Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Adiponectin, May Be a Potential Protective Factor for Obesity-Related Osteoarthritis
Authors Jiang H, Pu Y, Li ZH , Liu W , Deng Y, Liang R, Zhang XM , Zuo HD
Received 1 February 2022
Accepted for publication 8 April 2022
Published 27 April 2022 Volume 2022:15 Pages 1305—1319
DOI https://doi.org/10.2147/DMSO.S359330
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Hai Jiang, Yu Pu, Zeng-Hui Li, Wei Liu, Yan Deng, Rui Liang, Xiao-Ming Zhang, Hou-Dong Zuo
Medical Imaging Key Laboratory of Sichuan Province, Department of Radiology, Affiliated Hospital of North Sichuan Medical College, Nanchong, People’s Republic of China
Correspondence: Hou-Dong Zuo, Sichuan Key Laboratory of Medical Imaging, Department of Radiology, Affiliated Hospital of North Sichuan Medical College, Nanchong, People’s Republic of China, Tel +86-817-2587621, Email [email protected]
Abstract: Osteoarthritis (OA) is the most common joint disease in elderly individuals and seriously affects quality of life. OA has often been thought to be caused by body weight load, but studies have increasingly shown that OA is an inflammation-mediated metabolic disease. The current existing evidence suggests that OA is associated with obesity-related chronic inflammation as well as abnormal lipid metabolism in obesity, such as fatty acids (FA) and triglycerides. Adiponectin, a cytokine secreted by adipose tissue, can affect the progression of OA by regulating obesity-related inflammatory factors. However, the specific molecular mechanism has not been fully elucidated. According to previous research, adiponectin can promote the metabolism of FA and triglycerides, which indicates that it is a potential protective factor for OA through many mechanisms. This article aims to review the mechanisms of chronic inflammation, FA and triglycerides in OA, as well as the potential mechanisms of adiponectin in regulating chronic inflammation and promoting FA and triglyceride metabolism. Therefore, adiponectin may have a protective effect on obesity-related OA, which could provide new insight into adiponectin and the related mechanisms in OA.
Keywords: adiponectin, obesity, osteoarthritis, inflammation, fatty acid, triglyceride
Introduction
Osteoarthritis (OA) is the most common degenerative joint disease characterized by joint pain, swelling and dysfunction. According to its causes, OA can be divided into primary and secondary forms.1 Although the detailed pathogenesis pathways of primary OA are still unclear, most scholars agree that biomechanical, inflammatory, and metabolic factors (obesity) are main risk factors in the occurrence and development of the disease. In addition, aging, endocrine (estrogen deficiency) and muscle reduction factor has also been proposed.2 According to these different risk factors, Herero-Beaumont3 proposed the existence of four clinical phenotypes-biomechanical OA, osteoporotic OA, metabolic OA (obesity-related OA), and inflammatory OA. In the obese population, the occurrence of OA is related to joint overload as well as chronic inflammation and abnormal lipid metabolism (Figure 1).4–6 In obesity-related OA, there are many M1 macrophages in adipose tissue and high levels of inflammatory factors such as interleukin-1 (IL-1), IL-6 and tumor necrosis factor-α (TNF-α) produced by adipose tissue-derived M1 macrophages.7 These cytokines are considered to be the main causes of chondrocyte damage and cartilage matrix degeneration.8 According to the researches, high levels of fatty acids (FA) and triglycerides were found to be related to the occurrence and development of OA.9,10 FA can be divided into saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) according to the length of the carbon chain and the number of double chains. PUFAs can be divided into omega-3 (n-3) and omega-6 (n-6) PUFAs according to the double bond position.11 Although n-3 PUFAs can help reduce inflammation and protect joints,12 SFAs and n-6 PUFAs have strong proinflammatory effects, and they play an important role in promoting the secretion of inflammatory factors in chondrocytes and structural damage.13,14 Epidemiological studies have shown that triglycerides can increase the risk of OA in the hand joints.6 Vitro experiments have found that triglycerides can promote the release of chondrocyte inflammation markers and the breakdown of extracellular matrix (ECM).15 Therefore, FA and triglycerides may play an important role in the progression of OA.
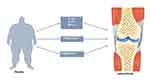 |
Figure 1 Possible mechanisms of obesity leading to osteoarthritis. IL-1β, interleukin 1 β. TNF-α, tumor necrosis factor-α. |
Adiponectin is a protein hormone derived from adipose tissue. Previous studies have found that adiponectin and its receptor are of great significance for the treatment of obesity-related diseases such as atherosclerosis and type 2 diabetes. Adiponectin can significantly improve insulin sensitivity and has anti-inflammatory properties on endothelial cells and macrophages.16,17 Meanwhile, adiponectin inhibits inflammation by regulating the proliferation and function of M1 and M2 macrophages, and also promotes FA metabolism in liver and skeletal muscle by activating amp-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor-α (PPAR-α).18 On the one hand, adiponectin can increase the activity and expression of enzymes that promote triglyceride hydrolysis, such as lipoprotein lipase (LPL), hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL); on the other hand, adiponectin can promote FA decomposition to reduce the materials for synthesizing triglycerides.18–20 Therefore, adiponectin can regulate lipid metabolism in obese people and reduce the circulating levels of FA as well as triglycerides. Therefore, adiponectin can reduce the risk factors for obesity-related OA.
This review summarizes the role of chronic inflammation, FA and triglycerides in the progression of OA in obese people, as well as the potential mechanism by which adiponectin to reduces chronic inflammation and promotes FA and triglyceride metabolism (Figure 2).
Obesity-Related Biological Processes and OA
Chronic Inflammation and OA
Osteoarthritis (OA)21 is one of the most common chronic joint diseases. Advances in epidemiology and basic medicine have given us a new definition of OA, metabolic OA, which is closely related to obesity.22 In addition to mechanical load, an increasing number of experiments have shown that inflammatory factors produced by adipose tissue play an increasingly important role in the occurrence of OA.23 As an endocrine organ, adipose tissue contains a variety of immune cells, including macrophages, T cells, B cells and neutrophils, and it is an important source of proinflammatory factors such as IL-1β, IL-6, and TNF-α.24 Macrophages are functionally classified into classically activated M1 macrophages and alternatively activated M2 macrophage.25 Pro-inflammatory M1 macrophages can secrete many inflammatory factors, such as IL-1β, IL-6, TNF-α, IL-8, IL-12 and IL-23,26 and the function of these cytokines in OA has been well established. Although anti-inflammatory M2 macrophages generate anti-inflammatory cytokines and anabolic factors, such as IL-4, IL-10, IGF-1 and transforming growth factor-β (TGF-β),27,28 these anti-inflammatory cytokines are insufficient to counteract the catabolic inflammatory response, particularly during the imbalance of a high pro-inflammatory (M1-like) to anti-inflammatory (M2-like) ratio.29,30 In obese tissues, anti-inflammatory M2 macrophages are converted into pro-inflammatory M1 macrophages.27,28 In a rat model of obesity-related OA, infiltrating macrophages in the synovium and bone marrow-derived macrophages in synovial fluid are of the M1 phenotype,29 and inducing the polarization of M2-phenotype macrophages contributes to reducing the progression of OA.31 Therefore, we have reasons to believe that M1 macrophages are the main causes of chronic inflammation in obese people.
Among the inflammatory factors released by fat cells and infiltrating macrophages, IL-1β, TNF-α and IL-6 are the key mediators involved in the progression of joint tissues in OA patients, although others also play very important roles, such as IL-15, IL-17, and IL-18.8 IL-1β and TNF-α can induce ECM degradation of chondrocytes and upregulate the expression of other factors such as iNOS, NO, COX-2, PGE2, MMPs and ADAMTS.8 These cytokines can regulate the anabolic activities of chondrocytes and destroy the cartilage structure. Studies have shown that increased concentrations of IL-1 and TNF-α can be observed in various anatomical structures of joints, such as synovial fluid, synovium, cartilage and subchondral bone layer.32 After treatment with IL-1β and TNF-α, the secretion of MMPs are significantly increased in chondrocytes, which have a key regulatory role in cartilage destruction.33 In articular cartilage, Type II collagen in cartilage and type I collagen in adjacent tendons and bones sustain joint structure strength. MMPs mediate the degradation of almost all collagen proteins.34 PGE2 can stimulate the expression and production of MMP-13 and ADAMTS-5 in human chondrocytes. This result is related to the signaling pathway mediated by EP4, which is a PGE2 receptor.35 In addition, PGE2 upregulates the expression of IL-6 mRNA and protein of T/C-28a2 chondrocytes through the cyclic adenosine monophosphate (cAMP) signaling pathway, and the level is time- and dose-dependent.36 Experimental results have shown that IL-1β and TNF-α can promote the expression of ADAMTS family members, such as ADAMTS-4 and ADAMTS-5.37 The ADAMTS family of enzymes can effectively cleave the aggregated proteins in the extracellular matrix of chondrocytes.36 In animal experiments, after deletion of the catalytic domains of ADAMTS4 and ADAMTS5, the destruction of mouse chondrocytes was significantly reduced.38,39 Studies have shown that IL-1 and TNF-α can promote iNOS activity, leading to over expression of NO in chondrocytes.40,41 On the one hand, NO can promote chondrocyte MMP expression and increase the sensitivity of chondrocytes to oxidants. On the other hand, NO indirectly participates in the production of inflammatory factors by promoting the production of TNF-α in synovial cells.42 In addition, TNF-α can recruit osteoclasts to promote bone destruction. Lam J reported that although TNF-α alone cannot induce the differentiation of macrophages into osteoclasts, TNF-α and RANKL, which are nuclear factor kappa-B (NF-κB) receptor activator ligands, can significantly enhance the activity of NF-κB and stress-activated protein kinase/c-Jun N-terminal kinase.43 These two signaling pathways are critical to the formation of osteoclasts. Therefore, TNF-α can promote macrophages with permissive levels of RANKL to generate osteoclasts.
High levels of IL-6 are detected in knee synovial fluid and surrounding tissues during the early stages of OA.44 Stannus et al reported that circulating levels of IL-6 were associated with JSN and cartilage loss in older adults.45 Latourte et al confirmed that IL-6 increases the production of the main proteases involved in OA pathogenesis, such as MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5, which are induced by Stat3 and ERK1/2 signaling pathway in chondrocytes. Blocking the effect of IL-6 with receptor blockers can reduce OA cartilage damage, osteophyte formation, and the degree of synovial inflammation.46 In addition, IL-6 is related to metabolic disorders of subchondral bone tissue. Peruzzi has reported that IL-6 inhibits the differentiation of osteoblasts into osteocytes through the SHP2/MEK2, SHP2/AKT2 and IGFBP5 signaling pathways. In addition, after treating primary mouse osteoblasts with IL-6, the expression of Runx2, collagen 1A2, and osteocalcin was reduced, which can be reversed by anti-IL-6 antibodies.47,48 RANKL and osteoprotegerin (OPG) are key regulators of osteoclast formation. Semi-quantitative RT-PCR analysis showed that the mRNA expressions of RANKL and OPG increased after 24 h incubation with human soluble IL-6 plus human soluble IL-6R (both 100ng/mL).49 This biological process involves the STAT3 signaling pathway.50
In addition to the above mentioned proinflammatory factors, the pathological mechanism of OA also involves several other chemokines, such as IL-15, IL-17, and IL-18. The level of IL-15 in joint fluids increases in early-stage OA patients, and IL-15 significantly increases the levels of MMP-1 (mean ± SEM MMP-1 release: 843% ± 222 of control) and MMP-3 (mean MMP-3: 206% ± 42 of control) secreted by articular cartilage. Moreover, IL-15 receptor α gene (IL15RA) has been associated with the development of neuropathic pain-like symptoms after nerve injury.51 In the serum and synovial fluid of OA patients, IL-17 levels are elevated and positively correlated with OA severity. Na et al reported that IL-17 stimulates chondrocytes and fibroblasts to secrete proinflammatory factors such as IL-1β, IL-6 and TNF-α, which further promote cartilage destruction.52 Bao et al reported that treating chondrocytes with different concentrations of IL-18 (0, 1, 10, 100 ng/mL) for 24 h, the mRNA expressions of Collagen II, Sox9 and Aggrecan were downregulated in a dose-dependent manner. They also find that IL-18 promotes the proapoptotic effect of rat chondrocytes by activating the PI3K/Akt/mTOR signaling pathway (Table 1).53
 |
Table 1 Inflammatory Factor Involved in the Pathophysiology of OA |
FAs and OA
In addition to low levels of systemic inflammation, another characteristic of obese patients is increased plasma levels of free FAs.54 Based on the above classification, FAs can be further classified (Figure 3). For example, SFAs include palmitic acid (PA) and stearic acid (SA), MUFAs include oleic acid (OL) and palmitoleic acid (POA), n-3 PUFAs include eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and n-6 PUFAs include linoleic acid (LA) and arachidonic acid (AA).55 Recent studies have shown that FAs are involved in the pathogenesis of OA and different types of FAs have different effects on OA.56
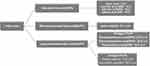 |
Figure 3 Fatty acids subtype. |
After measuring the deposited lipid composition in OA chondrocytes, it was found that the levels of FA, PA, SA, OL, LA and AA increased.13,57 In several vitro experiments, PA increased the levels of IL-1β, IL-6, MMP-13, and ADAMTS and reduced the expression of the anti-inflammatory factor IL-10.58–60 Sekar et al found that after feeding rats PA- and SA-rich feed for 16 weeks, cartilage degeneration and OA-like changes in subchondral bone occurred in the knee joints, indicated by surface irregularity, disorganization of the articular cartilage and loss of proteoglycans.61 In further research, vitro data demonstrated an enhanced expression of the hypertrophic markers ADAMTS4 and ADAMTS5 and the cartilage degenerative marker MMP-13 as well as a declining expression of ACAN, COL2, and SOX9 in MA-, PA-, and SA-treated chondrocytes compared with LA treatment.61 Insulin-like growth factor-1 (IGF-1) plays an important role in chondrocytes survival and ECM synthesis. PA can induce CHOP expression in human chondrocytes, which leads to activation of the JNK signaling pathway and inhibits IGF-1 function.62 PA and SA activate the typical NF-κB signaling pathway and promote autophagy in human chondrocytes.58 Miao et al found that SA can activate a novel lactate-HIF1α pathway to induce mice chondrocytes to express IL-1β, IL-6 and TNF-α.63 In a recent study, it was found that PA causes chondrocyte mitochondrial dysfunction and changes in glycolytic metabolism, and these effects can be partially reversed by MUFAs (such as OL).64 In a study of the relation between diet and OA progression in 2092 OA patients, with increasing levels of SFA, joint space width decreases were 0.25 mm, 0.26 mm, 0.33 mm, and 0.37 mm at 12, 24, 36, 48 months, respectively.65
Among MUFAs, the effect of OL on chondrocytes is controversial. On the one hand, OL can reduce the expression of COX-2 and MMP-1 in chondrocytes and inhibit the destruction of chondrocytes.66 On the other hand, coincubation of OL and PA stimulate chondrocytes the production of ROS and RNS such as 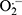 , H2O2, and NO, respectively, as well as the production of IL-6 and IL-8.67
, H2O2, and NO, respectively, as well as the production of IL-6 and IL-8.67
In recent years, the relationship between the plasma levels of N-3 PUFAs and N-6 PUFAs and the progression of OA has become a research hotspot. The most common N-6 PUFAs in the human diet are LA and AA, wherein LA is the precursor of AA, and AA is the precursor of PEG2 and leukotrienes.68 After analyzing lipids in plasma and synovial fluid, Wu found that most N-6 PUFAs were positively correlated with the severity of osteoarthritis and synovitis.69 Besides, in male patients with OA, plasma N-6 PUFA levels were positively correlated with joint effusion, knee joint structural damage,13 osteophyte formation, synovitis.70 An vitro experiment proved that after culturing chondrocytes with LA, the production of PGE2, IL-6 and NO increased.14,66,71 In addition, AA can upregulate the expression of PEG, ADAMTS72 and COX-273 in animal chondrocytes. In contrast, research on n-3 PUFAs found that they may have a protective effect on OA. Sibille et al reported that OA patients with a high n-6/n-3 ratio had more severe pain symptoms and functional limitations, while the levels of n-3 PUFAs were inversely correlated with the levels of inflammatory mediators and pain tolerance.74 The reduction in the n-3/n-6 PUFA ratio in IFP may contribute to the inflammation and cartilage degradation of early OA.75 Previous studies have shown that N-3 PUFAs significantly reduced the expression levels of ADAMTS-4, ADAMTS-5, MMP-3, MMP-13, TNF-α, IL1-β, IL-6, and COX-2. Among N-3 PUFAs, EPA is the most effective.76–78 Correspondingly, Dai et al reported that N-3 PUFAs can reduce the expression of Col-X and Runx2 to inhibit the hypertrophy and differentiation of chondrocytes.79 Moreover, Kubo et al reported that the preoperative serum EPA + DHA levels and (EPA + DHA)/AA ratio were found to be negatively associated with the serum d-ROMs (a biomarker of oxidative stress resulting from IR injury) at 96 h after surgery and ∆ d-ROMs (Table 2).80
 |
Table 2 FAs Involved in the Pathophysiology of OA |
Triglycerides and OA
Epidemiological studies of abnormal blood lipid metabolism and OA have found that elevated serum triglycerides are risk factors for the progression of OA.81 Davies-Tuck et al reported that triglycerides are associated with the prevalence of BMLs in asymptomatic middle-aged women for more than two years. The results of this study provide a basis for the hypothesis that vascular pathological changes are involved in the progression of OA.82 In another study, it was reported that triglycerides increased the risk of hand OA.6 Abourazzak et al found that hypertriglyceridemia was associated with a higher pain visual analogue scale and Lequesne index.83 Pan et al divided the pain into three groups according to the pain trajectory: minimal pain, mild pain, and moderate pain. After adjusting for potential confounding factors, it was found that hypertriglyceridemia was associated with moderate pain.84 Pan et al found that hypertriglyceridemia was independently associated with volume loss of the medial tibial cartilage on MRI. Moreover, triglycerides significantly increased the size of the BML in the medial compartment BML.85 Some studies have provided evidence for this link between hypertriglyceridemia and OA. High ECM catabolism and excessive chondrocyte death are the two main pathological features of OA.86,87 Research by Xie showed that triglycerides can promote NF-κB nuclear translation by inducing endoplasmic reticulum stress, which leads to the release of proinflammatory factors such as collagen-II and MMP-3, thereby enhancing the catabolism of the ECM.15,88
Biological Actions of Adiponectin on Obesity-Induced OA
Adiponectin
Adiponectin, also known as AdipoQ, Acrp30, ApM1, or GBP28, is a protein hormone produced exclusively by adipocytes that was first reported in 1995.89-91 This cytokine is a 28–30 kDa collagen-like protein composed of 244 amino acids. The interaction between the disulfide bonds at the amino terminus of adiponectin can form polymers of different molecular weights, including low-molecular-weight adiponectin (trimer), medium-molecular-weight adiponectin (hexamer) and high-molecular-weight adiponectin (HMW).92 Yamauchi T first confirmed AdipoR1 and AdipoR2 as adiponectin receptors in 2003.16 AdipoR1 is mainly expressed in skeletal muscle, while AdipoR2 is abundant in the liver. Adiponectin can activate AMPK, PPAR-α, PPAR-γ and other signaling pathways through these two receptors to participate in metabolic regulation.18
Adiponectin and Chronic Inflammation
Adiponectin, as a typical anti-inflammatory and antioxidant stress active substance, has been proven to have a protective effect on diabetes and atherosclerosis. However, the role of adiponectin in osteoarthritis has not been well studied.93 Recently, numerous animal and human studies have shown that adiponectin delays the progression of OA through anti-inflammatory factors. Honsawek et al94 reported that the concentration of adiponectin in blood and synovial fluid was significantly negatively correlated with the grade of OA by KL grading criteria, which suggested that it might have a potentially protective effect. Zheng and Chen et al94,95 found that plasma adiponectin levels were positively correlated with synovial fluid levels and that plasma adiponectin levels were significantly higher than synovial adiponectin levels. These results indicate that the main source of adiponectin in synovial fluid is blood. Moreover, Yusuf et al96 reported that patients with higher serum adiponectin triplets had a 70% reduced risk of developing hand OA compared with patients with the lowest serum adiponectin triplets over 6 years.
Many studies have shown that adiponectin can regulate the proliferation and function of M1 and M2 macrophages to exert its anti-inflammatory properties.97–99 In vitro, adiponectin promotes the production of IL-10 and IL-1 receptor antagonist in monocytes, monocyte derived macrophages and dendritic cells.100 Yokota et al reported that adiponectin inhibits the proliferation of bone marrow monocyte progenitor cells by inducing apoptosis and inhibite the functions of mature macrophages, including phagocytosis and the release of TNF-α.97 Wulster-Radcliffe et al found that adiponectin can inhibit the production of IL-6 and TNF-α in pig-derived adipocytes and macrophages. This biological effect is partly mediated by inhibiting the NF-κB signaling pathway and ERK1/2 activity.101,102 In adiponectin knockout mice, M1 markers, including TNF-α, IL-6 and MCP-1, were increased. Conversely, M2 markers, such as arginase-1, macrophage galactose N-acetyl-galactosamine specific lectin-1 and IL-10, were remarkably reduced.99 In addition, Wang et al previously reported that adiponectin inhibits proinflammatory cytokines, including IL-1, IL-6 and TNF-α in alveolar macrophages through the TLR2/4 signaling pathway and inhibits macrophage polarization through the COX-2/PGE2 signaling pathway.103 In subcutaneous white adipose tissue, adiponectin can promote the proliferation of M2 macrophages by activating AKT.98 Full-length adiponectin promotes the transformation of the M1 to M2 state through the AdipoR2→IL-4→STAT-6-dependent signaling pathway and AdipoR2→AMPK signaling pathway.104 In addition to the AMP-activated protein kinase and peroxisome proliferator activated receptor (PPAR)-γ, adiponectin also mediates the differentiation of monocyte macrophages into M2 macrophages through PPAR-α. Adiponectin can reduce macrophage markers in adiponectin knockout mice through these two signaling pathways.105 Therefore, adiponectin suppresses the activation of M1 subtype macrophages and enhances the proliferation of anti-inflammatory M2 subtype macrophages. However, the mechanism needs further exploration.
Chen et al reported that adiponectin can upregulate tissue inhibitor of matrix metalloproteinases-2 (TIMP-2) and downregulate IL-1β-induced MMP-13 in human chondrocyte.95 TIMP-2 has good inhibitory properties on the activity of MMPs and ADAMTS.106 After treating ATDC5 mouse chondrocytes with 0.5μg/mL adiponectin, increases in chondrocyte proliferation, proteoglycan synthesis and matrix mineralization were observed, which as reflected by the upregulation of type II collagen, aggrecan, Runx2 and type X collagen in chondrocytes. The underlying mechanism may be that adiponectin upregulated the expression of cartilage signaling molecules, such as IHH, PTHrP, Ptc1, FGF18, BMP7, IGF1 and P21.107 HU reported that subsequent to 6 h of H2O2 treatment, significant reduction in rat chondrocyte viability was demonstrated in the treated groups. However, the percentage of H2O2 induced apoptotic significantly reduced in chondrocytes pretreated with 0.5 µg/mL global adiponectin for 24 h by activating AMPK/ mTOR Signaling pathway.108 As mentioned above, adiponectin may have a protective effect on OA by reducing the inflammatory level in obese people and resisting the degradation of cartilage extracellular matrix.
In contrast, some studies have found that adiponectin has proinflammatory effects on OA. On the one hand, Korkmaz et al confirmed the positive correlation between adiponectin level and OA severity in Ahlback classification.109 On the other hand, Honsawek et al suggested that plasma adiponectin levels are negatively correlated with OA severity.94 Tang et al reported that treatment of osteoarthritis synovial fibroblast (OASF) with adiponectin (0.1–30μg/mL) for 24 h, the production of IL-6 is in a concentration-dependent manner and time-dependent manner. Further, mRNA levels of IL-6 and AdipoR1 subtype receptor were evidently increased after 12 h of adiponectin (3μg/mL) treatment. These results suggest that AdipoR1 may be involved in adiponectin-induced expression and release of IL-6 in OASF.110 Zuo et al showed that AdipoR1 may mediate adiponectin to induce synovial fibroblasts to produce PGE2 in a concentration-dependent manner.111 Tong et al reported that qPCR analysis showed that the expression of MMP-3 mRNA was significantly increased after the chondrocytes were incubated with adiponectin (30 ng/mL) for 24 h.112 Chen suggested that adiponectin up-regulates the production of Intercellular adhesion molecule-1 (ICAM-1) in human OASF via the LKB1/CaMKII, AMPK, c-Jun, and AP-1 signaling pathway.113 Besides, adiponectin could elicit perpetuate cartilage-degrading processes by inducing expression of vascular cell adhesion molecules-1 (VCAM-1) in chondrocytes, which is responsible for infiltration of leukocyte and monocyte into OA joints.114 These conflicting findings, showing protective and damaging properties, may also suggest a dual or more complex role for adiponectin in OA. Consequently, current evidence indicates that the role of adiponectin in the pathogenesis of OA has not been completely elucidated.
Adiponectin and FAs
As a candidate for the treatment of obesity-related metabolic syndrome, adiponectin can reduce plasma free FA levels after meals,115 increase plasma lipid clearance,116 and promote FA oxidation in the liver and muscle.117 Adiponectin can increase the activity of carnitine palmityl transferase I to enhance the oxidation of FAs in the liver, and it can also reduce the activities of two key enzymes involved in FA synthesis, including acetyl-CoA carboxylase (ACC) and FA synthase (FAS).118 Studies have found that full-length adiponectin can increase the level of AMPK in isolated liver cells and promote ACC phosphorylation and FA oxidation.119 Correspondingly, the production of malonyl-coenzyme A is reduced, which relieves the inhibition of carnitine palmitoyl transferase-1 (CPT-1) activity and enhances the entry of FA into the mitochondria for β oxidation.120 Schindler et al reported that adiponectin upregulated the mRNA levels of the FA transporters CD36, FATP4, FABP4 and HSL through the AMPK signaling pathway.121 The inhibition of FAS is due to adiponectin decreasing the expression of FA genes in the liver.118 CPT-1 is a potential regulator of FA β oxidation and can promote lipid inflow to the mitochondria for oxidation.122 Awazawa et al found that adiponectin inhibits the expression of sterol regulatory element binding protein (SREBP1c), which is the main regulator of enzymes related to FA synthesis in hepatocytes.123 They also found that adiponectin can inhibit SREBP1c through AdipoR1. For example, deleting LKB1, an upstream kinase of AMPK, can eliminate the negative effects of adiponectin on SREBP1c expression. These data indicate that adiponectin inhibits SREBP1c through the AdipoR1/LKB1/AMPK pathway, which suggests that adiponectin may inhibit the synthesis of FAs in the liver.123,124 In addition, adiponectin promotes FA oxidation by participating in the activation of peroxisome proliferator-activated receptor (PPAR-α).125,126 Adiponectin activates PPAR-α through AdipoR2 to promote triglyceride decomposition, intracellular FA transport, and mitochondrial FA β -oxidation.127,128 Blocking AdipoR1 and R2 leads to increased tissue triglyceride content, inflammation and oxidative stress.18 Fruebis et al reported that globular adiponectin can improve the systemic metabolic environment by promoting the oxidation of skeletal muscle FAs.116,117 Yamauchi etal reported that after treating C2C12 cardiomyocytes with adiponectin, the phosphorylation levels of FA oxidation, AMPK and ACC increased.119 Yoon et al reported that adiponectin promotes FA oxidation in muscle cells by sequentially activating AMPK, p38 MAPK and PPAR-α.125 Besides, adiponectin can increase the expression of PPAR-α target genes in C2C12 myotubes, such as ACO, CPT1 and FABP3.125,129,130 Overexpression of adiponectin can significantly upregulate the expression of genes involved in intracellular mitochondrial FA transport (LPL, CD36, CPT1B) and lipolysis (HSL, ATGL).20 Rice found that AdipoR1 overexpression in retinal pigment epithelial cells enhanced DHA uptake (Table 3).131 Since adiponectin can reduce the levels of local and circulating adiponectin by promoting FA oxidation, they are able to reduce the progression of OA. Future research may focus on revealing the relationship between adiponectin and various subtypes of FAs.
 |
Table 3 Adiponectin and FAs |
Adiponectin and Triglycerides
Most existing epidemiological studies have supported that circulating adiponectin is negatively correlated with serum triglycerides.132,133 Studies have shown that serum HMW adiponectin is negatively correlated with triglyceride-rich very low-density lipoprotein (VLDL) in univariate regression. Additionally, in multivariate regression analysis, adiponectin was the most significant predictor of plasma VLDL apolipoprotein B (apoB) concentration.134 Lipoprotein lipase (LPL) is highly expressed in tissues that use and store triglycerides, such as heart, skeletal muscle and fat, and LPL is an important target of adiponectin to regulate triglyceride catabolism.134 LPL can catalyze the hydrolysis of large amounts of triglycerides in VLDL.135 Studies have found that there is a positive correlation between LPL and adiponectin in normal subjects, patients with metabolic syndrome and T2DM136 After treatment of mice with globular adiponectin, LPL activity released by heparin was significantly increased. Additionally, the activity of LPL in epididymal white adipose tissue and heart also increased.137 Another study found that adiponectin can promote LPL and VLDL receptor (VLDLr) mRNA levels in mouse skeletal muscle, and the activity of LPL in mouse skeletal muscle was significantly increased by 41%.19 These results indicate that adiponectin promotes the catabolism of VLDL to reduce plasma triglyceride levels. The reduction of serum apo-CIII, a well-known LPL inhibitor, induced by adiponectin may be another mechanism by which adiponectin promotes triglyceride degradation. According to reports, there is a negative correlation between circulating adiponectin and serum apo-CIII.138,139 Moreover, after adiponectin treatment of human HepG2 hepatocytes, apo-CIII mRNA levels were downregulated.19 Lopez et al found that in vitro model, adiponectin can upregulate the mRNA levels of HSL and ATGL, which can promote the catabolism of triglycerides and release free FAs.20 Yamauchi et al reported that adiponectin stimulates FA oxidation through the AMPK and PPAR-α pathways, which may be a potential mechanism for adiponectin to reduce triglycerides.18 The mechanism is as described above.
Conclusion
In vivo andin vitro research continues to provide scientific evidence for the relationship between obesity and OA. The levels of inflammatory factors, FAs and triglycerides in obese people are higher than those in healthy people. Many M1 macrophages present in adipose tissue will replace M2 macrophages and release inflammatory factors, leading to cartilage cell destruction and matrix degradation. In addition, recent studies have shown that FAs and triglycerides play important roles in the progression of OA through different mechanisms. Adiponectin is a classic anti-inflammatory and antioxidant cytokine. On the one hand, adiponectin can reduce the level of inflammation in the body by regulating the proliferation and function of macrophages. On the other hand, adiponectin inhibits the secretion and function of MMPs after IL-1β treatment of chondrocytes. However, studies have shown that adiponectin is involved in the inflammation of synovial membrane and chondrocytes. Therefore, the relationship between adiponectin and OA is controversial. Moreover, adiponectin can promote the decomposition of FAs in the liver and skeletal muscle and can also promote the decomposition of triglycerides by regulating the metabolism of VLDL, a lipoprotein that contains the most triglycerides. Although the role of adiponectin in OA has not been elucidated, it has been indicated to be effective in decreasing inflammatory factors and promoting the metabolism of FA and triglyceride. Therefore, adiponectin may be one of the important factors involved in the molecular events that prevent the development of OA. Future studies need to explicitly study the anti-inflammatory activity of adiponectin in different clinical OA subtypes and levels. Investigating the clinical significance of adiponectin in the normal and pathological process of OA may provide a potential therapeutic target for disease treatment. In conclusion, adiponectin may have a protective effect on obesity-related OA.
Acknowledgments
Thanks American Journal Experts for editing this manuscript.
Funding
This review was supported by grants from the Nature Science Foundation of China (Grant no. 81701756), Sichuan Provincial Department of Education (Grant no. 18ZB0215), City-School Cooperation Project (Grant no. 18SXHZ0389).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Martel-Pelletier J, Barr AJ, Cicuttini FM, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2(1):16072. doi:10.1038/nrdp.2016.72
2. Berenbaum F. Deep phenotyping of osteoarthritis: a step forward. Ann Rheum Dis. 2019;78(1):3–5. doi:10.1136/annrheumdis-2018-213864
3. Herrero-Beaumont G, Roman-Blas JA, Bruyere O, et al. Clinical settings in knee osteoarthritis: pathophysiology guides treatment. Maturitas. 2017;96:54–57. doi:10.1016/j.maturitas.2016.11.013
4. Xie C, Chen Q. Adipokines: new therapeutic target for osteoarthritis? Curr Rheumatol Rep. 2019;21(12):71. doi:10.1007/s11926-019-0868-z
5. Van de Vyver A, Clockaerts S, van de Lest CHA, et al. Synovial fluid fatty acid profiles differ between osteoarthritis and healthy patients. Cartilage. 2020;11(4):473–478. doi:10.1177/1947603518798891
6. Garcia-Gil M, Reyes C, Ramos R, et al. Serum lipid levels and risk of hand osteoarthritis: the Chingford prospective cohort study. Sci Rep. 2017;7(1):3147. doi:10.1038/s41598-017-03317-4
7. Wang T, He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. doi:10.1016/j.cytogfr.2018.10.002
8. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. doi:10.1038/nrrheum.2010.196
9. Sanchez-Santos MT, Judge A, Gulati M, et al. Association of metabolic syndrome with knee and hand osteoarthritis: a community-based study of women. Semin Arthritis Rheum. 2019;48(5):791–798. doi:10.1016/j.semarthrit.2018.07.007
10. Mustonen AM, Nieminen P. Fatty acids and oxylipins in osteoarthritis and rheumatoid arthritis-A complex field with significant potential for future treatments. Curr Rheumatol Rep. 2021;23(6):41. doi:10.1007/s11926-021-01007-9
11. Loef M, Schoones JW, Kloppenburg M, Ioan-Facsinay A. Fatty acids and osteoarthritis: different types, different effects. Joint Bone Spine. 2019;86(4):451–458. doi:10.1016/j.jbspin.2018.07.005
12. Huang MJ, Wang L, Jin DD, et al. Enhancement of the synthesis of n-3 PUFAs in fat-1 transgenic mice inhibits mTORC1 signalling and delays surgically induced osteoarthritis in comparison with wild-type mice. Ann Rheum Dis. 2014;73(9):1719–1727. doi:10.1136/annrheumdis-2013-203231
13. Loef M, Ioan-Facsinay A, Mook-Kanamori DO, et al. The association of plasma fatty acids with hand and knee osteoarthritis: the NEO study. Osteoarthritis Cartilage. 2020;28(2):223–230. doi:10.1016/j.joca.2019.10.002
14. Frommer KW, Schaffler A, Rehart S, Lehr A, Muller-Ladner U, Neumann E. Free fatty acids: potential proinflammatory mediators in rheumatic diseases. Ann Rheum Dis. 2015;74(1):303–310. doi:10.1136/annrheumdis-2013-203755
15. Xie JJ, Chen J, Guo SK, et al. Panax quinquefolium saponin inhibits endoplasmic reticulum stress-induced apoptosis and the associated inflammatory response in chondrocytes and attenuates the progression of osteoarthritis in rat. Biomed Pharmacother. 2018;97:886–894. doi:10.1016/j.biopha.2017.10.068
16. Kovalchuk I, Kovalchuk O, Kalck V, et al. Pathogen-induced systemic plant signal triggers DNA rearrangements. Nature. 2003;423(6941):760–762. doi:10.1038/nature01683
17. Okamoto Y, Kihara S, Ouchi N, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106(22):2767–2770. doi:10.1161/01.CIR.0000042707.50032.19
18. Yamauchi T, Nio Y, Maki T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13(3):332–339. doi:10.1038/nm1557
19. Qiao L, Zou C, van der Westhuyzen DR, Shao J. Adiponectin reduces plasma triglyceride by increasing VLDL triglyceride catabolism. Diabetes. 2008;57(7):1824–1833. doi:10.2337/db07-0435
20. Lopez-Yus M, Lopez-Perez R, Garcia-Sobreviela MP, Del Moral-Bergos R, Lorente-Cebrian S, Arbones-Mainar JM. Adiponectin overexpression in C2C12 myocytes increases lipid oxidation and myofiber transition. J Physiol Biochem. 2021. doi:10.1007/s13105-021-00836-7
21. Fu K, Robbins SR, McDougall JJ. Osteoarthritis: the genesis of pain. Rheumatology. 2018;57(suppl_4):iv43–iv50. doi:10.1093/rheumatology/kex419
22. Lee SW, Rho JH, Lee SY, et al. Dietary fat-associated osteoarthritic chondrocytes gain resistance to lipotoxicity through PKCK2/STAMP2/FSP27. Bone Res. 2018;6(1):20. doi:10.1038/s41413-018-0020-0
23. Berenbaum F, Eymard F, Houard X. Osteoarthritis, inflammation and obesity. Curr Opin Rheumatol. 2013;25(1):114–118. doi:10.1097/BOR.0b013e32835a9414
24. Dam V, Sikder T, Santosa S. From neutrophils to macrophages: differences in regional adipose tissue depots. Obes Rev. 2016;17(1):1–17. doi:10.1111/obr.12335
25. Wang J, Xia J, Huang R, et al. Mesenchymal stem cell-derived extracellular vesicles alter disease outcomes via endorsement of macrophage polarization. Stem Cell Res Ther. 2020;11(1):424. doi:10.1186/s13287-020-01937-8
26. Nahrendorf M, Swirski FK. Abandoning M1/M2 for a network model of macrophage function. Circ Res. 2016;119(3):414–417. doi:10.1161/CIRCRESAHA.116.309194
27. Vieira-Potter VJ. Inflammation and macrophage modulation in adipose tissues. Cell Microbiol. 2014;16(10):1484–1492. doi:10.1111/cmi.12336
28. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. doi:10.1172/JCI29881
29. Sun AR, Panchal SK, Friis T, et al. Obesity-associated metabolic syndrome spontaneously induces infiltration of pro-inflammatory macrophage in synovium and promotes osteoarthritis. PLoS One. 2017;12(8):e0183693. doi:10.1371/journal.pone.0183693
30. Griffin TM, Scanzello CR. Innate inflammation and synovial macrophages in osteoarthritis pathophysiology. Clin Exp Rheumatol. 2019;37 Suppl 120(5):57–63.
31. Sun AR, Wu X, Liu B, et al. Pro-resolving lipid mediator ameliorates obesity induced osteoarthritis by regulating synovial macrophage polarisation. Sci Rep. 2019;9(1):426. doi:10.1038/s41598-018-36909-9
32. Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi:10.1155/2014/561459
33. Mueller MB, Tuan RS. Anabolic/Catabolic balance in pathogenesis of osteoarthritis: identifying molecular targets. PM R. 2011;3(6 Suppl 1):S3–11. doi:10.1016/j.pmrj.2011.05.009
34. Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11(1):529–543. doi:10.2741/1817
35. Attur M, Al-Mussawir HE, Patel J, et al. Prostaglandin E2 exerts catabolic effects in osteoarthritis cartilage: evidence for signaling via the EP4 receptor. J Immunol. 2008;181(7):5082–5088. doi:10.4049/jimmunol.181.7.5082
36. Wang P, Zhu F, Konstantopoulos K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-kappaB activation. Am J Physiol Cell Physiol. 2010;298(6):C1445–1456. doi:10.1152/ajpcell.00508.2009
37. Tortorella MD, Malfait AM, Deccico C, Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9(6):539–552. doi:10.1053/joca.2001.0427
38. Glasson SS, Askew R, Sheppard B, et al. Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis Rheum. 2004;50(8):2547–2558. doi:10.1002/art.20558
39. Glasson SS, Askew R, Sheppard B, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–648. doi:10.1038/nature03369
40. Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;427 Suppl:S27–36.
41. Akaraphutiporn E, Sunaga T, Bwalya EC, Yanlin W, Carol M, Okumura M. An insight into the role of apoptosis and autophagy in nitric oxide-induced articular chondrocyte cell death. Cartilage. 2020;13(2_suppl):826S–38S.
42. Vuolteenaho K, Moilanen T, Knowles RG, Moilanen E. The role of nitric oxide in osteoarthritis. Scand J Rheumatol. 2007;36(4):247–258. doi:10.1080/03009740701483014
43. Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106(12):1481–1488. doi:10.1172/JCI11176
44. Tsuchida AI, Beekhuizen M, Rutgers M, et al. Interleukin-6 is elevated in synovial fluid of patients with focal cartilage defects and stimulates cartilage matrix production in an in vitro regeneration model. Arthritis Res Ther. 2012;14(6):R262. doi:10.1186/ar4107
45. Stannus O, Jones G, Cicuttini F, et al. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18(11):1441–1447. doi:10.1016/j.joca.2010.08.016
46. Latourte A, Cherifi C, Maillet J, et al. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann Rheum Dis. 2017;76(4):748–755. doi:10.1136/annrheumdis-2016-209757
47. Kaneshiro S, Ebina K, Shi K, et al. IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J Bone Miner Metab. 2013;32(4):378–392. doi:10.1007/s00774-013-0514-1
48. Peruzzi B, Cappariello A, Del Fattore A, Rucci N, De Benedetti F, Teti A. c-Src and IL-6 inhibit osteoblast differentiation and integrate IGFBP5 signalling. Nat Commun. 2012;3(1):630. doi:10.1038/ncomms1651
49. Palmqvist P, Persson E, Conaway HH, Lerner UH. IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-kappa B ligand, osteoprotegerin, and receptor activator of NF-kappa B in mouse calvariae. J Immunol. 2002;169(6):3353–3362. doi:10.4049/jimmunol.169.6.3353
50. O’Brien CA, Lin SC, Bellido T, Manolagas SC. Expression levels of gp130 in bone marrow stromal cells determine the magnitude of osteoclastogenic signals generated by IL-6-type cytokines. J Cell Biochem. 2000;79(4):532–541. doi:10.1002/1097-4644(20001215)79:4<532::AID-JCB20>3.0.CO;2-U
51. Warner SC, Nair A, Marpadga R, et al. IL-15 and IL15RA in osteoarthritis: association with symptoms and protease production, but not structural severity. Front Immunol. 2020;11:1385. doi:10.3389/fimmu.2020.01385
52. Na HS, Park JS, Cho KH, et al. Interleukin-1-interleukin-17 signaling axis induces cartilage destruction and promotes experimental osteoarthritis. Front Immunol. 2020;11:730. doi:10.3389/fimmu.2020.00730
53. Bao J, Chen Z, Xu L, Wu L, Xiong Y. Rapamycin protects chondrocytes against IL-18-induced apoptosis and ameliorates rat osteoarthritis. Aging. 2020;12(6):5152–5167. doi:10.18632/aging.102937
54. Boden G. Obesity and free fatty acids. Endocrinol Metab Clin North Am. 2008;37(3):
55. Korbecki J, Gutowska I, Wiercioch M, et al. Sodium orthovanadate changes fatty acid composition and increased expression of stearoyl-coenzyme A desaturase in THP-1 macrophages. Biol Trace Elem Res. 2020;193(1):152–161. doi:10.1007/s12011-019-01699-2
56. Bao M, Zhang K, Wei Y, et al. Therapeutic potentials and modulatory mechanisms of fatty acids in bone. Cell Prolif. 2020;53(2):e12735. doi:10.1111/cpr.12735
57. Cillero-Pastor B, Eijkel G, Kiss A, Blanco FJ, Heeren RM. Time-of-flight secondary ion mass spectrometry-based molecular distribution distinguishing healthy and osteoarthritic human cartilage. Anal Chem. 2012;84(21):8909–8916. doi:10.1021/ac301853q
58. Sekar S, Wu X, Friis T, Crawford R, Prasadam I, Xiao Y. Saturated fatty acids promote chondrocyte matrix remodeling through reprogramming of autophagy pathways. Nutrition. 2018;54:144–152. doi:10.1016/j.nut.2018.02.018
59. Frommer KW, Hasseli R, Schäffler A, et al. Free fatty acids in bone pathophysiology of rheumatic diseases. Front Immunol. 2019;10:2757. doi:10.3389/fimmu.2019.02757
60. Ma H, Liu CM, Shao SQ, et al. Myriocin alleviates oleic/palmitate induced chondrocyte degeneration via the suppression of ceramide. Eur Rev Med Pharmacol Sci. 2020;24(24):12938–12947. doi:10.26355/eurrev_202012_24197
61. Sekar S, Shafie SR, Prasadam I, et al. Saturated fatty acids induce development of both metabolic syndrome and osteoarthritis in rats. Sci Rep. 2017;7(1):46457. doi:10.1038/srep46457
62. Nazli SA, Loeser RF, Chubinskaya S, Willey JS, Yammani RR. High fat-diet and saturated fatty acid palmitate inhibits IGF-1 function in chondrocytes. Osteoarthritis Cartilage. 2017;25(9):1516–1521. doi:10.1016/j.joca.2017.05.011
63. Miao H, Chen L, Hao L, et al. Stearic acid induces proinflammatory cytokine production partly through activation of lactate-HIF1alpha pathway in chondrocytes. Sci Rep. 2015;5(1):13092. doi:10.1038/srep13092
64. Vazquez-Mosquera ME, Fernandez-Moreno M, Cortes-Pereira E, et al. Oleate prevents palmitate-induced mitochondrial dysfunction in chondrocytes. Front Physiol. 2021;12:670753. doi:10.3389/fphys.2021.670753
65. Lu B, Driban JB, Xu C, Lapane KL, McAlindon TE, Eaton CB. Dietary fat intake and radiographic progression of knee osteoarthritis: data from the osteoarthritis initiative. Arthritis Care Res. 2017;69(3):368–375. doi:10.1002/acr.22952
66. Bastiaansen-Jenniskens YM, Siawash M, van de Lest CHA, et al. Monounsaturated and saturated, but not n-6 polyunsaturated fatty acids decrease cartilage destruction under inflammatory conditions. Cartilage. 2013;4(4):321–328. doi:10.1177/1947603513494401
67. Medina-Luna D, Santamaria-Olmedo MG, Zamudio-Cuevas Y, et al. Hyperlipidemic microenvironment conditionates damage mechanisms in human chondrocytes by oxidative stress. Lipids Health Dis. 2017;16(1):114. doi:10.1186/s12944-017-0510-x
68. Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2018;132:41–48. doi:10.1016/j.plefa.2018.03.004
69. Wu C-L, Kimmerling KA, Little D, Guilak F. Serum and synovial fluid lipidomic profiles predict obesity-associated osteoarthritis, synovitis, and wound repair. Sci Rep. 2017;7(1):1. doi:10.1038/s41598-016-0028-x
70. Wu CL, Jain D, McNeill JN, et al. Dietary fatty acid content regulates wound repair and the pathogenesis of osteoarthritis following joint injury. Ann Rheum Dis. 2015;74(11):2076–2083. doi:10.1136/annrheumdis-2014-205601
71. Shen CL, Dunn DM, Henry JH, Li Y, Watkins BA. Decreased production of inflammatory mediators in human osteoarthritic chondrocytes by conjugated linoleic acids. Lipids. 2004;39(2):161–166. doi:10.1007/s11745-004-1214-6
72. Adler N, Schoeniger A, Fuhrmann H. Polyunsaturated fatty acids influence inflammatory markers in a cellular model for canine osteoarthritis. J Anim Physiol Anim Nutr. 2018;102(2):e623–e632. doi:10.1111/jpn.12804
73. Hurst S, Rees SG, Randerson PF, Caterson B, Harwood JL. Contrasting effects of n-3 and n-6 fatty acids on cyclooxygenase-2 in model systems for arthritis. Lipids. 2009;44(10):889–896. doi:10.1007/s11745-009-3347-x
74. Sibille KT, King C, Garrett TJ, et al. Omega-6: omega-3 PUFA ratio, pain, functioning, and distress in adults with knee pain. Clin J Pain. 2018;34(2):182–189. doi:10.1097/AJP.0000000000000517
75. Mustonen AM, Kakela R, Finnila MAJ, et al. Anterior cruciate ligament transection alters the n-3/n-6 fatty acid balance in the lapine infrapatellar fat pad. Lipids Health Dis. 2019;18(1):67. doi:10.1186/s12944-019-1008-5
76. Caron JP, Gandy JC, Brown JL, Sordillo LM. Omega-3 fatty acids and docosahexaenoic acid oxymetabolites modulate the inflammatory response of equine recombinant interleukin1β-stimulated equine synoviocytes. Prostaglandins Other Lipid Mediat. 2019;142:1–8. doi:10.1016/j.prostaglandins.2019.02.007
77. Zainal Z, Longman AJ, Hurst S, et al. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthritis Cartilage. 2009;17(7):896–905. doi:10.1016/j.joca.2008.12.009
78. Curtis CL, Hughes CE, Flannery CR, Little CB, Harwood JL, Caterson B. n-3 fatty acids specifically modulate catabolic factors involved in articular cartilage degradation. J Biol Chem. 2000;275(2):721–724. doi:10.1074/jbc.275.2.721
79. Dai Y, Zhang L, Yan Z, et al. A low proportion n-6/n-3 PUFA diet supplemented with Antarctic krill (Euphausia superba) oil protects against osteoarthritis by attenuating inflammation in ovariectomized mice. Food Funct. 2021;12(15):6766–6779. doi:10.1039/D1FO00056J
80. Kubo Y, Ikeya M, Sugiyama S, et al. Association between preoperative long-chain polyunsaturated fatty acids and oxidative stress immediately after total knee arthroplasty: a pilot study. Nutrients. 2021;13(6):2093. doi:10.3390/nu13062093
81. Chang HW, Sudirman S, Yen YW, Mao CF, Ong AD, Kong ZL. Blue mussel (Mytilus edulis) water extract ameliorates inflammatory responses and oxidative stress on osteoarthritis in obese rats. J Pain Res. 2020;13:1109–1119. doi:10.2147/JPR.S244372
82. Davies-Tuck ML, Hanna F, Davis SR, et al. Total cholesterol and triglycerides are associated with the development of new bone marrow lesions in asymptomatic middle-aged women - A prospective cohort study. Arthritis Res Ther. 2009;11(6):R181. doi:10.1186/ar2873
83. Abourazzak FE, Talbi S, Lazrak F, et al. Does metabolic syndrome or its individual components affect pain and function in knee osteoarthritis women? Curr Rheumatol Rev. 2015;11(1):8–14. doi:10.2174/1573397111666150522093337
84. Pan F, Tian J, Cicuttini F, Jones G. Metabolic syndrome and trajectory of knee pain in older adults. Osteoarthritis Cartilage. 2020;28(1):45–52. doi:10.1016/j.joca.2019.05.030
85. Pan F, Tian J, Mattap SM, Cicuttini F, Jones G. Association between metabolic syndrome and knee structural change on MRI. Rheumatology. 2020;59(1):185–193. doi:10.1093/rheumatology/kez266
86. Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(7):412–420. doi:10.1038/nrrheum.2016.65
87. Heraud F. Apoptosis in normal and osteoarthritic human articular cartilage. Ann Rheum Dis. 2000;59(12):959–965. doi:10.1136/ard.59.12.959
88. Chen J, Xie JJ, Shi KS, et al. Glucagon-like peptide-1 receptor regulates endoplasmic reticulum stress-induced apoptosis and the associated inflammatory response in chondrocytes and the progression of osteoarthritis in rat. Cell Death Dis. 2018;9(2):212. doi:10.1038/s41419-017-0217-y
89. Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (adipose most abundant gene transcript 1). 1996. Biochem Biophys Res Commun. 2012;425(3):556–559. doi:10.1016/j.bbrc.2012.08.023
90. Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270(45):26746–26749. doi:10.1074/jbc.270.45.26746
91. Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120(4):803–812. doi:10.1093/oxfordjournals.jbchem.a021483
92. Pajvani UB, Du X, Combs TP, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278(11):9073–9085. doi:10.1074/jbc.M207198200
93. Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18(6):6. doi:10.3390/ijms18061321
94. Honsawek S, Chayanupatkul M. Correlation of plasma and synovial fluid adiponectin with knee osteoarthritis severity. Arch Med Res. 2010;41(8):593–598. doi:10.1016/j.arcmed.2010.11.007
95. Chen TH, Chen L, Hsieh MS, Chang CP, Chou DT, Tsai SH. Evidence for a protective role for adiponectin in osteoarthritis. Biochim Biophys Acta. 2006;1762(8):711–718. doi:10.1016/j.bbadis.2006.06.008
96. Yusuf E, Ioan-Facsinay A, Bijsterbosch J, et al. Association between leptin, adiponectin and resistin and long-term progression of hand osteoarthritis. Ann Rheum Dis. 2011;70(7):1282–1284. doi:10.1136/ard.2010.146282
97. Yokota T, Oritani K, Takahashi I, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96(5):1723–1732. doi:10.1182/blood.V96.5.1723
98. Hui X, Gu P, Zhang J, et al. Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab. 2015;22(2):279–290. doi:10.1016/j.cmet.2015.06.004
99. Ohashi K, Parker JL, Ouchi N, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285(9):6153–6160. doi:10.1074/jbc.M109.088708
100. Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323(2):630–635. doi:10.1016/j.bbrc.2004.08.145
101. Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-kappaB activation and IL-6 production and increases PPARgamma2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol. 2005;288(5):R1220–1225. doi:10.1152/ajpregu.00397.2004
102. Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316(3):924–929. doi:10.1016/j.bbrc.2004.02.130
103. Wang D, Zhang S, Liu B, Wang B, He S, Zhang R. Anti-inflammatory effects of adiponectin in cigarette smoke-activated alveolar macrophage through the COX-2/PGE2 and TLRs signaling pathway. Cytokine. 2020;133:155148. doi:10.1016/j.cyto.2020.155148
104. Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem. 2011;286(15):13460–13469. doi:10.1074/jbc.M110.204644
105. Lovren F, Pan Y, Quan A, et al. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am J Physiol Heart Circ Physiol. 2010;299(3):H656–663. doi:10.1152/ajpheart.00115.2010
106. Cabral-Pacheco GA, Garza-Veloz I, Castruita-de la Rosa C, et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int J Mol Sci. 2020;21(24):9739. doi:10.3390/ijms21249739
107. Challa TD, Rais Y, Ornan EM. Effect of adiponectin on ATDC5 proliferation, differentiation and signaling pathways. Mol Cell Endocrinol. 2010;323(2):282–291. doi:10.1016/j.mce.2010.03.025
108. Hu J, Cui W, Ding W, Gu Y, Wang Z, Fan W. Globular adiponectin attenuated H2O2-induced apoptosis in rat chondrocytes by inducing autophagy through the AMPK/ mTOR pathway. Cell Physiol Biochem. 2017;43(1):367–382. doi:10.1159/000480416
109. Korkmaz C. Response to ‘Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways’. Arthritis Res Ther. 2012;14(3):402. doi:10.1186/ar3862
110. Tang CH, Chiu YC, Tan TW, Yang RS, Fu WM. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-kappa B pathway. J Immunol. 2007;179(8):5483–5492. doi:10.4049/jimmunol.179.8.5483
111. Zuo W, Wu ZH, Wu N, et al. Adiponectin receptor 1 mediates the difference in adiponectin- induced prostaglandin E2 production in rheumatoid arthritis and osteoarthritis synovial fibroblasts. Chin Med J. 2011;124(23):3919–3924.
112. Tong KM, Chen CP, Huang KC, et al. Adiponectin increases MMP-3 expression in human chondrocytes through AdipoR1 signaling pathway. J Cell Biochem. 2011;112(5):1431–1440. doi:10.1002/jcb.23059
113. Chen HT, Tsou HK, Chen JC, Shih JM, Chen YJ, Tang CH. Adiponectin enhances intercellular adhesion molecule-1 expression and promotes monocyte adhesion in human synovial fibroblasts. PLoS One. 2014;9(3):e92741. doi:10.1371/journal.pone.0092741
114. Conde J, Scotece M, Lopez V, et al. Adiponectin and leptin induce VCAM-1 expression in human and murine chondrocytes. PLoS One. 2012;7(12):e52533. doi:10.1371/journal.pone.0052533
115. Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13(2):84–89. doi:10.1016/S1043-2760(01)00524-0
116. Fruebis J, Tsao TS, Javorschi S, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98(4):2005–2010. doi:10.1073/pnas.98.4.2005
117. Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–946. doi:10.1038/90984
118. Xu A, Wang Y, Keshaw H, Xu LY, Lam KSL, Cooper GJS. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112(1):91–100. doi:10.1172/JCI200317797
119. Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–1295. doi:10.1038/nm788
120. Stern JH, Rutkowski JM, Scherer PE. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016;23(5):770–784. doi:10.1016/j.cmet.2016.04.011
121. Schindler M, Pendzialek M, Grybel KJ, et al. Adiponectin stimulates lipid metabolism via AMPK in rabbit blastocysts. Hum Reprod. 2017;32(7):1382–1392. doi:10.1093/humrep/dex087
122. Dai J, Liang K, Zhao S, et al. Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis. Proc Natl Acad Sci U S A. 2018;115(26):E5896–E5905. doi:10.1073/pnas.1801745115
123. Awazawa M, Ueki K, Inabe K, et al. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun. 2009;382(1):51–56. doi:10.1016/j.bbrc.2009.02.131
124. Miyamoto L, Ebihara K, Kusakabe T, et al. Leptin activates hepatic 5’-AMP-activated protein kinase through sympathetic nervous system and α1-adrenergic receptor: a potential mechanism for improvement of fatty liver in lipodystrophy by leptin. J Biol Chem. 2012;287(48):40441–40447. doi:10.1074/jbc.M112.384545
125. Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55(9):2562–2570. doi:10.2337/db05-1322
126. Holland WL, Xia JY, Johnson JA, et al. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol Metab. 2017;6(3):267–275. doi:10.1016/j.molmet.2017.01.002
127. Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62(3):720–733. doi:10.1016/j.jhep.2014.10.039
128. Ishtiaq SM, Rashid H, Hussain Z, Arshad MI, Khan JA. Adiponectin and PPAR: a setup for intricate crosstalk between obesity and non-alcoholic fatty liver disease. Rev Endocr Metab Disord. 2019;20(3):253–261. doi:10.1007/s11154-019-09510-2
129. Shabani P, Emamgholipour S, Doosti M. CTRP1 in liver disease. Adv Clin Chem. 2017;79:1–23.
130. Nakamura MT, Yudell BE, Loor JJ. Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res. 2014;53:124–144. doi:10.1016/j.plipres.2013.12.001
131. Rice DS, Calandria JM, Gordon WC, et al. Adiponectin receptor 1 conserves docosahexaenoic acid and promotes photoreceptor cell survival. Nat Commun. 2015;6(1):6228. doi:10.1038/ncomms7228
132. Shim CY, Park S, Kim JS, et al. Association of plasma retinol-binding protein 4, adiponectin, and high molecular weight adiponectin with insulin resistance in non-diabetic hypertensive patients. Yonsei Med J. 2010;51(3):375–384. doi:10.3349/ymj.2010.51.3.375
133. Yamamoto Y, Hirose H, Saito I, et al. Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population. Clin Sci (Lond). 2002;103(2):137–142. doi:10.1042/CS20010336
134. Ng TW, Watts GF, Farvid MS, Chan DC, Barrett PH. Adipocytokines and VLDL metabolism: independent regulatory effects of adiponectin, insulin resistance, and fat compartments on VLDL apolipoprotein B-100 kinetics? Diabetes. 2005;54(3):795–802. doi:10.2337/diabetes.54.3.795
135. Arora R, Nimonkar AV, Baird D, et al. Structure of lipoprotein lipase in complex with GPIHBP1. Proc Natl Acad Sci U S A. 2019;116(21):10360–10365. doi:10.1073/pnas.1820171116
136. Shirakawa T, Nakajima K, Yatsuzuka S, et al. The role of circulating lipoprotein lipase and adiponectin on the particle size of remnant lipoproteins in patients with diabetes mellitus and metabolic syndrome. Clin Chim Acta. 2015;440:123–132. doi:10.1016/j.cca.2014.10.029
137. Li X, Zhang D, Vatner DF, et al. Mechanisms by which adiponectin reverses high fat diet-induced insulin resistance in mice. Proc Natl Acad Sci U S A. 2020;117(51):32584–32593. doi:10.1073/pnas.1922169117
138. Chan DC, Watts GF, Ng TW, et al. Adiponectin and other adipocytokines as predictors of markers of triglyceride-rich lipoprotein metabolism. Clin Chem. 2005;51(3):578–585. doi:10.1373/clinchem.2004.045120
139. Tsubakio-Yamamoto K, Sugimoto T, Nishida M, et al. Serum adiponectin level is correlated with the size of HDL and LDL particles determined by high performance liquid chromatography. Metabolism. 2012;61(12):1763–1770. doi:10.1016/j.metabol.2012.05.011
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.