tissues, creating strategies to restore their functionality and architecture. One of the potential approaches in animal studies is the use of mesenchymal stem cells (MSCs).13–16 MSCs can be obtained from various sources.17,18 Although MSCs can hypothetically be obtained from almost any tissue within the human body, there are practical limitations concerning the difficulty and invasiveness of the procurement process and various donor characteristics. Currently, the main sources of MSCs are bone marrow and adipose tissue. Another sources include; dental pulp, umbilical cord tissue (Wharton’s Jelly) or amniotic fluid.19
Adipose-derived stem cells (ASCs) is one of MSCs derived from body fat tissue and is often referred to adipose-derived mesenchymal stem cells (ADMSCs).17,18,20,21 ADMSCs have almost the same characteristics as bone marrow derived mesenchymal stem cells (BMSCs), which can be transformed into tissues in the mesodermal pathway such as bone, cartilage, muscle, and adipose. BMSCs are MSCs often used, but bone marrow has the disadvantage of being limited in number in the body and the retrieval procedure is complicated.20,21 Research of ASCs in regenerative medicine continues to grow, including for IVD regeneration.17 The abundant source in the human body and the low risk of harvesting are the advantages of ASCs as an alternative source of MSCs.21–23 The uniqueness and potential of ASCs in IDD regeneration is an important power to explore further. This study aims to review the potential use of ASCs to regenerate the intervertebral disc degeneration.
Intervertebral Disc Structures
IVD is a fibrocartilage structure that connects the vertebral bodies.24–26 The total IVDs in the human body are 25 discs.24,25 This structure plays a role in spine mobility and has a function as a shock absorber.6,24,25 The nucleus pulposus (NP), the annulus fibrosus (AF), and the cartilaginous endplates (CEPs) are the structures that make up a healthy IVD.6,26–28
NP is the core of the IVD structure. The cell composition of NP is NP stem/progenitor cells (NPPCs), notochordal cells, chondrocyte-like cells, water, and extracellular matrix (ECM).29,30 The ECM composition of NP contains proteoglycan and collagen (COL) type II. Proteoglycan attracts water into the ECM so that the NP is very hydrated. NP plays a role in resisting pressure from the body on the spine structure.6,30
The NP is wrapped by AF with CEPs bordering the vertebral bodies on the outer side6,24,28(AMSU 10,11; Kepler 2013). The most composition of AF is COL type I concentrically arranged.6,30 There are two parts of AF, inner and outer. The inner AF is the part adjacent to the NP which contains dominant COL type II and proteoglycans, while the outer parts of the COL type I are more dominant24,31. Compared to NP, AF has less COL type II. The CEPs is an avascular organ with a capillaries network in the middle that is associated with the vascularization of the vertebral bodies.29 CEPs become a gateway for nutritional supply by a diffusion mechanism.31
Change in Intervertebral Disc Degeneration
Over time, IVD will degenerate and there will be structural and biochemical changes.32 CEPs will calcify and notochordal cells will disappear, triggering the degeneration process to occur in IVD.33,34 Calcification in IVD will disrupt the diffusion mechanism causing the supply of oxygen and nutrients for IVD to be disturbed.6,35 The consequence is an increase in lactic acid causes the IVD environment to become acidic and IVD cells undergo apoptosis.6,24,34 The loss of notochordal cells and IVD cell apoptosis caused a decrease in ECM production, whereas the production of matrix metalloproteinases (MMPs) as a degradation enzyme did not decrease and even increased. This process accelerated the degeneration process of IVD.7,34
Inflammatory mediators, such as interleukin (IL) and tumor necrosis factor (TNF)-α, influence the IDD process. Inflammatory mediators involve cell permeability thereby blocking the synthesis of proteoglycans and collagen. In contrast to proteoglycans and COL, inflammatory mediators increase MMPs activity.34,36 TNF-α also triggers the secretion of IL-6, IL-8, and various related cytokines stimulating cell movement and inducing inflammatory reactions.37
The interaction between TNF-α and IL with nerve fibers triggers pain in IDD.36,38 In normal IVD, innervation is limited to the outer AF.30 In the IVD degeneration process, there is an increase in sensory innervation, including in CEPs becomes deeper (Wuertz and Haglund, f 2013; Lyu et al, 2021). This neuronal ingrowth is caused by a brain-derived neurotrophic factor secreted by degenerating IVD cells, and nerve growth factor secreted by vascular tissue.6
Mesenchymal Stem Cell
Stem cells can be classified into an embryonic, fetal, adult, and induced pluripotent stem cells.39,40 Among all groups of stem cells, MSCs are the most widely developed stem cells in the world of research. MSCs are part of adult stem cells.39,41 The use of MSCs is considered to have no ethical problems and has no genomic stability problems.42 In addition, MSCs have the ability to differentiate into various connective tissue cells.39,41
MSCs can differentiate into the various lineage of mesoderm, ectoderm, and endoderm under specific in vitro conditions.39 Mesoderm differentiation is the easiest because it comes from the same embryonic origin. Adipogenesis, osteogenic, and chondrogenesis differentiation are included in mesoderm differentiation.43 The differentiation ability MSCs into ectoderm tissue is utilized for wound healing, cutaneous repair, hair regeneration, sweat gland restoration, corneal restoration, and neuron differentiation.43,44 With a multistep-protocol, MSCs can differentiate into endoderm tissues such as hepatic and pancreatic tissues.43,45
Definition and Function of ASCs
The definition of ASCs should look at the difference between the definition of a progenitor cell and a stem cell. Progenitors are cells that have the ability to differentiate into one or several specific cell types but have limited proliferative capabilities. While stem cells have the ability to self-renew and proliferate into other cells that are wider, so it’s called multipotent cell.46 Multipotent MSCs are able to differentiate into bone, fat, and cartilage.20 MSCs can be obtained from various tissues.18,39
One of the common source tissues for human MSCs is adipose tissue. ASCs are stem cells derived from fat tissue.18,20 As a stem cell, ASCs have multipotent ability to proliferate and differentiate into several other cells such as adipocytes, chondrocytes, and osteoblasts. ASCs have experienced an exponential increase in their use to treat various types of disorder, especially the muscle and bone system.47
Plenty of sources and minimum risk of taking is an advantage of ASCs over MSCs from other sources.21–23 The source of ASCs can come from different areas all through the body that contains fat. Subcutaneous fat tissue gotten from surgery has restorative potential after reimplantation into the body at the location of the damage.48,49 Fat tissues are a rich source of multipotent stem cells.50 In the human body, there are two common types of adipose tissue, brown adipose tissue and white adipose tissue.51,52
White and Brown Adipose Tissue
Adipose tissues are spread throughout the body and in normal people makeup approximately 20–30% of body composition. This number can differ depending on the body mass index, gender, and muscle mass in each person. In obese people, the amount of adipose tissue can be more and cause some adverse side effects.49 However, it turns out that fat tissue can be used as an alternative to stem cell-based treatment that is better and less invasive than bone marrow-derived mesenchymal stem cells (BMSCs), known as adipose-derived stem cells (ASCs).20,22 Based on their components and functions, there are two types of adipose tissue, brown and white adipose tissues.51–53 White adipocytes, or white fat cells, have some differences from brown adipocytes, or brown fat cells.52,53
Brown fat cells are multilocular with small lipid vacuoles. Vascularization of brown fat tissue is obvious because it requires large amounts of oxygen.54 The distribution of brown fat is widely in the neck, mediastinum, and interscapular area, while white fat is more in the waist and thighs. Physiologically, brown fat is commonly found in newborns and decreases with age.49,53 Brown fat has a higher number of mitochondria and contains a lot of iron, which makes brown fat dark red to brown in color. Mitochondria are more stout making brown fat oxygen demand more so that the capillaries are also more than white fat. Brown fat also has many unmyelinated nerves, providing sympathetic stimulation to fat cells.52,53
Brown adipocytes tissue plays a role in the thermogenesis process that can burn calories and prevent a person from obesity. This roles make brown fat better than white fat.49,52,53 The thermogenesis ability of brown adipose tissue because of its rich content of mitochondria.54 Thermogenesis in brown adipose tissue causes glucose and fat to be burned to generate heat through the action of uncoupling protein (UCP) 1, and mitochondrial membrane protein which interferes with the adenosine triphosphate synthesis process during oxidative phosphorylation by lowering the mitochondrial membrane potential.49,53 UCP1 is a brown adipose tissue-specific marker regulated by adrenergic signaling through sympathetic innervations, and this signaling is responsible for thermogenesis.54
White fat cells are spread in the subcutaneous and visceral areas. White fat is unilocular shaped, contains large lipid vacuoles, and is colored ivory or yellowish.54 White fats are considered a bad fat because they can affect the body’s metabolism and are a calorie accumulation (triglycerides) from excessive calorie consumption.49 Different from brown adipose tissue which expresses UCP1, white adipose tissue expresses isoform UCP2.54 White adipose can supply energy by lipolysis of triglycerides into fatty acids, so if there is too much white fat and lipolysis occurs on a large scale, it can cause insulin resistance.49
White adipose tissue, especially inguinal white adipose tissue, has recently been discovered to include beige adipocytes.54 Beige fat (precursors or mature cells) arises from white fat. Despite its common origin, beige fat has a different metabolic role than white fat, as well as a different transcriptional program.55 The beige adipocyte is a fat cell with features similar to the white fat cell, which stores energy, and the brown fat cell, which creates heat for thermogenesis.56
ASCs Harvesting
The harvesting procedure of adipose tissue to be used as ASCs is important for regenerative medicine.46,57 ASCs isolated from white adipose tissue differ from those extracted from brown adipose tissue, and also ASCs isolated from various anatomical sites possess differ.54 The total number of viable cells that can be retrieved from subcutaneous fatty tissue is unaffected by the anatomical location of the adipose tissue.58 The latter approach was chosen because is a safe, well-tolerated, and slightly invasive procedure but provides a high amount of stromal/stem cells.57 The largest source adipose tissue is abdominal fat.21,23
Adipose tissue can be harvested in the form of solid adipose tissue or lipoaspirate.59 Harvesting adipose tissue approaches which can be used are surgical resection, power-assisted liposuction (PAL), and laser-assisted liposuction (LAL).57,59 Surgical resection was used to obtain solid adipose tissue, while PAL and LAL harvested adipose tissue in the form of lipoaspirate.46,59 Lipoaspiration facilitates extracting subcutaneous tissues easier. The liposuction technique has no consequence on ASCs function.54 Due to better multiplication potential and slow degradation of isolated cells, PAL is an excellent method of ASCs collection for clinical purposes.57,59
Despite the lack of a standard approach, sliced adipose tissue is often digested by one or more of the following options, such as collagenase, dispase, trypsin, and other enzymes.46,58 Temperature (37°C), digesting time (ranging, from 30 minutes – >1 hour), and tissue mass to volume ratios are all suggested; however, protease concentrations are considerably more varied.46,57 Single-layer cultures on standard tissue dishes with a basal medium containing 10% fetal bovine serum are commonly used to grow isolated ASCs.58 Aspirated adipose tissue provides approximately 3.5×105 to 1×106 ASCs each gram.54
The Process of ASCs Formation
MSCs can differentiate into several cell lines such as osteogenesis, chondrogenesis, myogenesis, marrow stroma, tendogenesis, lipogenesis, and several other pathways such as the dermal pathway. This pathway changes according to the environment and physiological processes that the body needs. If MSCs are in an adipose environment, they can differentiate into endothelium, smooth muscle, white fat cells, and brown fat.48 Initially. MSCs will develop into adipoblast, then become pre-adipocytes and when the pre-adipocytes go to fat tissue then they will turn into adiposity. However, during ASCs change process did not occur in the presence of stem cell precursors, namely myogenic factor (Myf) 5-positive and Myf5-negative. Myf5-positive precursors converted MSCs to brown adipose tissue whereas Myf5-negative converted MSCs to white adipose tissue. This MSCs pathway in adipose tissue shows that ASCs can assist in the process of tissue wound regeneration through endothelial differentiation and the effect of ASCs on increasing Vascular endothelial growth factor (VEGF).49,58,60
ASCs Identification
Clinical interest and scientific approach in the transplant of ASC-derived secretome or purified exosomes is increasing nowadays due to its promising result for regenerative medicine. The secretome itself is a complex of microvesicles and exosomes secreted by living cell, it can be isolated from almost all body fluids and carrying lots of biologically active proteins, lipid, and nucleic acids. The standardized handling method to ensure a quantity of ASCs secretome product for therapeutic approach is still not established until now. Exosomes is more stable and easily storable compared with the cells, have lower maintenance cost, and having a lower possibility for immune rejection during in vivo transplantation, the molecules is also more protected from degradation. The characterization of ASCs secretomic profile is mostly done by proteomic approach, but the mechanism of ASC isolation and expansion could affect the composition of secretome is not fully understood.57
Flow cytometric examination of cell surface markers is routinely used to check the presence of ADSC features. The International Society for Cellular Therapy (ISCT) and the International Federation for Adipose Therapeutics and Science (IFATS) describe ASCs following three minimal criteria: (1) cells must be plastic-adherent; (2) they must express CD73, CD90, and CD105 but not CD14, CD11b, CD45, CD19, CD79, nor human leukocyte antigen-DR (HLA-DR); and (3) they have to be able to differentiate into preadipocytes, chondrocytes, or osteoblasts.46,57,58 Based on another recommendations, ASCs should not express the hematopoietic markers (90%), such as CD13, CD73 CD90. To distinguish from bone marrow MSCs, it is recommended to use at least two additional marker such as CD36 (GPIIIb) and CD106 (VCAM-1). The other reports suggested that in contrast to MSCs, ASCs do not express CD106 but CD36 positive.59 The ISCT also recommended that MSCs should lack or even be negative for CD117 and CD34 expression; nevertheless, definite markers for efficiently identifying ASCs are still argued.46,61 ASCs can express CD34, according to existing studies.46,62 Early passage ASCs express higher amounts of CD117, HLA-DR, and CD34 than late passage ASCs.46,63 Despite the fact that isolation and culture processes differ, the immunophenotype is constant among facilities.57
It is recommended the basic characteristic phenotype of ASCs should include at least two positive and two negative markers in one analysis. A study about a cell surface marker screening analysis conclusing that sample collection method did not significantly influence the expression of surface markers profile. A study analyzing phenotype stability of ASCs in long-term culture in several markers such as CD90, CD44, CD34, CD45 that conducted at the 1st, 3rd and 5th passage. All tested groups, were characterized by high (~90%) expression of CD90 and CD44.64 Furthermore, surface markers expression was similar among all ASCs groups and was stable during various stages of culture. These results confirmed our previous findings.59
ASCs for Regeneration of Intervertebral Disc Regeneration
The number of disc cells decreases as the IVD degeneration process progresses. Based on this, cell transplantation is one of the potential biology approaches for IDD.65 However, the biochemical microenvironment of IVD is rough, with low oxygen concentrations, inadequate nutrition, excessive osmolarity, and high acidity.35,66 Although autologous disc cells are a promising cell source, they have a number of weaknesses in the therapeutic setting: (1) Autologous disc cell extraction, whether by image-guided aspiration or open surgical collection, is an intrusive procedure; (2) collecting disc cells from a healthy IVD may hasten IVD degeneration; (3) disc cells from a degenerated disc may not be functionally perfect for re-implantation.66
Because of their ease of access, low donor site morbidity, and high proliferation rate, ASCs appear to be a superior choice for tissue engineering in IVD regeneration.66 ASCs offer excellent differentiation abilities and are well applicable to the treatment of IDD.61 The transformation of ASCs into ectodermal, endodermal, and mesodermal cells has been reported in the previous study.39 As ASCs were from the mesodermal origin, development into adipogenic, chondrogenic, and osteogenic cells becomes less contentious.47,54 This stem cell outperforms BMSCs.54
ASCs have the ability to restore degenerated IVDs. ASCs have been shown to enhance disc regeneration by forming a chondrogenic lineage and enhancing aggrecan and COL type II synthesis.9 By interacting with local chondrocytes or cartilage explants in cartilage defects, transplanted ASCs play a vital role in the success of cell-based therapies for cartilage regeneration.58 Upregulation of collagen type IIA, type IIB, and aggrecan gene expression is associated with ASCs development along the cartilaginous lineage and is linked to cocultures with NP cells and type II hydrogel. COL type II provides a suitable medium for ASCs attachment and a favorable microenvironment when combined with soluble substances released by NP cells to induce cartilage/NP lineage development. COL type II, the dominant collagen in the nucleus pulposus ECM, has been demonstrated to sustain and even induce the chondrogenic phenotype in MSCs.25
Increased quantities of IVD cells and ECM components were reported when ASCs were cultured with TGF-3 in vitro. At the same time, ASCs possessed a stronger ability to develop into NP cells than BMSCs when induced by TGF-1, growth differentiation factor (GDF)-5, or GDF-6, and the expression levels of ECM components such as sulfated glycosaminoglycans and COL II were much higher.67 ECM forms up a large portion of IVD, injected stem cells are expected to interact with ECM components after being injected into the disc. ECM has been found to be important in the regulation of stem cell differentiation into several lineages, cell proliferation, and cell migration.25 We try to summarize the mechanism of IVD degeneration and role of ASCs (Figure 1).
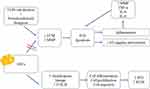 |
Figure 1 Mechanism of intravertebral disc (IVD) degeneration and role of ASCs increasing regeneration of IVD and extracellular matrix. |
ASCs are considered to play a part in the regeneration process by releasing cytokines and growth factors (GFs) that promote healing via paracrine signals.48,49,68 Several factors influence angiogenesis, including paracrine factors, stromal cell-derived factor-1 (SDF-1) chemokines, and vascular endothelial growth factors (VEGF), that causes more ASCs to migrate to damaged cells.48,49 The effect of angiogenesis/neovascularization on brown adipose tissue has been explored in various studies on rats that reported an enhanced VEGF. Endothelial cell proliferation and angiogenesis activation are induced by VEGF, which aids homeostasis.69
Animal Studies and Clinical Trials
An animal study with an IDD model injected with ASCs showed reduced disc height loss and restoration of disc signal intensity on magnetic resonance imaging.70 These stem cells can develop into NP-like cells and release an ECM composed of anionic proteoglycans, COL type II, and aggrecan.25,61,66 A set of traits distinguish nucleus pulposus cells from chondrocytes in articular cartilage.25
A study evaluated the effects of matrilin-3-primed adipose-derived MSCs (Ad-MSCs) on the repair of the degenerated disc in vitro and in vivo in rabbits model disc degeneration is searching for an optimal priming concentration and duration and developed an optimal protocol for Ad-MSC spheroid generation. Priming with 10 ng/mL matrilin-3 for 5 days resulted in the highest mRNA expression of type 2 collagen and aggrecan in vitro, it also showed the increased secretion of favorable growth factors such as transforming growth factor beta (TGF-β1), TGF-β2, interleukin-10 (IL10), granulocyte colony-stimulating factor (G-CSF), and matrix metalloproteinase 1 (MMP1) and decreased secretion of hypertrophic ECM components. Matrilin-3-primed Ad-MSC spheroid implantation was associated with optimal repair in a rabbit model. This result suggested that priming MSCs with matrilin-3 and spheroid formation could be an effective strategy to overcome the challenges associated with the use of MSCs for the treatment of IVD degeneration.71
The experiment used hematopoietic stem cells (HSCs) on IDD in animal models and clinical trials in patients. The animals experiments showed that the IVD could be regenerated by injection of HSCs, but in clinical trials none of the ten patients had significantly improved symptoms of discogenic low back pain at the 6-month and 1-year follow-up visits.72 Another research analyzed small case studies found that stem cell therapy for patients with IDD may be useful in alleviating pain or improving IVD function, but the overall data on efficacy and safety did not reveal any major findings, and it was not clear whether the changes in symptoms were clinically important.53 In general, there are no multicenter clinical studies and studies have only been on small numbers of patients. Also, the study protocols widely differ in the choice of inclusion criteria, the chosen cell sources for MSCs, the methods of transplantation and in the follow‐up conditions.35
Although many reports have demonstrated the advantages of stem cells in the treatment of IDD, there are some reports that described deficiencies the application of stem cells. A clinical trials found that cell injection therapy exceeding the normal range of cell dose was ineffective in delaying IDD or led to worse outcomes.73 Local injection of excessive numbers of cells may cause cell accumulation and death, thus triggering an inflammatory response. When injecting stem cells, the amount of injection is of great concern. MSCs may undergo unnecessary cell migration or cell leakage after IVD injection, resulting in ineffective treatment and osteophyte formation. An improper puncture operation can lead to disk infection or diskitis.61
Conclusion
ASCs appear to be a superior choice for tissue engineering in IVD regeneration. ASCs are one of the MSCs that are continuously being developed and have various advantages over autologous disc cells, BMSCs, and other MSCs. These advantages like ease of obtaining, low donor site morbidity, high proliferation rate, and excellent differentiation abilities. ASCs also have the ability to act as immunomodulators by stimulating the migration of immune cells to damaged tissues. With the harvesting process to the right and appropriate application, ASCs can be used for IVD regeneration in extreme IDD microenvironments.
The clinical trials of ASCs application is still lacking and some of the result is not linier with the animal experiments result. Therefore, further research is needed to use ASCs in regular clinical applications. Research on specific markers for ASCs, ex vivo genetic modification of cells, and regulators of differentiation, migration, and cell survival after transplantation must be clearly explained. Along with the development of scientific awareness regarding the regenerative ability and potential use of these cells, along with the development of scientific awareness regarding the regenerative ability and potential use of these cells, potentially dangerous consequences must be clarified, and the regulatory system that regulates their clinical usage must mature. The microenvironment during treatment may also play role as prognostic indicators, further research is needed to create a best result.
Abbreviations
ADMSCs, adipose-derived mesenchymal stem cells; AF, annulus fibrosus; ASCs, adipose-derived stem cells; BMSCs, bone marrow-derived mesenchymal stem cells; CEPs, cartilaginous endplates; COL, collagen; ECM, extracellular matrix; GDF, growth differentiation factor; HLA-DR, human leukocyte antigen-DR; IDD, intervertebral disc degeneration; IFATS, International Federation for Adipose Therapeutics and Science; IL, interleukin; ISCT, International Society for Cellular Therapy; IVD, intervertebral disc; LAL, laser-assisted liposuction; LBP, low back pain; MMPs, matrix metalloproteinases; MSCs, mesenchymal stem cells; Myf, myogenic factor; NP, nucleus pulposus; NPPCs, nucleus pulposus progenitor cells; PAL, power-assisted liposuction; SDF-1, stromal cell-derived factor-1; TNF, tumor necrosis factor; UCP, uncoupling protein; VEGF, vascular endothelial growth factors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Husky MM, Ferdous Farin F, Compagnone P, Fermanian C, Kovess-Masfety V. Chronic back pain and its association with quality of life in a large French population survey. Health Qual Life Outcomes. 2018;16(1):548. doi:10.1186/s12955-018-1018-4
2. Driscoll T, Jacklyn G, Orchard J, et al. The global burden of occupationally related low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(6):975–981. doi:10.1136/annrheumdis-2013-204631
3. Clouet J, Fusellier M, Camus A, Le Visage C, Guicheux J. Intervertebral disc regeneration: from cell therapy to the development of novel bioinspired endogenous repair strategies. Adv Drug Deliv Rev. 2019;146:306–324. doi:10.1016/j.addr.2018.04.017
4. Cheung KMC, Karppinen J, Chan D, et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine. 2009;34(9):934–940. doi:10.1097/BRS.0b013e3181a01b3f
5. Siemionow K, An H, Masuda K, Andersson G, Cs-Szabo G. The effects of age, sex, ethnicity, and spinal level on the rate of intervertebral disc degeneration: a review of 1712 intervertebral discs. Spine. 2011;36(17):1333–1339. doi:10.1097/BRS.0b013e3181f2a177
6. Kepler CK, Ponnappan RK, Tannoury CA, Risbud MV, Anderson DG. The molecular basis of intervertebral disc degeneration. Spine J. 2013;13(3):318–330. doi:10.1016/j.spinee.2012.12.003
7. Romaniyanto CRS, Tinduh D, et al. The potential of mesenchymal stem‐cell secretome for regeneration of intervertebral disc: a review article. Indones J Biotechnol. 2021;26(2):61–75. doi:10.22146/IJBIOTECH.63318
8. Roberts S, Evans H, Trivedi J, Menage J. Histology and Pathology of the Human Intervertebral Disc. J Bone Jt Surg. 2006;88(suppl_2):10–14. doi:10.2106/jbjs.f.00019
9. Vadalà G, Russo F, Ambrosio L, Loppini M, Denaro V. Stem cells sources for intervertebral disc regeneration. World J Stem Cells. 2016;8(5):185–201. doi:10.4252/wjsc.v8.i5.185
10. Van Den Eerenbeemt KD, Ostelo RW, Van Royen BJ, Peul WC, Van Tulder MW. Total disc replacement surgery for symptomatic degenerative lumbar disc disease: a systematic review of the literature. Eur Spine J. 2010;19(8):1262–1280. doi:10.1007/s00586-010-1445-3
11. Ishiguro H, Kaito T, Yarimitsu S, et al. Intervertebral disc regeneration with an adipose mesenchymal stem cell-derived tissue-engineered construct in a rat nucleotomy model. Acta Biomater. 2019;87:118–129. doi:10.1016/j.actbio.2019.01.050
12. Henry N, Clouet J, Le Bideau J, Le Visage C, Guicheux J. Innovative strategies for intervertebral disc regenerative medicine: from cell therapies to multiscale delivery systems. Biotechnol Adv. 2018;36(1):281–294. doi:10.1016/j.biotechadv.2017.11.009
13. Vadalà G, Ambrosio L, Russo F, Papalia R, Denaro V. Interaction between Mesenchymal Stem Cells and Intervertebral Disc Microenvironment: from Cell Therapy to Tissue Engineering. Stem Cells Int. 2019;2019:1–15. doi:10.1155/2019/2376172
14. Bach FC, Willems N, Penning LC, Ito K, Meij BP, Tryfonidou MA. Potential regenerative treatment strategies for intervertebral disc degeneration in dogs. BMC Vet Res. 2014;10(1):3. doi:10.1186/1746-6148-10-3
15. Freeman BJC, Kuliwaba JS, Jones CF, et al. Allogeneic mesenchymal precursor cells promote healing in postero-lateral annular lesions and improve indices of lumbar intervertebral disc degeneration in an ovine model. Spine. 2016;41(17):1331–1339. doi:10.1097/BRS.0000000000001528
16. Romaniyanto F, Sigit Prakoeswa CR, et al. An update of current therapeutic approach for Intervertebral Disc Degeneration: a review article. Ann Med Surg. 2022;77:103619. doi:10.1016/j.amsu.2022.103619
17. Seo Y, Shin TH, Kim HS. Current strategies to enhance adipose stem cell function: an update. Int J Mol Sci. 2019;20(15):3827. doi:10.3390/ijms20153827
18. Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. npj Regen Med. 2019;4(1). doi:10.1038/s41536-019-0083-6
19. Berebichez-Fridman R, Montero-Olvera PR. Sources and clinical applications of mesenchymal stem cells state-of-The-art review. Sultan Qaboos Univ Med J. 2018;18(3):e264–e277. doi:10.18295/squmj.2018.18.03.002
20. Feisst V, Meidinger S, Locke MB. From bench to bedside: use of human adipose-derived stem cells. Stem Cells Cloning Adv Appl. 2015;8:149–162. doi:10.2147/SCCAA.S64373
21. Francis SL, Duchi S, Onofrillo C, Di Bella C, Choong PFM. Adipose-derived mesenchymal stem cells in the use of cartilage tissue engineering: the need for a rapid isolation procedure. Stem Cells Int. 2018;2018:1–9. doi:10.1155/2018/8947548
22. Huri PY, Hamsici S, Ergene E, Huri G, Doral MN. Infrapatellar fat pad-derived stem cell-based regenerative strategies in orthopedic surgery. Knee Surg Relat Res. 2018;30(3):179–186. doi:10.5792/ksrr.17.061
23. Hye Kim J, Gyu Park S, Kim WK, Song SU, Sung JH. Functional regulation of adipose-derived stem cells by PDGF-D. Stem Cells. 2015;33(2):542–556. doi:10.1002/stem.1865
24. Rustenburg CME, Emanuel KS, Peeters M, Lems WF, Vergroesen PPA, Smit TH. Osteoarthritis and intervertebral disc degeneration: quite different, quite similar. JOR Spine. 2018;1(4):e1033. doi:10.1002/jsp2.1033
25. Hoogendoorn RJW, Lu ZF, Kroeze RJ, Bank RA, Wuisman PI, Helder MN. Adipose stem cells for intervertebral disc regeneration: current status and concepts for the future: tissue Engineering Review Series. J Cell Mol Med. 2008;12(6A):2205–2216. doi:10.1111/j.1582-4934.2008.00291.x
26. Dowdell J, Erwin M, Choma T, Vaccaro A, Iatridis J, Cho SK. Intervertebral Disk Degeneration and Repair. Neurosurgery. 2017;80(3S):S46–S54. doi:10.1093/NEUROS/NYW078
27. Pattappa G, Li Z, Peroglio M, Wismer N, Alini M, Grad S. Diversity of intervertebral disc cells: phenotype and function. J Anat. 2012;221(6):480–496. doi:10.1111/j.1469-7580.2012.01521.x
28. Iatridis JC, Nicoll SB, Michalek AJ, Walter BA, Gupta MS. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013;13(3):243–262. doi:10.1016/j.spinee.2012.12.002
29. Erwin WM, Hood KE. The cellular and molecular biology of the intervertebral disc: a clinician’s primer. J Can Chiropr Assoc. 2014;58(3):246–257.
30. Ohtori S, Inoue G, Miyagi M, Takahashi K. Pathomechanisms of discogenic low back pain in humans and animal models. Spine J. 2015;15(6):1347–1355. doi:10.1016/J.SPINEE.2013.07.490
31. Oichi T, Taniguchi Y, Oshima Y, Tanaka S, Saito T. Pathomechanism of intervertebral disc degeneration. JOR Spine. 2020;3(1). doi:10.1002/jsp2.1076
32. Kos N, Gradisnik L, Velnar T, Brief A. Review of the Degenerative Intervertebral Disc Disease. Med Arch. 2019;73(6):421–424. doi:10.5455/medarh.2019.73.421-424
33. Rodrigues-Pinto R, Richardson SM, Hoyland JA. An understanding of intervertebral disc development, maturation and cell phenotype provides clues to direct cell-based tissue regeneration therapies for disc degeneration. Eur Spine J. 2014;23(9):1803–1814. doi:10.1007/s00586-014-3305-z
34. Wuertz K, Haglund L. Inflammatory mediators in intervertebral disk degeneration and discogenic pain. Glob Spine J. 2013;3(3):175–184. doi:10.1055/S-0033-1347299
35. Loibl M, Wuertz-Kozak K, Vadala G, Lang S, Fairbank J, Urban JP. Controversies in regenerative medicine: should intervertebral disc degeneration be treated with mesenchymal stem cells? JOR Spine. 2019;2(1):e1043. doi:10.1002/jsp2.1043
36. Lyu FJ, Cui H, Pan H, et al. Painful intervertebral disc degeneration and inflammation: from laboratory evidence to clinical interventions. Bone Res. 2021;9(1):1–14. doi:10.1038/s41413-020-00125-x
37. Feng C, Liu H, Yang M, Zhang Y, Huang B, Zhou Y. Disc cell senescence in intervertebral disc degeneration: causes and molecular pathways. Cell Cycle. 2016;15(13):1674–1684. doi:10.1080/15384101.2016.1152433
38. Papavassiliou AG, Pneumaticos SG, Evangelopoulos DS. Biologic treatment of mild and moderate intervertebral disc degeneration. Mol Med. 2014;20:400–409. doi:10.2119/molmed.2014.00145
39. Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20(1):5–14. doi:10.3727/096368910X
40. Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - Current trends and future prospective. Biosci Rep. 2015;35. doi:10.1042/BSR20150025
41. Richardson SM, Hoyland JA, Mobasheri R, Csaki C, Shakibaei M, Mobasheri A. Mesenchymal stem cells in regenerative medicine: opportunities and challenges for articular cartilage and intervertebral disc tissue engineering. J Cell Physiol. 2010;222(1):23–32. doi:10.1002/jcp.21915
42. Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013;34(6):747–754. doi:10.1038/aps.2013.50
43. Miana VV, Prieto González EA. Adipose tissue stem cells in regenerative medicine. Ecancermedicalscience. 2018;12. doi:10.3332/ecancer.2018.822
44. Jadalannagari S, Aljitawi OS. Ectodermal Differentiation of Wharton’s Jelly Mesenchymal Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Tissue Eng - Part B Rev. 2015;21(3):314–322. doi:10.1089/ten.teb.2014.0404
45. Azandeh S, Gharravi AM, Orazizadeh M, Khodadi A, Tabar MH. Improvement of mesenchymal stem cell differentiation into the endoderm lineage by four step sequential method in biocompatible biomaterial. BioImpacts. 2016;6(1):9–13. doi:10.15171/bi.2016.02
46. Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International So. Cytotherapy. 2013;15(6):641–648. doi:10.1016/j.jcyt.2013.02.006
47. Li H, Zimmerlin L, Marra KG, Donnenberg VS, Donnenberg AD, Rubin JP. Adipogenic potential of adipose stem cell subpopulations. Plast Reconstr Surg. 2011;128(3):663–672. doi:10.1097/PRS.0b013e318221db33
48. Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 2017;6(6):1445–1451. doi:10.1002/sctm.17-0051
49. Hutchings G, Janowicz K, Moncrieff L, et al. The proliferation and differentiation of adipose-derived stem cells in neovascularization and angiogenesis. Int J Mol Sci. 2020;21(11):1–25. doi:10.3390/ijms21113790
50. Baer PC, Overath JM, Urbschat A, et al. Effect of different preconditioning regimens on the expression profile of murine adipose-derived stromal/stem cells. Int J Mol Sci. 2018;19(6):1719. doi:10.3390/ijms19061719
51. Wald D, Teucher B, Dinkel J, et al. Automatic Quantification of Subcutaneous and Visceral Adipose Tissue From Whole-Body Magnetic Resonance Images Suitable for Large Cohort Studies. Journal of Magnetic Resonance Imaging. 2012;36(6):1421–1434. doi:10.1002/jmri.23775
52. Peng XG, Ju S, Fang F, et al. Comparison of brown and white adipose tissue fat fractions in ob, seipin, and Fsp27 gene knockout mice by chemical shift-selective imaging and 1H-MR spectroscopy. Am J Physiol. 2013;304(2):160–167. doi:10.1152/ajpendo.00401.2012
53. Rosell M, Kaforou M, Frontini A, et al. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. Am J Physiol. 2014;306(8):E945–E964. doi:10.1152/ajpendo.00473.2013
54. Tsuji W. Adipose-derived stem cells: implications in tissue regeneration. World J Stem Cells. 2014;6(3):312. doi:10.4252/wjsc.v6.i3.312
55. Wang QA, Tao C, Jiang L, et al. Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat Cell Biol. 2015;17(9):1099–1111. doi:10.1038/ncb3217
56. Min SY, Kady J, Nam M, et al. Human “brite/beige” adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat Med. 2016;22(3):312–318. doi:10.1038/nm.4031
57. Palumbo P, Lombardi F, Siragusa G, Cifone MG, Cinque B, Giuliani M. Methods of isolation, characterization and expansion of human adipose-derived stem cells (ASCs): an overview. Int J Mol Sci. 2018;19(7):1897. doi:10.3390/ijms19071897
58. Frese L, Dijkman PE, Hoerstrup SP. Adipose tissue-derived stem cells in regenerative medicine. Transfus Med Hemotherapy. 2016;43(4):268–274. doi:10.1159/000448180
59. Bajek A, Gurtowska N, Olkowska J, et al. Does the Harvesting Technique Affect the Properties of Adipose-Derived Stem Cells?-The Comparative Biological Characterization. J Cell Biochem. 2017;118(5):1097–1107. doi:10.1002/JCB.25724
60. Cawthorn WP, Scheller EL, Macdougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. Journal of Lipid Research. 2012;53(2):227–246. doi:10.1194/jlr.R021089
61. Zhang J, Liu Y, Chen Y, et al. Adipose-Derived Stem Cells: current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020;2020:1–26. doi:10.1155/2020/8810813
62. Suzuki E, Fujita D, Takahashi M, Oba S, Nishimatsu H. Adipose tissue-derived stem cells as a therapeutic tool for cardiovascular disease. World J Cardiol. 2015;7(8):454. doi:10.4330/wjc.v7.i8.454
63. Mizuno H. Adipose-derived stem cells for regenerative medicine in the field of plastic and reconstructive surgery. J Oral Biosci. 2013;55(3):132–136. doi:10.1016/j.job.2013.04.005
64. Bajek A, Gurtowska N, Gackowska L, et al. Does the liposuction method influence the phenotypic characteristic of human adipose-derived stem cells? Biosci Rep. 2015;35(3):1–9. doi:10.1042/BSR20150067
65. Sakai D, Schol J, Watanabe M. Clinical development of regenerative medicine targeted for intervertebral disc disease. Med. 2022;58(2). doi:10.3390/medicina58020267
66. Liang C, Li H, Tao Y, et al. Responses of human adipose-derived mesenchymal stem cells to chemical microenvironment of the intervertebral disc. J Transl Med. 2012;10(1):49. doi:10.1186/1479-5876-10-49
67. Clarke LE, McConnell JC, Sherratt MJ, Derby B, Richardson SM, Hoyland JA. Growth differentiation factor 6 and transforming growth factor-beta differentially mediate mesenchymal stem cell differentiation, composition, and micromechanical properties of nucleus pulposus constructs. Arthritis Res Ther. 2014;16(2):R67. doi:10.1186/ar4505
68. Sharma A. The role of adipokines in intervertebral disc degeneration. Med Sci. 2018;6(2):34. doi:10.3390/medsci6020034
69. Zhao L, Johnson T, Liu D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res Ther. 2017;8(1):1–9. doi:10.1186/s13287-017-0578-2
70. Jeong JH, Lee JH, Jin ES, Min JK, Jeon SR, Choi KH. Regeneration of intervertebral discs in a rat disc degeneration model by implanted adipose-tissue-derived stromal cells. Acta Neurochir. 2010;152(10):1771–1777. doi:10.1007/s00701-010-0698-2
71. Muttigi MS, Kim BJ, Kumar H, et al. Efficacy of matrilin-3-primed adipose-derived mesenchymal stem cell spheroids in a rabbit model of disc degeneration. Stem Cell Res Ther. 2020;11(1):1–12. doi:10.1186/s13287-020-01862-w
72. Haufe SMW, Mork AR. Intradiscal injection of hematopoietic stem cells in an attempt to rejuvenate the intervertebral discs. Stem Cells Dev. 2006;15(1):136–137. doi:10.1089/scd.2006.15.136
73. Meisel HJ, Agarwal N, Hsieh PC, et al. Cell therapy for treatment of intervertebral disc degeneration: a systematic review. Glob Spine J. 2019;9(1_suppl):39S–52S. doi:10.1177/2192568219829024
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
