Back to Journals » Patient Preference and Adherence » Volume 16
Adherence to r-hGH Therapy in Pediatric Growth Hormone Deficiency: Current Perspectives on How Patient-Generated Data Will Transform r-hGH Treatment Towards Integrated Care
Authors Savage MO, Fernandez-Luque L, Graham S, van Dommelen P, Araujo M, de Arriba A , Koledova E
Received 21 April 2022
Accepted for publication 8 July 2022
Published 11 July 2022 Volume 2022:16 Pages 1663—1671
DOI https://doi.org/10.2147/PPA.S271453
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Martin O Savage,1 Luis Fernandez-Luque,2 Selina Graham,3 Paula van Dommelen,4 Matheus Araujo,5 Antonio de Arriba,6 Ekaterina Koledova7
1Centre for Endocrinology, William Harvey Research Institute, Barts and the London School of Medicine & Dentistry, London, UK; 2Adhera Health Inc., Palo Alto, CA, USA; 3King’s College London, London, UK; 4The Netherlands Organization for Applied Scientific Research TNO, Leiden, the Netherlands; 5Neurological Institute; Cleveland Clinic, Cleveland, OH, USA; 6Paediatric Endocrinology, Hospital Universitario Miguel Servet, Zaragoza, Spain; 7Global Medical Affairs Cardiometabolic & Endocrinology, Merck Healthcare KGaA, Darmstadt, Germany
Correspondence: Martin O Savage, Centre for Endocrinology, William Harvey Research Institute, Barts and the London School of Medicine & Dentistry, Charterhouse Square, London, EC1M 6BQ, UK, Tel +44 7803084491, Email [email protected]
Abstract: Pediatric growth hormone (GH) deficiency is a licensed indication for replacement therapy with recombinant human growth hormone (r-hGH). Treatment, consisting of daily subcutaneous injections, extends from the time of diagnosis until cessation of linear growth at completion of puberty. Suboptimal adherence to r-hGH therapy is common and has been well documented to substantially impair the growth response and achievement of the optimal goal which is attainment of adult height within the genetic target range. The causes of poor adherence are complex and include disease-, patient-, doctor-, and treatment-related factors. Interventions for suboptimal adherence are important for a long-term successful outcome and can include both face-to-face and digital strategies. Face-to-face interventions include behavioral change approaches such as motivational interviewing and non-judgmental assessment. Medical and nursing staff require training in these techniques. Digital solutions are rapidly advancing as evidenced by the electronic digital auto-injector device, easypod® (Merck Healthcare KGaA, Darmstadt, Germany), which uses the web-based easypod® connect platform allowing adherence data to be transmitted electronically to healthcare professionals (HCPs), who can then access GH treatment history, enhancing clinical decisions. Over the past 10 years, the multi-national Easypod® Connect Observational Study has reported high levels of adherence (> 85%) from up to 40 countries. The easypod® connect system can be supported by a smartphone app, growlink™, which facilitates the interactions between the patients, their care team, and patient support services. HCPs are empowered by new digital techniques, however, the human–digital partnership remains essential for optimal growth management. The pediatric patient on r-hGH therapy will benefit from these innovations to enhance adherence and optimize long-term response.
Keywords: behavioral support, data, digital, growth, participatory medicine
Introduction
Adherence to a therapeutic regimen is an essential component of the success of any prescribed therapy. In the case of treatment of pediatric growth disorders, prescribed therapy in the form of recombinant human growth hormone (r-hGH) will generally be started in early childhood when the child presents with short stature and continued for many years. The aim is to normalize height during childhood and adolescence and achieve an adult height consistent with the genetic target of the family.1 Such a therapeutic regimen, consisting of daily subcutaneous injections lasting for many years, places a considerable psychological and physical burden on the patient to adhere. There is also a pressure on healthcare professionals (HCPs) responsible for this care to induce a beneficial long-term result.2
Two important considerations linked to good adherence to r-hGH are necessary for optimal outcomes. These are, first, the extent to which the patient’s behavior matches agreed recommendations from their HCP and, second, persistence with the therapy, ie lack of discontinuation.3 Adherence can be defined as the extent to which the patient follows a prescribed therapeutic regimen and, in the case of r-hGH, the extent to which daily r-hGH injections are taken. The success of r-hGH therapy, as in other chronic conditions, is thought to be dependent on the patient’s ability to maximally adhere to their treatment regimen.2,3
In this review, we will discuss the challenges, both to the patient and HCP, of maintaining a high level of adherence to r-hGH, and the factors which have been shown to influence adherence both negatively and positively. We will summarize feedback data from both HCPs and patients, and discuss knowledge from other more advanced therapeutic areas regarding the importance of data generation and analysis to understand how to positively support adherence. Our aim is to look forward to future developments in digital health which will positively impact on adherence. We will discuss the contribution of behavioral support and its digitalization as a means of supporting the family and patient, and conclude by debating the importance of design of adherence support, with continuous evaluation cycles of new digital tools, in order to achieve maximal personalized impact on the adherence paradigm and the patient’s journey.
Identifying Factors That Affect Adherence
Since the development and widespread clinical use of r-hGH in 1985, a range of growth disorders have been approved for this treatment by regulatory organizations such as the US Food and Drug Administration and European Medicines Agency.4 Initially, GH deficiency (GHD) was approved, followed by non-GH-deficient disorders such as Turner syndrome, short stature related to birth size small for gestational age (SGA), and idiopathic short stature.5 Treatment of these disorders until adult height is reached is, by definition, demanding and the issue of good adherence to the prescribed therapy is highly relevant to the final outcome.6
Thus, begins a multi-year journey involving daily injections and regular consultation visits (typically every 6 months) to assess growth and metabolic parameters. Along this journey and depending on the healthcare setting, the child and his/her parents receive various levels of information, support, and encouragement to comply with the therapy regimen. HCPs may also be involved in dealing with clinical, emotional, and behavioral issues that may arise during teenage years. Adolescents with GHD may require transitional care and continued r-hGH therapy through into adulthood to optimize body composition maturation and metabolic factors that could adversely affect their cardiovascular health.7,8
Factors adversely affecting adherence which are encountered by HCPs include managing clinical, emotional, and behavioral issues arising during teenage years.9 Other factors shown to be strongly associated with non-adherence and lack of persistence include poor understanding of both the condition and consequences of missed r-hGH doses, injection discomfort, dissatisfaction with growth outcomes compared with pediatric endocrinologist predictions, and inadequate or problematic contact with HCPs.10
Participatory Medicine and Digital Health Interventions
Digital health technologies have become an essential part of daily life and, consequently, they have high potential to support patients and caregivers in their health management. As early as 1996, research showed the positive impact of digital tools in diabetes patient education for children.11 However, the wider adoption and implementation of such technologies is still a major challenge.12 The scientific community has been looking into many factors that address adoption and acceptance of technologies and these often highlight human factors such as usability, perceived usefulness, and literacy levels.13,14 These factors related to the adoption of technology have some similarities to drivers for medication adherence,15–17 including education or how the medication is being introduced.
Addressing the patients’ and caregivers’ perspectives is crucial, especially in areas where digital health interventions are supporting medication adherence or other long-term self-management behaviors. In the case of digital interventions in pediatrics, a key aspect to consider is the interplay between caregivers and patients especially during the transition to adult care18 or patient-initiated medication. For example, a key moment to intervene is when injections are transitioning from being delivered by the caregivers to the children themselves.
In recent years, a lot of effort has been put into the use of new methodologies to capture end-user’s feedback when using digital interventions, including participatory research and design research.19 Such methodologies facilitate the capture of feedback and the perspective of patients and caregivers for adjusting behavioral interventions.20 This feedback can then be used to adjust digital interventions to minimize adoption challenges. For example, the project Sisom (from the Norwegian phrase “Si det Som det er”, meaning “Tell it how it is”) focused on capturing the feedback of children with chronic conditions using a child-friendly patient-reported outcome mobile solution designed to enhance nurse–patient relationships.21 Another example is the mobile solution Pain Squad™ for children with oncologic pain, for example, where patients were heavily involved in the design to maximize adherence to the use of the mobile-based pain diary.22 A more recent example, explained below, is the CARING study which focuses on the feasibility of supporting the emotional wellbeing of caregivers in a mobile-based digital intervention.23
Several factors are important when capturing feedback from patients and caregivers for the development of digital health interventions. Studies have shown that socio-cultural factors such as gender, ethnicity, and education level are relevant in the adoption of such digital health technologies.24–26 Also, caregivers’ and patients’ perspectives concerning digital health interventions should be included in the analysis of healthcare delivery since, in most cases, the roll-out and implementation of such solutions will impact the provision of healthcare. To address this “service delivery” angle, methods aligned with service design are often applied.14 Finally, emerging research highlights the relevance of addressing digital health literacy as an enabler for adoption. Consequently, it represents a major aspect to consider when studying the patients’ and caregivers’ perspectives. For example, high levels of digital health literacy reduce risks regarding the adoption and safe usage of digital health tools by both caregivers and patients.27
Improving Adherence with Digital Solutions in Growth Disorders Treated with r-hGH
There are several ways of administering r-hGH to pediatric patients, including syringes, pens, and auto-injector devices. One such device, the easypod® autoinjector, transmits data to a web-based platform that allows HCPs to monitor adherence and access longitudinal patient data. To test the impact of this digital ecosystem on adherence, the Easypod® Connect Observational Study (ECOS) was performed across multiple countries.28 The ECOS demonstrated how a digital health ecosystem, that records dose, date, and time of r-hGH administration, can help to maintain high adherence (≥85%; mg injected/mg prescribed) over the course of several years in different countries.28 Real-world data extracted from the easypod® connect ecosystem support these findings. In an analysis performed from 2007 to the end of 2020, adherence data were available for 20,264 patients from 38 countries.29,30 Levels of high adherence increased over time in European (76% in 2010; 82–84% in 2015–2019; 86% in 2020), North American (Canadian) (65% in 2010; 68% in 2015; 88% in 2019–2020), and Asian (58–62% in 2014–2015; 68–73% in 2016–2020) patients.29,30 No consistent change in adherence was found among Latin-American and Caribbean patients.29,30 Importantly, the observed adherence levels also had a statistically significant effect on change in Height Standard Deviation Scores (∆HSDS) from treatment start. Mean ∆HSDS were 0.4, 0.7, 1.0, and 1.1 after 12, 24, 36, and 48 months treatment, respectively, in patients with high (≥85%) monthly adherence, 0.3, 0.6, 0.8, and 0.9 in patients with intermediate (>56–84%) monthly adherence and 0.2, 0.5, 0.6, and 0.7 in patients with low (≤56%) monthly adherence.29,30
The Use of Patient-Generated Data to Develop Digital Tools
Expansion of digital health ecosystems, like easypod® connect, through addition of new digital tools that have been co-created with HCPs and patients, offers an exciting opportunity to further improve both adherence and clinical outcomes for patients with growth disorders. When developing such digital tools, we propose following an iterative cycle that leverages the use of patient-generated data (Figure 1). The approach implies that defined hypotheses are validated based on patient-generated data prior to the design of prototypes, which are then tested in a clinical setting as the basis for future hypotheses. This continuous feedback loop can help pinpoint areas for improvement based on pre-defined patient populations. First, a team of interdisciplinary HCPs defines a hypothesis to improve management towards an optimal outcome based on their clinical experience. For example, they propose a mathematical model that predicts future therapy response based on experience and demographic information. Once the hypothesis has been thoroughly defined, data scientists use information from connected devices and other data sources, such as electronic health records, to develop, analyze, and validate data-driven models in an experimental setting. If successful, an experimental model (prototype) is designed and tested in collaboration with the HCP team, taking end-user feedback into consideration. An enhanced digital ecosystem is then established as the basis for real-world evaluation of (determinants) of use and outcome, for example, in prospective clinical trials. This enhanced ecosystem not only has the potential to improve disease management, but also serves as the basis for hypothesis generation within the next iterative loop. Over time, with increased patient-generated datasets, improved synergy between experienced teams, and new assumptions and hypotheses, this agile and incremental approach to the development of digital ecosystems will reflect the evolution of healthcare provision.
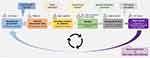 |
Figure 1 Continuous feedback loop based on patient-generated data. The data provided by patients, the HCP team and data scientists contribute to the development of an enhanced ecosystem. |
Importance of Behavioral Support for Adherence
To complement digital solutions, the use of psychology-based approaches within the healthcare environment can be beneficial to support HCPs in learning how to help patients to make healthy choices and decisions in their lives. HCPs are uniquely positioned within clinical settings to monitor, support, and promote adherence behaviors due to their existing supporting relationships with patients and their families.31 Importantly, HCPs are trusted by their patients and are often the people patients will turn to when they are thinking about making a health-related change. Addressing adherence-related issues within routine clinical practice can be a struggle, as patients and/or their families generally find it difficult to talk openly about adherence and are often reluctant or apprehensive to disclose treatment non-adherence.32 Thus, it is important for medical and nursing HCPs to be supported in core training to develop and reinforce key consultation behaviors and skills, ie motivational interviewing (MI).33
Motivational Interviewing
MI is a skill which can benefit both medical and nursing HCPs. Examples of the benefits of MI can be taken from experience in making healthy life choices. When considering these choices, reaction to the individual can be unhelpful, such as not listening or negatively encouraging regressive behavior. By contrast, a helpful response to the same life choices would consist of positive reactions such as genuine empathetic listening and exploration of the individual’s feelings without judgement. This behavior typifies the spirit of MI, the key principles of which are partnership, acceptance, compassion, and evocation (PACE). Collaboration is important because partnership on an equal level with the patient is a key aim. Acceptance leads to better understanding of the decisions and choices that patients and families are making without judgement. These choices are accepted and the HCP responds with guidance. Compassion is a further component that is combined with Evocation, which means drawing out a patient’s inner motivation and commitment, and building on this to effect change.2,33
The OARS Approach
Core skills in MI can be discussed under the acronym OARS, which stands for Open questions, Affirmations, Reflective listening, and Summarising.2,33 The conversation can be structured by following these headings. Open questions such as “what”, “how”, and “why” will open conversations and evoke dialogue. Other examples would be “what are your hopes for your consultation today?” and “I am curious to learn how you have been getting on with your injections?” These questions can be prefaced by saying “help me understand … ” and the conversation can develop by inviting the patient or family to talk about what is on their mind and what their needs and their priorities are. Affirmations are about helping patients to recognize their own strengths and positive beliefs that are going to help them to adhere to r-hGH therapy. Examples could be to say to a patient, “I can see it took courage for you to try this out today” or to a parent, “your creative ideas around this are very helpful”. Reflective listening consists of not only listening and reflecting back what is said, it also helps in verbalizing the thinking and feelings that lie underneath, showing a depth of empathy that leads to further conversations. The last skill here is summarizing, which serves the useful purpose of wrapping up conversations and can be started by saying “let me see if I have got this right, you are feeling this on one hand and perhaps feeling this on the other?”.
Psychologically-Based Patient Support Programs
Pediatric endocrinology nurse specialists can play a key role in addressing and managing the needs of patients prescribed r-hGH treatment and their families within their medical consultations. In view of this, psychologically-based patient support programs (PSPs) have been designed to help support patients and families to better manage their condition and treatment, with the purpose to optimize treatment adherence and improve clinical outcomes. These programs have demonstrated improved outcomes in a wide variety of diseases, through multidisciplinary HCP training and coaching; therefore, it is crucial for HCPs to begin to implement these new approaches within clinical practice in order to make a positive impact.34,35
One such PSP is TuiTek®, a digital, multicomponent, personalized program designed to support the needs of patients, caregivers, and HCPs throughout the treatment care pathway. The intervention comprises two key service components: 1) a PSP training session, which aims to provide the HCP with the tools and strategies to deliver the TuiTek® PSP and 2) a PSP Manual, consisting of A) a personalization screener, for HCPs to identify the key issues and challenges faced by patients and caregivers, and tailor the patient support; and B) a set of personalized one-to-one telephone call guides and resource packs which utilize a range of behavior change techniques (BCTs) and principles of MI, to support the HCP to engage in high-quality adherence-focused conversations with the patient during scheduled outbound calls with caregivers.
HCP-led calls which use BCTs and implement MI principles have been shown to affect meaningful behavior change across different health conditions such as increasing physical activity and improving diet,36,37 as well as demonstrating a positive impact on treatment adherence.38 This aligns with the findings of the TuiTek® PSP which has been shown to positively address disease- and treatment-related barriers amongst caregivers regarding optimal adherence of their children to GH treatment; this, in turn, has the potential to improve adherence levels and patient clinical health outcomes.
ADHERA CARING: An Example of Digitalized Patient Support
Caregiver emotional distress has been found to be a driver of poor adherence and self-management skills in pediatrics and growth disorders.39–41 This includes aspects related to anxiety and fear of the medication itself, but also aspects such as poor communication between parents and children. Overall, poor emotional wellbeing has a direct impact on the self-efficacy of both caregivers and patients themselves which ultimately will drive poor self-management behaviors.
There is a body of literature in pediatrics showing the efficacy of interventions to address the emotional wellbeing of caregivers of children living with chronic conditions. These include the use of techniques such as cognitive behavioral therapy and mindfulness.42,43 As a result, caregivers are better equipped to handle emotional stressors. Also, there is evidence of the positive impact of enhancing parenting skills such as communication to help cope with stressful situations related to the self-management of a chronic condition.44
In the CARING study, a digitally enabled intervention was designed and implemented to complement the work of the pediatric endocrinology unit in the University Hospital of Miguel Servet in Zaragoza, Spain.23 The clinicians identified children with suboptimal adherence using the easypod® connect platform, their caregivers were then invited to participate in a study that includes the use of a digital program to deliver an intervention designed to improve the mental wellbeing of caregivers.
The digital intervention was powered by the ADHERA CARING platform that incorporates educational content to improve self-management skills, including gamification elements (eg quizzes), and is designed to ensure understandability and usability. This is complemented by content addressing mental-wellbeing based on cognitive behavioral therapy, including content such as videos of relaxation techniques aimed at helping families to reduce anxiety before injections. Furthermore, tailored motivational messages were sent to caregivers to reinforce engagement and therapeutic effectiveness. The behavioral design of the intervention was based on the Integrated Model for Behavioral Change (I-CHANGE).20
The first phase of the study included the recruitment of 10 caregivers who tested the program for a month and provided feedback in a semi-structured interview. The qualitative feedback data was used to identify areas for improvement and adjustment of the intervention prior to starting the second phase of the study which is aimed at quantifying the clinical impact of such an intervention. The preliminary results achieved in the first phase of the study showed high engagement and positive feedback; in addition, participants highlighted the importance of such interventions not only when adherence is suboptimal but also at the initiation of the treatment.23
Unmet Needs in Growth Disorder Management: Digital Solutions
There are several unmet clinical needs related to the management of a child with GHD. The first is the late age of diagnosis. In a recent study of 39 children with GHD, the mean age of diagnosis was 4.6 years in Germany, 7.0 years in the UK, and 9.4 years in the USA.45 The late age of diagnosis has a negative impact on the adult height achieved after r-hGH therapy.1 The subjects with abnormal variables are sent for investigation and diagnosis.46 Such a technique of height screening has not yet been demonstrated to work in a real-world busy inner-city environment.
A second unmet need relates to the poor quality of growth response to r-hGH therapy, for which there are a wide range of causes. Therapy needs to be individualized, in terms of starting dose, for every child starting therapy. The “one-dose-fits-all” philosophy which was widely practiced in the 1980s and 1990s can no longer be defended and is inconsistent with the current standards of precision medicine.47 Many children are receiving inadequate doses of r-hGH with a lack of sophisticated dose individualization taking into consideration the known predictive factors.48 In addition, GH responsiveness may be affected by influences outside the GH-IGF-1 axis such as genetic variants which can induce a degree of GH resistance.49 In these subjects, r-hGH therapy should logically be discontinued. The range of responses to r-hGH also extends to children with more severe GH deficiency, who respond well to r-hGH doses below the recommended dose. A third unmet need relates to patients displaying poor adherence to r-hGH therapy, as discussed in this article.
Finally, the standard of transitional care of the adolescent with GHD after completion of linear growth from pediatric to adult care is highly variable between centers and countries.50 Several digital tools are available to assess a young patient’s readiness for transition, including the Transition Readiness Assessment Questionnaire. This has been used in endocrinology to compare young people with Turner syndrome to those with type 1 diabetes, and revealed that those with Turner syndrome are less mature in the management of their healthcare and may find the process of transfer to adult services difficult.51 This aspect is, however, also connected with national healthcare policies. Mobile devices, such as smartphone apps (e.g. Tiny Medical Apps’ Digital Health Passport app), have been developed that can assist young people in self-managing their condition.
Conclusions
We believe that supporting patients across their disease journey means more than just providing them and their physicians with an effective therapy. Beyond the prescription of r-hGH, it means providing all stakeholders involved with the tools, information, services, and support needed to achieve the goal of effective treatment and clinical benefit. Methods for assessment of adherence need to be standardized, both from the point of view of definition of adherence and its measurement.52 For GH-deficient patients, caregivers, and HCPs, this has meant a change in the attitude towards r-hGH adherence and embracing the concept of a successful human–digital partnership which is essential to achieve these goals.2 The relationship between poor adherence and poor response to r-hGH therapy is well established.53 While enthusiasm and support for digital health technologies was slow at first, these efforts have accelerated with broader awareness and acceptance amongst both patients and HCPs. New digital technologies will evolve and the introduction of innovations and new technologies, while providing challenges for patients and HCPs, have the potential to further improve the personalized management of the GH-deficient patient receiving r-hGH therapy. The development of digital ecosystems reflecting the evolution of healthcare provision and an agile incremental approach of their enhancements by Iterative loops has the potential to improve disease management.
Acknowledgments
Editorial assistance was provided by Amy Evans of inScience Communications, Springer Healthcare Ltd, UK, and was funded by Merck Healthcare KGaA, Darmstadt, Germany.
Funding
This study was sponsored by Merck (CrossRef Funder ID: 10.13039/100009945).
Disclosure
MOS has consultancy agreements with Merck Healthcare KGaA Darmstadt and Pfizer as well as honoraria for lectures from Ipsen, GeneSciences, and Sandoz. LF-L is Chief Scientific Officer at Adhera Health Inc., Palo Alto, CA, USA. SG and PvD have consultancy agreements with Merck. MA has previously had a consultancy agreement with Merck. AdA does not have any conflicts of interest to declare. EK is an employee of Merck Healthcare KGaA, Darmstadt, Germany and holds shares in the company. The authors report no other conflicts of interest in this work.
References
1. Wit JM, Deeb A, Bin-Abbas B, Al Mutair A, Koledova E, Savage MO. Achieving optimal short- and long-term responses to paediatric growth hormone therapy. J Clin Res Pediatr Endocrinol. 2019;11(4):329–340. doi:10.4274/jcrpe.galenos.2019.2019.0088
2. Child J, Davies C, Frost K, et al. Managing paediatric growth disorders: integrating technology into a personalised approach. J Clin Res Pediatr Endocrinol. 2020;12(3):225–232.
3. Fisher BG, Acerini CL. Understanding the growth hormone therapy adherence paradigm: a systematic review. Horm Res Paediatr. 2013;79(4):189–196. doi:10.1159/000350251
4. Dattani M, Malhotra N. A review of growth hormone deficiency. Paediatr Child Health. 2019;29:285–292. doi:10.1016/j.paed.2019.04.001
5. Ranke MB, Wit JM. Growth hormone - past, present and future. Nat Rev Endocrinol. 2018;14(5):285–300. doi:10.1038/nrendo.2018.22
6. Dimitri P, Fernandez-Luque L, Banerjee I, et al. An eHealth framework for managing pediatric growth disorders and growth hormone therapy. J Med Internet Res. 2021;23(5):e27446. doi:10.2196/27446
7. Kuromaru R, Kohno H, Ueyama N, Hassan HM, Honda S, Hara T. Long-term prospective study of body composition and lipid profiles during and after growth hormone (GH) treatment in children with GH deficiency: gender-specific metabolic effects. J Clin Endocrinol Metab. 1998;83(11):3890–3896. doi:10.1210/jcem.83.11.5261
8. Johannsson G, Albertsson-Wikland K, Bengtsson BA. Discontinuation of growth hormone (GH) treatment: metabolic effects in GH-deficient and GH-sufficient adolescent patients compared with control subjects. Swedish study group for growth hormone treatment in children. J Clin Endocrinol Metab. 1999;84(12):4516–4524. doi:10.1210/jcem.84.12.6176
9. Taddeo D, Egedy M, Frappier JY. Adherence to treatment in adolescents. Paediatr Child Health. 2008;13(1):19–24. doi:10.1093/pch/13.1.19
10. Graham S, Weinman J, Auyeung V. Identifying potentially modifiable factors associated with treatment non-adherence in paediatric growth hormone deficiency: a systematic review. Horm Res Paediatr. 2018;90(4):221–227. doi:10.1159/000493211
11. Brown SJ, Lieberman DA, Germeny BA, Fan YC, Wilson DM, Pasta DJ. Educational video game for juvenile diabetes: results of a controlled trial. Med Inform. 1997;22(1):77–89. doi:10.3109/14639239709089835
12. Stome LN, Wilhelmsen CR, Kvaerner KJ. Enabling guidelines for the adoption of eHealth solutions: scoping review. JMIR Form Res. 2021;5(4):e21357. doi:10.2196/21357
13. El Benny M, Kabakian-Khasholian T, El-Jardali F, Bardus M. Application of the eHealth literacy model in digital health interventions: scoping review. J Med Internet Res. 2021;23(6):e23473. doi:10.2196/23473
14. Shaw J, Agarwal P, Desveaux L, et al. Beyond “implementation”: digital health innovation and service design. NPJ Digit Med. 2018;1:48. doi:10.1038/s41746-018-0059-8
15. Acerini CL, Segal D, Criseno S, et al. Shared decision-making in growth hormone therapy-implications for patient care. Front Endocrinol. 2018;9:688. doi:10.3389/fendo.2018.00688
16. Assefi AR, Roca F, Rubstein A, Chareca C. Positive impact of targeted educational intervention in children with low adherence to growth hormone treatment identified by use of the easypod electronic auto-injector device. Front Med Technol. 2021;3:609878. doi:10.3389/fmedt.2021.609878
17. World Health Organization. Adherence to long-term therapies: evidence for action; 2003. Available from: https://www.who.int/chp/knowledge/publications/adherence_report/en/.
18. Tornivuori A, Tuominen O, Salantera S, Kosola S. A systematic review on randomized controlled trials: coaching elements of digital services to support chronically ill adolescents during transition of care. J Adv Nurs. 2020;76(6):1293–1306. doi:10.1111/jan.14323
19. Syed-Abdul S, Zhu X, Fernandez-Luque L. Digital Health. Mobile and Wearable Devices for Participatory Health Applications.
20. Cheung KL, Hors-Fraile S, de Vries H. How to use the integrated-change model to design digital health programs. In: Syed-Abdul S, Zhu X, Fernandez-Luque L, editors. Digital Health. Mobile and Wearable Devices for Participatory Health Applications.
21. Arvidsson S, Gilljam BM, Nygren J, Ruland CM, Nordby-Boe T, Svedberg P. Redesign and validation of sisom, an interactive assessment and communication tool for children with cancer. JMIR Mhealth Uhealth. 2016;4(2):e76. doi:10.2196/mhealth.5715
22. Stinson JN, Jibb LA, Nguyen C, et al. Development and testing of a multidimensional iPhone pain assessment application for adolescents with cancer. J Med Internet Res. 2013;15(3):e51. doi:10.2196/jmir.2350
23. Signorelli G, Nunez-Benjumea FJ, Munoz AA, et al. Digital health platform for emotional and self-management support of caregivers of children receiving growth hormone treatment. Stud Health Technol Inform. 2022;289:371–375. doi:10.3233/SHTI210936
24. Mitchell UA, Chebli PG, Ruggiero L, Muramatsu N. The digital divide in health-related technology use: the significance of race/ethnicity. Gerontologist. 2019;59(1):6–14. doi:10.1093/geront/gny138
25. O’Connor S, Hanlon P, O’Donnell CA, Garcia S, Glanville J, Mair FS. Understanding factors affecting patient and public engagement and recruitment to digital health interventions: a systematic review of qualitative studies. BMC Med Inform Decis Mak. 2016;16(1):120. doi:10.1186/s12911-016-0359-3
26. Rahimi B, Nadri H, Lotfnezhad Afshar H, Timpka T, Systematic A. Review of the technology acceptance model in health informatics. Appl Clin Inform. 2018;9(3):604–634. doi:10.1055/s-0038-1668091
27. Magsamen-Conrad K, Wang F, Tetteh D, Lee YI. Using technology adoption theory and a lifespan approach to develop a theoretical framework for eHealth literacy: extending UTAUT. Health Commun. 2020;35(12):1435–1446. doi:10.1080/10410236.2019.1641395
28. Koledova E, Stoyanov G, Ovbude L, Davies PSW. Adherence and long-term growth outcomes: results from the easypod connect observational study (ECOS) in paediatric patients with growth disorders. Endocr Connect. 2018;7(8):914–923. doi:10.1530/EC-18-0172
29. Koledova E, Bagha M, Arnaud L, Piras F, van Dommelen P. Optimising adherence using a connected injection device can improve growth outcomes: evidence from real-world data on 11 million injections in 20,000 patients with growth disorders. Horm Res Paediatr. 2021;94:1–445.
30. Tornincasa V, Dixon D, Le Masne Q, et al. Integrated digital health solutions in the management of growth disorders in pediatric patients receiving growth hormone therapy: a retrospective analysis. Front Endocrinol. 2022. doi:10.3389/fendo.2022.882192
31. Abdel-Tawab R, James DH, Fichtinger A, Clatworthy J, Horne R, Davies G. Development and validation of the Medication-Related Consultation Framework (MRCF). Patient Educ Couns. 2011;83(3):451–457. doi:10.1016/j.pec.2011.05.005
32. Engel T, Ungar B, Ben-Haim G, Levhar N, Eliakim R, Ben-Horin S. Re-phrasing the question: a simple tool for evaluation of adherence to therapy in patients with inflammatory bowel disease. United European Gastroenterol J. 2017;5(6):880–886. doi:10.1177/2050640616687838
33. Miller W, Rollnick S. Applications of Motivational Interviewing.
34. Weingarten SR, Henning JM, Badamgarav E, et al. Interventions used in disease management programmes for patients with chronic illness-which ones work? Meta-analysis of published reports. BMJ. 2002;325(7370):925. doi:10.1136/bmj.325.7370.925
35. Bennett HD, Coleman EA, Parry C, Bodenheimer T, Chen EH. Health coaching for patients with chronic illness. Fam Pract Manag. 2010;17(5):24–29.
36. Cook PF, Emiliozzi S, El-Hajj D, McCabe MM. Telephone nurse counseling for medication adherence in ulcerative colitis: a preliminary study. Patient Educ Couns. 2010;81(2):182–186. doi:10.1016/j.pec.2009.12.010
37. McBride CM, Rimer BK. Using the telephone to improve health behavior and health service delivery. Patient Educ Couns. 1999;37(1):3–18. doi:10.1016/S0738-3991(98)00098-6
38. Turner AP, Sloan AP, Kivlahan DR, Haselkorn JK. Telephone counseling and home telehealth monitoring to improve medication adherence: results of a pilot trial among individuals with multiple sclerosis. Rehabil Psychol. 2014;59(2):136–146. doi:10.1037/a0036322
39. Silva N, Bullinger M, Sommer R, Rohenkohl A, Witt S, Quitmann J. Children’s psychosocial functioning and parents’ quality of life in paediatric short stature: the mediating role of caregiving stress. Clin Psychol Psychother. 2018;25(1):e107–e118. doi:10.1002/cpp.2146
40. Gerain P, Zech E. Does informal caregiving lead to parental burnout? Comparing parents having (or not) children with mental and physical issues. Front Psychol. 2018;9:884. doi:10.3389/fpsyg.2018.00884
41. Alsaigh R, Coyne I. Mothers’ experiences of caring for children receiving growth hormone treatment. J Pediatr Nurs. 2019;49:e63–e73. doi:10.1016/j.pedn.2019.09.005
42. Segal Z, Teasdale J, Williams J. Mindfulness-based cognitive therapy: theoretical rationale and empirical status. In: Hayes SC, Follette VM, Linehan MM, editors. Mindfulness and Acceptance: Expanding the Cognitive-Behavioral Tradition. Guilford Press; 2004:45–65.
43. Townshend K, Jordan Z, Stephenson M, Tsey K. The effectiveness of mindful parenting programs in promoting parents’ and children’s wellbeing: a systematic review. JBI Database System Rev Implement Rep. 2016;14(3):139–180. doi:10.11124/JBISRIR-2016-2314
44. Okafor M, Sarpong D, Ferguson A, Satcher D. Improving health outcomes of children through 10 effective parenting: model and methods. Int J Environ Res Public Health. 2014;11:296–311. doi:10.3390/ijerph110100296
45. Brod M, Alolga SL, Beck JF, Wilkinson L, Hojbjerre L, Rasmussen MH. Understanding burden of illness for child growth hormone deficiency. Qual Life Res. 2017;26(7):1673–1686. doi:10.1007/s11136-017-1529-1
46. Savage MO, Backeljauw PF, Calzada R, et al. Early detection, referral, investigation, and diagnosis of children with growth disorders. Horm Res Paediatr. 2016;85(5):325–332. doi:10.1159/000444525
47. Fernandez-Luque L, Al Herbish A, Al Shammari R, et al. digital health for supporting precision medicine in pediatric endocrine disorders: opportunities for improved patient care. Front Pediatr. 2021;9:715705. doi:10.3389/fped.2021.715705
48. Polak M, Konrad D, Tonnes Pedersen B, Puras G, Snajderova M. Still too little, too late? Ten years of growth hormone therapy baseline data from the NordiNet(R) international outcome study. J Pediatr Endocrinol Metab. 2018;31(5):521–532. doi:10.1515/jpem-2017-0489
49. Argente J, Tatton-Brown K, Lehwalder D, Pfaffle R. Genetics of growth disorders-which patients require genetic testing? Front Endocrinol. 2019;10:602. doi:10.3389/fendo.2019.00602
50. Colver A, Rapley T, Parr JR, et al. Facilitating transition of young people with long-term health conditions from children’s to adults’ healthcare services - implications of a 5-year research programme. Clin Med. 2020;20(1):74–80. doi:10.7861/clinmed.2019-0077
51. Culen C, Herle M, Ertl DA, et al. Less ready for adulthood?-Turner syndrome has an impact on transition readiness. Clin Endocrinol (Oxf). 2020;93(4):449–455. doi:10.1111/cen.14293
52. Gomez R, Ahmed SF, Maghnie M, Li D, Tanaka T, Miller BS. Treatment adherence to injectable treatments in pediatric growth hormone deficiency compared with injectable treatments in other chronic pediatric conditions: a systematic literature review. Front Endocrinol. 2022;13:795224. doi:10.3389/fendo.2022.795224
53. Loftus J, Miller BS, Parzynski CS, et al. Association of daily growth hormone injection adherence and height among children with growth hormone deficiency. Endocr Pract. 2022;28:565–571. doi:10.1016/j.eprac.2022.02.013
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
