Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Adherence to roflumilast under dose-escalation strategy in Korean patients with COPD
Authors Park TS , Kang J , Lee JS , Oh YM , Lee SD, Lee SW
Received 16 October 2018
Accepted for publication 28 December 2018
Published 16 April 2019 Volume 2019:14 Pages 871—879
DOI https://doi.org/10.2147/COPD.S191033
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Tai Sun Park,1,* Jieun Kang,2,* Jae Seung Lee,2 Yeon-Mok Oh,2 Sang-Do Lee,2 Sei Won Lee2
1Department of Internal Medicine, Hanyang University College of Medicine, Seoul, South Korea; 2Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea
*These authors contributed equally to this work
Background: Frequent development of adverse events and consequent low adherence are major barriers in the wide use of roflumilast. Asian patients may be more susceptible to adverse events due to low BMI. In this study, we aimed to determine if a dose-escalation strategy is useful to improve the drug adherence rate.
Methods: This was a randomized, prospective, open-label, single-blind study to compare the adherence rate to roflumilast according to a dose-escalation or conventional dose strategy in patients with COPD in South Korea. Patients were randomized into two groups (1:1), either roflumilast 500 µg once daily for 12 weeks or roflumilast 250 µg once daily for 4 weeks, and then 500 µg for 12 weeks. The primary outcome was the percentage of patients prematurely discontinuing roflumilast due to adverse events.
Results: A total of 55 patients were randomly assigned to either a conventional-dose group (n=28) or a dose-escalation group (n=27). Discontinuation rates of roflumilast due to adverse events were 46.4% for the conventional-dose group and 59.3% for the dose-escalation group. The median time to discontinuation was not different between groups (58 days for the conventional-dose group, 56 days for the dose-escalation group, p=0.629). In a multivariate analysis, older age was a significant risk factor for drug discontinuation.
Conclusion: High discontinuation rates of roflumilast were observed in both groups regardless of the dose-escalation strategy. The frequent discontinuation suggests that the dose-escalation strategy may not be useful in Asian patients.
Clinical trial: This study is registered at www.ClinicalTrials.gov with identifier number NCT02018432.
Keywords: COPD, roflumilast, adherence
Introduction
Roflumilast is an oral phosphodiesterase-4 (PDE4) inhibitor used for the treatment of patients with severe or very severe COPD, with chronic bronchitis symptoms and a history of exacerbation.1,2 Roflumilast reduces acute exacerbation rates,3,4 which helps improve patient mortality and quality of life.5–7 It has been shown to provide an additive effect to bronchodilator treatment,3,8,9 being a good add-on therapy option for frequent exacerbators. However, low adherence rate to the medication is a major barrier of its wide use in daily practice.10,11
Low adherence to roflumilast is mostly due to adverse effects at the beginning of therapy, including diarrhea, nausea, loss of appetite, abdominal pain, and headache,12,13 although these adverse events are known to resolve within a few weeks.14 In contrast to previous studies that reported a relatively small proportion of patients suffering from adverse events,9,15 it appears that adverse events that lead to discontinuation of the medication are more frequent in real clinical practice.16 Therefore, methods to improve adherence are imperative to benefit from the reduction in exacerbation rates, which will improve patient’s outcomes.
Recently, Watz et al17 reported a dose-escalation method as a useful strategy to improve roflumilast adherence (OPTIMIZE study). In their trial, patients were randomized at a ratio of 1:1:1 into groups corresponding to a 4-week treatment of roflumilast 250 μg once daily, 500 μg every other day, or 500 μg once daily, all followed by roflumilast 500 μg once daily. A significantly lower discontinuation rate was demonstrated when the drug was administered in a dose-escalation manner (from 250 μg to 500 μg). However, it is not known whether this strategy is useful in other regions or races, as this study was conducted among European patients whose mean BMI was approximately 26 kg/m2, higher than that of general Asian patients.18 Low BMI was reported as a significant risk factor for discontinuation of roflumilast.19 Patients with a low BMI may be more susceptible to adverse events leading to discontinuation, even at a lower dosage. In the present study, we aimed to determine if the dose-escalation strategy is useful for Korean patients whose BMI is generally lower and who may be less tolerable to roflumilast compared to Caucasian patients. To evaluate this, we compared the discontinuation rates of roflumilast in patients who received roflumilast 500 μg from the beginning and those who were under dose-escalation strategy (250 μg for the first 4 weeks and then 500 μg).
Materials and methods
Study design
This was a randomized, prospective, open-label, single-blind study to compare adherence rate to roflumilast according to dose-escalation or conventional dose strategy (ClinicalTrials.gov: NCT02018432). The study was conducted between April 2014 and January 2016 at Asan Medical Center, Seoul, South Korea. Patients were randomized into two groups (1:1), using a randomization table generated by SAS software (version 6.1; SAS Institute, Inc., Cary, NC, USA) as follows: 1) roflumilast 500 μg once daily for 12 weeks or 2) roflumilast 250 μg once daily for 4 weeks and then 500 μg for 12 weeks (Figure 1). Patients in the dose-escalation group were followed for 16 weeks, whereas those in the conventional-dose group were followed for 12 weeks, to monitor the development of adverse events during the 12-week use of roflumilast 500 μg.
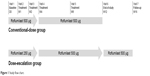  | Figure 1 Study flow chart. |
Figure 1 illustrates patient visit schedule. If a patient decided to stop roflumilast, further follow-up visits were not mandatory. The following clinical data were obtained at the baseline: demographics (birth date, gender, height, weight, smoking history, etc.), findings of physical examination, laboratory test, chest radiograph, electrocardiogram, pulmonary function test, and concurrent medication use. At each visit, patients were interviewed to monitor compliance to the medication and development of adverse events. A follow-up pulmonary function test was performed at weeks 4 and 12. Short-acting bronchodilators for on-demand symptom relief were allowed during the study; however, methylxanthine derivatives (ie, theophylline) were discontinued at least 2 weeks prior to enrollment to the study. Medications for COPD, including inhaled long-acting muscarinic antagonist (LAMA), long-acting beta-2 agonist (LABA), or combinations of LAMA and LABA, LABA and inhaled corticosteroid (ICS), LAMA, LABA, and ICS, were continued. Systemic corticosteroids were not allowed except for the treatment of acute exacerbations.
As the use of 250 μg of roflumilast was off-label, approval from Ministry of Food and Drug Safety was obtained before the initiation of the study (approval no. 20130204957). Roflumilast 250 μg was provided in the form of a 1/2 tablet. This study was conducted in accordance with the Declaration of Helsinki. The Asan Medical Center Institutional Review Board approved the study protocol (IRB no. 2013-0979). All the patients provided written informed consent for this study.
Study patients
Patients aged 40 years or older who met the following criteria were recruited: 1) clinical diagnosis of severe or very severe COPD (post-bronchodilator FEV1/FVC ratio <70% and post-bronchodilator FEV1 <50% of the predicted value) at least 4 weeks prior to the study, 2) smoking history of ≥10 pack-years, 3) at least one episode of exacerbation (visits to outpatient clinic and/or emergency room or hospitalization due to purulent sputum, increase in sputum amount, or worsened dyspnea) in the previous year, and 4) chronic bronchitis (cough and sputum production for at least 3 months within 2 years). Only those who were able to provide written informed consent were included.
Patients were excluded if they had an exacerbation or respiratory infection within 4 weeks prior to the baseline visit, known as alpha-1 antitrypsin deficiency, need for long-term oxygen therapy, or moderate-to-severe liver function impairment (Child–Pugh class B or C). Please refer to Supplementary materials for the full list of the exclusion criteria.
Study outcomes
The primary outcome was the proportion of patients who prematurely discontinued roflumilast due to adverse events during the entire study period. The secondary outcomes included the proportion of patients prematurely discontinuing roflumilast due to adverse events during the first 8 weeks, time to discontinuation due to adverse events during the study period, and changes in pre- and post-bronchodilator FEV1 from Visit 1 (baseline) to Visits 4 (week 4) and 6 (week 12).
Safety assessments included regular examination for potential adverse events using the WHO classification at each visit. Adverse events and serious adverse events were graded and summarized according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Investigators assessed the relationship between the treatment and adverse events.
Statistical analysis
Assuming the discontinuation rate of patients in the dose-escalation group as 10% and that of patients in the conventional-dose group as 35%, the required sample size per each group was calculated to be 40, with a significance level of 5% and desired power of test of 80%. Considering discontinuations due to other causes rather than adverse events, the total target sample size was estimated to be 84. Rates of discontinuation and incidence rates of adverse events were compared using the chi-squared test or Fisher’s exact test. Cox proportional hazard model was used to identify risk factors for discontinuation of roflumilast using age, BMI, dose-escalation strategy, baseline post-bronchodilator FEV1, current smoking status, amount of smoking, dyspnea grade, and type of medications as covariates. Any differences in results were considered significant if the p-value was <0.05. All data were analyzed using SPSS, version 21 (IBM Corporation, Armonk, NY, USA).
Results
Patients
The number of patients initially aimed to be recruited was 84; however, because of similarly high rates of dropout in both groups, early termination of the study was recommended by the Data Monitoring Committee after an interim analysis. Therefore, in total we studied 55 patients who were randomized into either the conventional-dose group (n=28) or the dose-escalation group (n=27).
Table 1 shows the baseline clinical characteristics of the two groups. Mean ages were 69.8 and 70.5 years, and mean BMIs were 22.2 and 21.2 kg/m2, for the conventional-dose group and the dose-escalation group, respectively. There were no significant differences in baseline characteristics between the conventional-dose and dose-escalation groups (Table 1).
  | Table 1 Baseline clinical characteristics |
Discontinuation rates of roflumilast
In the conventional-dose group, 12 patients completed the study, with 46.4% discontinuing due to adverse events. Similarly, nine dose-escalation patients finished the study, with 59.3% discontinuing due to adverse events (Table 2). There was no significant difference in discontinuation rate overall (p=0.549) or during the first 8 weeks (52% vs 64%, p=0.567). The median time to discontinuation was not significantly different between groups (58 days in the conventional-dose group and 56 days in the dose-escalation group, Figure 2, p=0.629).
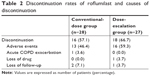  | Table 2 Discontinuation rates of roflumilast and causes of discontinuation |
  | Figure 2 Adherence rates during the study period. |
Multivariate analysis was performed to identify risk factors for discontinuation of roflumilast using the Cox proportional hazard model (Table 3). Older age was a statistically significant risk factor for discontinuation (HR, 1.082; 95% CI 1.006–1.164, p=0.034), whereas others including BMI, baseline FEV1, and dose-escalation strategy were not.
Changes in pulmonary function
Changes in pre- and post-bronchodilator FEV1 from the baseline to week 4 and week 12 were measured for patients who were on roflumilast. In the conventional-dose group, 15 patients performed spirometry at week 4 and 12 performed spirometry at week 12. In the dose-escalation group, 18 performed spirometry at week 4 and 11 patients performed spirometry at week 12. Changes in FEV1 did not significantly differ between groups (Table S1).
Adverse events
Table 4 shows the type and frequency of adverse events reported by the study patients. The most common adverse events were diarrhea (n=24), anorexia (n=17), headache (n=9), weight loss (n=8), and nausea (n=6). In both groups, diarrhea was the most common adverse event, followed by anorexia. Insomnia occurred in two patients in the dose-escalation group.
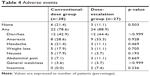  | Table 4 Adverse events |
Figure 3 shows the percentage of remaining patients with adverse events during the study period. Diarrhea was reported as early as the first week of the administration. Even after excluding patients who were intolerable to roflumilast, the percentage of patients who complained of diarrhea remained high. Approximately 25% of the patients in the conventional-dose group and 11% in the dose-escalation group reported diarrhea at the end of the study. Weight loss was reported at as early as week 2 and remained until week 12.
  | Figure 3 (A) Percentage of the patients with adverse events in the conventional-dose group and (B) percentage of the patients with adverse events in the dose-escalation group. |
Discussion
Our study found high discontinuation rates of roflumilast regardless of dose-escalation strategy. The study dropout rate due to adverse events was 46.4% and 59.3% for the conventional-dose and dose-escalation groups, respectively. Although the number of patients included in this study was small, the high discontinuation rate of roflumilast is noteworthy.
In a previous study by Rabe et al,20 discontinuations due to adverse events were more frequent in patients receiving roflumilast 500 μg than in those receiving roflumilast 250 μg. Considering that a lower dose of medication is associated with lower rate of adverse events and that most side effects resolve within few weeks, dose-escalation strategy may be an attractive method to improve the drug adherence rate. In this context, a study conducted in South Korea retrospectively assessed the adherence rate of roflumilast in patients treated with this strategy,10 demonstrating that adherence rate was 73% in patients who received roflumilast 500 μg from the beginning and 80% in those who started at 250 μg and subsequently increased to 500 μg, resulting in a lower discontinuation rate with the dose-escalation strategy. However, six patients (14%) had to decrease their dose back to 250 μg because of the increased side effects. Furthermore, it took totally 80 days for dose escalation, which was not explained by the authors, but it may be due to drug intolerance, preventing physicians from escalating the dose in real world practice.
Although the dose-escalation method was an effective strategy to improve the adherence rate in the OPTIMIZE study,17 this has not been evaluated in Asian patients. In our study, about 80% of the patients encountered any adverse events and about half of our patients discontinued roflumilast due to these adverse events. These rates were not abnormally high compared with other studies conducted in Asian patients.21–24 In a Phase III randomized trial in 411 Asian patients, the overall incidence of adverse events was 66%.22 In the OPTIMIZE study, the discontinuation rates were around 20%, much lower than observed in our study. This difference suggests that the dose-escalation strategy is not effective in Asian patients. If the probability of adverse events becomes too high, a half dose may not be enough to reach adaptation, and thus patients are still at higher risk of discontinuation when the full dose is administered. In our study, about 15% of the patients discontinued roflumilast additionally at 500 μg in the dose-escalation group.
One plausible explanation for these high discontinuation rates is a low BMI in our study patients (mean 21.7 kg/m2); low BMI has previously been shown to be a risk factor for roflumilast intolerance.19 However, a previous study reported a relatively low incidence of adverse events even among low BMI patients,25 suggesting that BMI is not the only factor contributing to high discontinuation rate in our study. Another possible explanation is a high mean age in our study patients. In fact, age was a significant risk factor for the drug discontinuation in the Cox proportional hazard model (Table 3). Different drug metabolisms by ethnicity may also explain high dropout rates. CYP enzymes account for approximately 3/4 of metabolic reactions.26 Roflumilast is converted to an active metabolite, roflumilast N-oxide, by CYP450 3A4 and 1A2, and the active metabolite is then eliminated by CYP3A4.27,28 Frequency of genetic polymorphisms for CYP3A4 differs among ethnicities, possibly leading to a divergence of drug-related reactions among different ethnicities.29 In the OPTIMIZE study, total PDE4 inhibitory activity levels were slightly higher among patients who discontinued roflumilast due to adverse events. Unfortunately, we have not measured plasma drug levels in this study. Measuring drug levels among different ethnicities may help to clarify the difference in adverse event rates between ethnicities. Further studies are needed to elucidate this association.
Considering the low tolerability to the 500 μg of roflumilast as shown in our study, one may question if 250 μg can replace 500 μg as a therapeutic dose for Asian patients or patients with low BMI. Roflumilast 500 μg, the approved dose for the treatment of COPD, was shown with lower rates of exacerbations compared with 250 μg in a clinical trial.20 However, there is a possibility that a lower dose such as 250 μg or less may be as effective as 500 μg in certain populations. To the best of our knowledge, the efficacy of 250 μg in Asian patients in terms of reducing exacerbations has not been studied. Further studies are warranted to evaluate if a lower dose is as effective as the conventional dose in Asian patients or low BMI patients.
Consistent with previous reports,1,12,25 the most common adverse event in our study was diarrhea, which was reported in almost half of the patients. Moreover, diarrhea remained highly frequent in the remaining patients after excluding those who dropped out of the study. Some patients reported continued diarrhea until the end of the study (Figure 3). According to previous work by Watz et al, discontinuation continued until day 80 as well. The persistence of adverse events may decrease long-term adherence to roflumilast; therefore, appropriate management for persistent adverse events is important.17
Limitations
Some limitations should be addressed in this study. First, this study included a relatively small number of patients; however, the similarly high rates of discontinuation in both groups prompted early termination of the study. After reaching 65% of the target patient recruitment, the interim analysis revealed that patient dropout rate due to adverse events was high regardless of the dose-escalation strategy. To demonstrate a significant difference in the drug discontinuation rate, an extreme result had to be obtained from the remained patients; in other words, at least 85% of the additional patients included in the dose-escalation group would be needed to complete the study, and meanwhile, every additional patient in the conventional-dose group would drop out of the study. This is highly unlikely, and we concluded that increasing sample size would not change our findings. Despite the small number of patients, our study provided important insights that the dose-escalation strategy, which was useful for certain population, may not be effective in other areas or ethnicities. Second, the sample size calculation might have been incorrect. Because there was no preceding study that could be used as a reference, we assumed the discontinuation rates of patients out of experience with a help from a statistician. Finally, plasma drug levels were not measured; thus, the effect of drug level or the difference of drug metabolism could not be analyzed in this study.
Conclusion
High discontinuation rates of roflumilast were observed in both groups regardless of the dose-escalation strategy. This study suggests that the dose-escalation strategy may not be useful depending on ethnicities or characteristics of patients. Given the paucity of studies focusing on Asian patients, further studies to improve the drug adherence rate of roflumilast are warranted in this population.
Data sharing statement
Participant data (clinical characteristics including age, sex, BMI, pulmonary function test results, mMRC grade, and compliance records) can be obtained on direct request to the corresponding author.
Acknowledgment
This study was supported by grants (2018-7043 and 2018-0352) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
Disclosure
The authors report no conflicts of interest in this work.
References
Beghe B, Rabe KF, Fabbri LM. Phosphodiesterase-4 inhibitor therapy for lung diseases. Am J Respir Crit Care Med. 2013;188(3):271–278. doi:10.1164/rccm.201301-0021PP | ||
Rennard SI, Calverley PM, Goehring UM, Bredenbroker D, Martinez FJ. Reduction of exacerbations by the PDE4 inhibitor roflumilast–the importance of defining different subsets of patients with COPD. Respir Res. 2011;12:18. doi:10.1186/1465-9921-12-122 | ||
Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015;385(9971):857–866. doi:10.1016/S0140-6736(14)62410-7 | ||
Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(2):154–161. doi:10.1164/rccm.200610-1563OC | ||
Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi:10.1164/ajrccm.157.5.9709032 | ||
Seneff MG, Wagner DP, Wagner RP, Zimmerman JE, Knaus WA. Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA. 1995;274(23):1852–1857. | ||
Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi:10.1136/thx.2005.040527 | ||
Bateman ED, Rabe KF, Calverley PM, et al. Roflumilast with long-acting beta2-agonists for COPD: influence of exacerbation history. Eur Respir J. 2011;38(3):553–560. doi:10.1183/09031936.00178710 | ||
Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374(9691):685–694. doi:10.1016/S0140-6736(09)61255-1 | ||
Hwang H, Shin JY, Park KR, et al. Effect of a dose-escalation regimen for improving adherence to roflumilast in patients with chronic obstructive pulmonary disease. Tuberc Respir Dis (Seoul). 2015;78(4):321–325. doi:10.4046/trd.2015.78.4.321 | ||
Munoz-Esquerre M, Diez-Ferrer M, Monton C, et al. Roflumilast added to triple therapy in patients with severe COPD: a real life study. Pulm Pharmacol Ther. 2015;30:16–21. doi:10.1016/j.pupt.2014.10.002 | ||
Michalski JM, Golden G, Ikari J, Rennard SI. PDE4: a novel target in the treatment of chronic obstructive pulmonary disease. Clin Pharmacol Ther. 2012;91(1):134–142. doi:10.1038/clpt.2011.266 | ||
Pinner NA, Hamilton LA, Hughes A. Roflumilast: a phosphodiesterase-4 inhibitor for the treatment of severe chronic obstructive pulmonary disease. Clin Ther. 2012;34(1):56–66. doi:10.1016/j.clinthera.2011.12.008 | ||
Calverley PM, Martinez FJ, Fabbri LM, Goehring UM, Rabe KF. Does roflumilast decrease exacerbations in severe COPD patients not controlled by inhaled combination therapy? The REACT study protocol. Int J Chron Obstruct Pulmon Dis. 2012;7:375–382. doi:10.2147/COPD.S31100 | ||
Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374(9691):695–703. doi:10.1016/S0140-6736(09)61252-6 | ||
Gupta S. Side-effects of roflumilast. Lancet. 2012;379(9817):710–711; author reply 711–712. DOI:10.1016/S0140-6736(12)60304-3 | ||
Watz H, Bagul N, Rabe KF, et al. Use of a 4-week up-titration regimen of roflumilast in patients with severe COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:813–822. doi:10.2147/COPD.S154012 | ||
Lim JU, Lee JH, Kim JS, et al. Comparison of World Health Organization and Asia-Pacific body mass index classifications in COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2465–2475. doi:10.2147/COPD.S141295 | ||
Kim KH, Kang HS, Kim JS, Yoon HK, Kim SK, Rhee CK. Risk factors for the discontinuation of roflumilast in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:3449–3456. doi:10.2147/COPD.S143967 | ||
Rabe KF, Bateman ED, O’Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast–an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366(9485):563–571. doi:10.1016/S0140-6736(05)67100-0 | ||
Lee JS, Hong YK, Park TS, Lee SW, Oh YM, Lee SD. Efficacy and safety of roflumilast in Korean patients with COPD. Yonsei Med J. 2016;57(4):928–935. doi:10.3349/ymj.2016.57.4.928 | ||
Lee SD, Hui DS, Mahayiddin AA, et al. Roflumilast in Asian patients with COPD: A randomized placebo-controlled trial. Respirology. 2011;16(8):1249–1257. doi:10.1111/j.1440-1843.2011.02038.x | ||
Liu D-Y, Wang Z-G, Gao Y, et al. Effect and safety of roflumilast for chronic obstructive pulmonary disease in Chinese patients. Medicine (Baltimore). 2018;97(7):e9864. doi:10.1097/MD.0000000000009864 | ||
Joo H, Han D, Lee JH, Rhee CK. Incidence of adverse effects and discontinuation rate between patients receiving 250 micrograms and 500 micrograms of roflumilast: A comparative study. Tuberc Respir Dis (Seoul). 2018;81(4):299–304. doi:10.4046/trd.2018.0015 | ||
Zheng J, Yang J, Zhou X, et al. Roflumilast for the treatment of COPD in an Asian population: a randomized, double-blind, parallel-group study. Chest. 2014;145(1):44–52. doi:10.1378/chest.13-1252 | ||
Guengerich FP. Cytochrome p450 and chemical toxicology. Chem Res Toxicol. 2008;21(1):70–83. doi:10.1021/tx700079z | ||
Lahu G, Nassr N, Hünnemeyer A. Pharmacokinetic evaluation of roflumilast. Expert Opin Drug Metab Toxicol. 2011;7(12):1577–1591. doi:10.1517/17425255.2011.632409 | ||
Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163(1):53–67. doi:10.1111/j.1476-5381.2011.01218.x | ||
Lee JS, Cheong HS, Kim LH, et al. Screening of genetic polymorphisms of CYP3A4 and CYP3A5 Genes. Korean J Physiol Pharmacol. 2013;17(6):479–484. doi:10.4196/kjpp.2013.17.6.479 |
Supplementary materials
Appendix
Adherence to roflumilast under dose-escalation strategy in patients with COPD
Exclusion criteria
- COPD exacerbation or respiratory infection 4 weeks prior to baseline visit
- Known a1-antitrypsin deficiency
- Need for long-term oxygen therapy
- Moderate-to-severe liver impairment (Child–Pugh B or C)
- Severe immunological diseases (eg, HIV infection, multiple sclerosis, lupus erythematosus, etc.)
- Severe acute infectious diseases
- Cancers
- Subjects being treated with immunosuppressive medicinal products (ie, methotrexate, azathioprine, infliximab, etanercept, or oral corticosteroids to be taken long-term) except for short-term systemic corticosteroids
- Latent infections such as tuberculosis, viral hepatitis, herpes viral infection, and herpes zoster
- Subjects with congestive heart failure (NYHA grades 3 and 4)
- Subjects with a history of depression associated with suicidal ideation or behavior
- Clinically meaningful bronchiectasis
- Pregnancy or breast feeding, or female subjects of childbearing potential unwilling to use effective contraception
- Patients with known hypersensitivity to roflumilast or rescue medication and their ingredients
- Patients with previous roflumilast therapy within past 3 months
- Patients with rare hereditary problems of galactose intolerance, Lapp lactase deficiency, or glucose-galactose malabsorption.
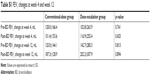  | Table S1 FEV1 changes at week 4 and week 12 |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

