Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Adherence to Antiretroviral Treatment Among People Who Started Treatment on the Same-Day of HIV Diagnosis in Ethiopia: A Multicenter Observational Study
Authors Ahmed I, Demissie M , Worku A, Gugsa S, Berhane Y
Received 1 September 2021
Accepted for publication 30 October 2021
Published 10 November 2021 Volume 2021:13 Pages 983—991
DOI https://doi.org/10.2147/HIV.S337073
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 7
Editor who approved publication: Professor Bassel Sawaya
Ismael Ahmed,1 Meaza Demissie,2 Alemayehu Worku,3 Salem Gugsa,4 Yemane Berhane2
1University of Gondar, Gondar, Ethiopia; 2Addis Continental Institute of Public Health, Addis Ababa, Ethiopia; 3Department of Preventive Medicine, School of Public Health, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 4Department of Global Health, University of Washington, Seattle, WA, USA
Correspondence: Ismael Ahmed Tel +251911126852
Email [email protected]
Introduction: Since the launch of universal “test and treat” approach in 2016, there has been a significant increase in persons initiated on antiretroviral therapy (ART) on the same-day of human immunodeficiency virus (HIV) diagnosis in low-income settings. However, there are limited studies that investigated the effect of rapid treatment initiation on adherence. In this study, we compared adherence to ART in people who started ART on the same-day of HIV diagnosis and those started more than 7 days after HIV diagnosis.
Methods: We conducted a retrospective cohort analysis using routinely collected data from multiple ART clinics. Participants were at least 15 years old, were newly diagnosed and started on ART between October 2016 and July 2018 in the Amhara region of Ethiopia. We used doubly-robust multivariable logistic regression model to estimate the adjusted effects on adherence.
Results: A total of 415 individuals who started ART on the same-day of HIV diagnosis and 527 individuals who started 7 days after their HIV diagnosis were included in the analysis. The proportion of participants who reported optimal adherence was significantly lower in the same-day group at 6- and 12-months (absolute risk difference of 6.5%; 95% CI: 1.1%, 11.9% and 6.8%; 95% CI: 1.2%, 12.5%, respectively) compared to the > 7 days group. After adjusting for baseline and non-baseline covariates, the same-day group was less likely to have optimal adherence both at 6- and 12-months (adjusted RR=0.90; 95% CI: 0.86, 0.94 and RR=0.89; 95% CI: 0.83, 0.95, respectively) compared to the > 7 days group.
Conclusion: We observed lower optimal adherence among individuals who started ART on the same-day of HIV diagnosis compared to those who started ART > 7 days after their HIV diagnosis. Our findings highlight the importance of identifying adherence barriers, providing support, and ensuring treatment readiness before initiating individuals on same-day ART.
Keywords: same-day antiretroviral therapy, rapid ART, adherence, test and treat, Africa, Ethiopia
Introduction
There has been a tremendous achievement in the fight against human immunodeficiency virus (HIV) followed by a rapid scale-up of free antiretroviral treatment (ART). The increased access to ART has averted an estimated 12.1 million AIDS-related deaths since 2010.1 However, worldwide, suboptimal ART adherence (<95% adherence level) has been one of the challenges of achieving optimal results from ART. Treatment adherence is a backbone for the success of ART program – that aims to reduce HIV-related morbidity and mortality and new HIV transmission through viral suppression. Durable viral suppression allows for maximal reconstitution of immune function and minimizes the emergence of drug-resistant virus.2 It also reduces the risk of sexual HIV transmission between heterosexual serodiscordant couples.3 However, without good or optimal treatment adherence (≥95% adherence), it is impossible to realize these benefits of ART.
A metanalysis that synthesized several studies across >26 countries before universal “test and treat” strategy showed a mean rate of 63.4% optimal adherence.4 Another systematic review of ART adherence in sub-Saharan Africa reported average adherence score of 72.9%.5 In Ethiopia, based on recent observational studies that assessed self-reported adherence using clinician’s-record, optimal adherence ranged between 60.3% and 94.8% and varied by region.6–8 On the other hand, a meta-analysis of studies conducted in sub-Saharan Africa identified that use of alcohol, male gender, use of traditional/herbal medicine, dissatisfaction with healthcare facility and healthcare workers, depression, discrimination and stigmatization, and poor social support as factors associated with non-adherence.5 A study conducted in other setting also showed that gender and marital status have significant association with ART adherence.9
Different methods have been used to measure adherence. Clinician recorded self-reported adherence is the most common method and a standard of care adherence measurement tool in low resource settings due to its relative ease of use of documenting self-reported adherence to ART by a clinician during routine patient follow-up. Earlier on, self-report was believed to overestimate adherence level due to response bias.10,11 However, studies from various settings have demonstrated that measures of adherence using self-report had no evidence of greater overestimation12 or was highly correlated with viral suppression.4,11,13,14
Unlike previous extensive research, limited studies have investigated ART adherence post-universal “test and treat” policy implementation. A recent cross-sectional study that estimated the proportion of optimal adherence among PLHIV enrolled during universal “test and treat” approach in Ethiopia reported 49.3% and 95.9% optimal adherence measured by self-report using Morisky scale and seven-day recall, respectively.15 In Nigeria,16 a cohort study that evaluated the national scale-up of “test and treat” reported <70% good adherence among PLHIV who started ART within two weeks of HIV diagnosis, according to their pharmacy refill. However, despite the increasing trend in the proportions of PLHIV enrolling for ART on the same-day of diagnosis,17–19 evidence of ART adherence among same-day ART initiators is lacking in low resource settings. In our observational study, we aimed to compare the proportion of optimal adherence between PLHIV who were initiated on ART on the same-day of HIV diagnosis and those initiated >7 days after HIV diagnosis.
Methods
Study Design
We conducted a retrospective cohort analysis using routinely collected data from multiple ART clinics to compare ART adherence among PLHIV who were initiated on same-day ART with those initiated >7 days after HIV diagnosis.
Study Setting and Participants
This study was part of the study that evaluates the effectiveness of same-day ART initiation. The detail of the study setting and participants has been published elsewhere.20 Briefly, the study was conducted at 11 public health facilities in Bahir Dar and Gondar, two towns in the Amhara region of northwest Ethiopia. The study included PLHIV who were ≥15 years old and started ART on the same-day of HIV diagnosis or >7 days after the initial diagnosis between 20 October 2016 and 18 July 2018. We excluded medical records of individuals who were initiated on ART 1–7 days after diagnosis, aged <15 years old, pregnant, dead, transferred-in from another health facility, and transferred-out to another health facility within 12-months of ART initiation. In addition, we excluded medical records of patients whose rapid ART initiation was delayed due to management of tuberculosis or cryptococcal meningitis21 and those who had died, due to missing adherence assessment.
Variables and Measurement
We defined optimal ART adherence (≥95% adherence) at 6- and 12-months following ART initiation as having clinician-recorded “good” adherence level at every visit in the previous 6-months of ART follow-up. Clinicians’ assessment of adherence is based on patients’ response about the number of missed antiretroviral (ARV) drug pills during the past month. The national guideline recommends clinicians to label ART adherence as “good” if the person missed ≤2 doses (≥95% adherence), “fair” if the person missed 3–5 doses (85–94% adherence), and “poor” if the person missed ≥6 doses (<85% adherence) out of the 30 doses to be taken during each month of ART follow-up.21
Individuals with at least one “poor” or ”fair” adherence measures in any of the last 6 months22 or those who missed their ART refill follow-up for a period of ≥30 days (labeled as loss to follow-up (LTFU)) between any of the last 6 months13 were categorized as having sub-optimal adherence.
We included both baseline and follow-up independent factors (biological, sociodemographic and clinical factors) in the analysis. We described the details of these factors including their measurements elsewhere.20
Study Size and Sampling Method
The sample size of this study was 988 (433 same-day and 555 after 7 days), which includes all eligible PLHIV who were newly diagnosed and started on ART between 20 October 2016 and 18 July 2018 at study sites.20
We calculated a required sample size of 427 study participants for both groups to be able to detect a 10% absolute difference in the proportion of individuals with optimal adherence between the two groups, based on results from studies conducted in the Amhara region of Ethiopia on self-reported optimal adherence among PLHIV who started ART before universal “test and treat” approach.23,24 We used P1 of 98% (proportion of PLHIV having optimal adherence among individuals in the same-day group) and P2 of 88% (proportion of PLHIV having optimal adherence among individuals in the control group).25 A 1:1 allocation with α of 0.05, 80% power, a design effect of 1.5 to account for the effect of clustering in multicenter design,26 and a 15% allowance for anticipated limitations with regard to missing medical record data in Ethiopia27 were used to calculate the sample size. We used StatCalc for cohort studies using Epi InfoTM version 7 (developed by Centers for Disease Control and Prevention) to determine the sample size.
Statistical Analysis
Sociodemographic and clinical characteristics of study participants were summarized by group. Chi-square test was used to compare baseline and follow-up covariates between the two groups. We assumed the likelihood of a covariate data to be missing completely at random (MCAR) based on our knowledge of the data and the HIV program coupled with Little’s chi-square MCAR test.28 Because of this assumption and having a fairly large sample size that is higher than the minimum required sample, we used complete-case analysis. However, due to high proportion and differential missing data, we excluded baseline CD4 data during analysis. Accordingly, the final model for the adjusted analysis included 551 and 822 number of observations for the 6- and 12-months adherence assessments, respectively.
Proportions of individuals with optimal adherence at 6- and 12-months after ART initiation were compared with absolute risk difference (RD) between the two groups using a chi-square test. We estimated the adjusted optimal adherence using risk ratio (RR) with 95% confidence interval (CI) for each study group. In the adjusted analysis, we first balanced the two groups by estimating the propensity score using a logistic regression model29,30 to adjust for the baseline covariates including age, sex, marital status, education, place of residence, BMI, WHO clinical stage, functional status and OI at enrollment. One observation was trimmed due to very low propensity score.
A doubly-robust multivariable logistic regression model that included both the propensity score and the baseline covariates were used to ensure sufficient covariate balance.29,30 Additionally, other non-baseline covariates such as cotrimoxazole preventive therapy (CPT), isoniazid preventive therapy (IPT), type of ARV regimen initiated, disclosure of HIV status and partner’s HIV status were included in the analysis. A cluster-robust standard error was used to account for the effect of clustering at health facility level.31,32 Possible significant interaction terms between the main effect variables were checked for inclusion in the model. Model diagnostics were conducted for the final model that included the propensity score and all significant variables (p<0.25) using the Hosmer-Lemeshow goodness-of-fit test supplemented by c-statistics for the area under the receiver operator characteristic curve.33 Adjusted RR with 95% CI was computed from the adjusted odds ratio after running the final logistic regression model.34 All statistical analyses were performed using Stata version 13.0 (StataCorp., College Station, TX).
Ethics
Ethical clearance was obtained from the Institutional Review Board of the University of Gondar with Ref. No. V/P/RCS/05/2488/2019. The ethical clearance with the protocol was shared to Amhara Public Health Institute for further clearance. The institute provided a support letter to town health offices requesting access to the medical records at study sites. Participants were not directly contacted nor were their personal identifiers collected. While reviewing patients’ medical records, non-personal identifiers such as unique ART number or medical record number were used to distinguish study subjects. Only data collectors and supervisors had access to the medical records and both groups signed confidentiality agreements before commencing data collection. The study was conducted in accordance with the Declaration of Helsinki.
Results
Characteristics of Study Participants
Of the 988 medical records reviewed, 942 (95.3%) participants (415 PLHIV who started ART on the same-day of HIV diagnosis and 527 PLHIV who started more than 7 days after their HIV diagnosis) had documented clinician recorded ART adherence information at 6- or 12-months after initiating ART; and were included in the current analysis. Of the 942 participants, 96 (63 in the same-day group and 33 in the >7 days group) and 149 (87 in the same-day group and 62 in the >7 days group) were LTFU at 6- and 12-months, respectively, and assumed to have sub-optimal adherence.
Participants who were initiated on ART on same-day of and more than 7 days after HIV diagnosis were similar in educational status, marital status, religion and HIV status of their partner. The median age of participants was 31 (IQR 27.0–39.0) in the same-day and 33 (IQR 27.0–40.0) in the over 7 days group (p=0.04). Compared to the >7 days group, participants in the same-day group were more likely to be women (p=0.03), live within town (p=0.003), disclose their HIV status (p=0.001), have good (working) functional status (p<0.001), have missing baseline CD4 cell count (p<0.001), be at WHO clinical stage I (p<0.001), not be eligible for CPT (p<0.001), and take IPT (p<0.001) (Tables 1 and 2).
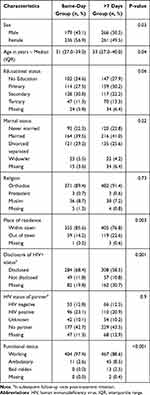 |
Table 1 Sociodemographic Characteristics of Study Participants by Group in Bahir Dar and Gondar, Ethiopia, 20 October 2016–18 July 2018 |
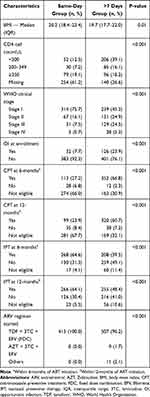 |
Table 2 Bio-Clinical Characteristics of Study Participants by Group in Bahir Dar and Gondar, Ethiopia, 20 October 2016–18 July 2018 |
All participants (100%, n=415) in the same-day group were prescribed with a Non-nucleoside Reverse Transcriptase Inhibitors (NNRTI)-based first-line regimen (a fixed-dose combination (FDC) of Tenofovir (TDF) + Lamivudine (3TC) + Efavirenz (EFV)) compared to 96.2% (n=507) of the >7 days group (p<0.001). However, 11 individuals in the >7 days group had their regimen changed to a different first-line regimen, and 8 of the 11 had been switched to another first-line NNRTI-based regimen within 12-months of ART initiation (Table 2). Additionally, one person’s ART in each group was changed to second-line ART within 12-months of ART initiation.
ART Adherence Outcomes at 6- and 12-Months of Follow-Up
As reported in Table 3, the proportion of study participants who reported optimal adherence at 6 months was significantly lower in the same-day group (75.1%, n=304) compared to the >7 days group (81.6%, n=425) with an absolute RD of 6.5% (95% CI: 1.1%, 11.9%; p=0.02). At 12 months, similarly, the proportion of study participants reported optimal adherence was significantly lower in the same-day (72.2%, n=292) compared to those initiated >7 days (78.1%, n=402) with an absolute RD of 6.8% (95% CI: 1.2%, 12.5%; p=0.02).
 |
Table 3 ART Adherence Outcomes at 6- and 12-Months ART Follow-Up by Group in Bahir Dar and Gondar, Ethiopia, 20 October 2016–18 July 2018 |
Compared to the >7 days group, the unadjusted and adjusted RR of 6-months optimal adherence for same-day group were 0.92 (95% CI: 0.86, 0.99; p=0.02) and 0.90 (95% CI: 0.86, 0.94; p<0.001), respectively. At 12 months, the unadjusted RR of optimal ART adherence for the same-day group was 0.91 (95% CI: 0.85, 0.99; p=0.02) compared to the >7 days group; the adjusted RR for this comparison was 0.89 (95% CI: 0.83, 0.95; p=0.001) (Table 4).
 |
Table 4 Adjusted ART Adherence Outcomes for Same-Day ART Group in Bahir Dar and Gondar, Ethiopia, 20 October 2016–18 July 2018 |
Discussion
In this study, we found that individuals who started ART on the same-day of HIV diagnosis had lower proportion of optimal ART adherence at 6-months and 12-months of ART follow-up compared to those who started ART more than 7 days after their HIV diagnosis. Similarly, in the adjusted analysis, same-day group had significantly lower optimal ART adherence compared to the >7 days group at 6- and 12-months post ART initiation.
We measured self-reported optimal adherence (≥95% adherence) using clinician assessment during routine clinical care follow-up. Our study found that PLHIV who started ART on the day of HIV diagnosis had a reduced optimal ART adherence at 6- and 12-months, compared to those who started ART more than 7 days after their HIV diagnosis. Our ART adherence results correlate with the lower viral suppression we found among same-day group in our previous publication that focused on virologic outcomes.35 Viral load measurement has been used as another way of assessing ART adherence36 — sometimes considered as the gold standard for monitoring ART adherence.37
Our finding was consistent with a recent observational study that evaluated the national scale-up of “test and treat” in Nigeria. This study highlighted that PLHIV who started ART within two weeks had <70% good adherence based on pharmacy refill data.16 The lower optimal ART adherence among same-day initiators in our study could be directly related with the higher LTFU reported among same-day group at 6- and 12-months post-initiation of ART.20 All LTFU individuals in this study were assumed to have sub-optimal adherence due to treatment interruption. These findings may indicate the need for intensified counseling and monitoring to PLHIV who started treatment on the initial date of HIV diagnosis.
Furthermore, the lower optimal ART adherence among same-day initiators could be related with failure to ensure the readiness of individuals for immediate ART initiation after a positive HIV diagnosis. Some individuals may need more time to comprehend the result and cope with the news of being HIV positive before deciding to start a lifelong treatment. Evidence from a randomized trial in South Africa and Kenya showed that, when given an option, only half of newly diagnosed PLHIV were eligible and ready for the same-day initiation of ART.19 Therefore, preference to start ART on a later date may be one of the reasons for high rate of early treatment interruption among same-day initiators during “test and treat” era. For instance, in South Africa18 and Haiti,17 35% and 20% of same-day initiators did not return to refill their ART after the initial ART dispensation, respectively, resulting in a phenomenon of “failed initiation” that program managers need to explore to understand and find resolutions for. Additionally, previous studies have showed that fear of drug side effects, inadvertent HIV status disclosure and discrimination38 are few of the items that need to be addressed prior to ART initiation to ensure good ART adherence.
ART clinicians should provide adequate support to individuals to help them understand their result and its implication, and assess and address possible risk factors or barriers to treatment adherence39,40 before putting PLHIV on immediate ART. Individuals who started ART on the same-day of HIV diagnosis should be prioritized for enhanced adherence counseling and follow-up especially during the early phase of treatment. Additionally, individuals with potential risk of suboptimal adherence should be linked for family-,41 facility- or community-based adherence support systems.42
Our study was limited to secondary data available on medical records of PLHIV, and hence we could not address some important individual-level factors including social, psychological, behavioral and mental health issues, and other provider- and system-level independent factors that may affect ART adherence in low resource settings.5 Incomplete information on some of the variables in the secondary data may also affect the analysis, though it was compensated with the large sample size we used compared to the minimum required size. In addition, we have excluded baseline CD4 result from the analysis due to differential missing data. However, according to a study in South Africa,43 this may not have an effect on the result due to no evidence of association between baseline CD4 and ART adherence. Individuals who transferred their care to another facility within 12 months of ART initiation were excluded from the study due to lack of information about their outcomes. Since there was evidence of a higher chance of discontinuing treatment among transferred-out individuals44 and with increased proportion of transferred-out cases among PLHIV who started ART under universal “test and treat” strategy,16 especially among same-day initiators,18 our optimal adherence estimates among same-day group may have been overestimated.
Despite these limitations, the strength of our study was its design. It is a multi-center study with a fairly large sample size that applied strict inclusion criteria to select participants to reduce selection bias. We used propensity scores to balance the two groups and conducted a doubly-robust method to ensure sufficient covariate balance29,30 and generate unbiased estimates.
Conclusions
Individuals who started ART on the same-day of HIV diagnosis had lower optimal adherence at 6-months and 12-months of ART follow-up compared to those who started ART more than 7 days after their HIV diagnosis. Patient-centered health care provision that assesses possible barriers to ART adherence and provide support accordingly can ensure the benefit aimed by rapid ART initiation. PLHIV initiated on the same-day of HIV diagnosis should receive enhanced adherence counseling and follow-up and be linked to other adherence support systems.
Abbreviations
AIDS, Acquired Immunodeficiency Syndrome; ART, Antiretroviral Therapy; ARV, Antiretroviral; AZT, Zidovudine; CPT, Cotrimoxazole Preventive Therapy; FDC, Fixed Dose Combination; HIV, Human Immunodeficiency Virus; IPT, Isoniazid Preventive Therapy; LTFU, Loss to Follow-up; NNRTI, Non-nucleoside Reverse Transcriptase Inhibitors; OI, Opportunistic Infections; PLHIV, People Living with HIV; 3TC, Lamivudine; TDF, Tenofovir; WHO, World Health Organization.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors gratefully acknowledge Amhara Public Health Institute, health facilities and data collectors for their support during the conduct of this study. The authors also thank Gizachew Tadesse for his special support on Open Data Kit electronic system. Finally, our special thanks go to Addis Continental Institute of Public Health and University of Gondar for giving the opportunity to study doctoral training program.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. UNAIDS. Global AIDS update 2020: seizing the moment; 2020. Available from: https://aids2020.unaids.org/report/.
2. UCSF. Adherence to HIV antiretroviral therapy; 2006. Available from: http://hivinsite.ucsf.edu/InSite?page=kb-03-02-09#S3X.
3. LeMessurier J, Traversy G, Varsaneux O, et al. Risk of sexual transmission of human immunodeficiency virus with antiretroviral therapy, suppressed viral load and condom use: a systematic review. CMAJ. 2018;190(46):E1350–E1360. doi:10.1503/cmaj.180311
4. Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM. Adherence to antiretroviral therapy and virologic failure: a meta-analysis. Medicine (Baltimore). 2016;95(15):e3361. doi:10.1097/MD.0000000000003361
5. Heestermans T, Browne JL, Aitken SC, Vervoort SC, Klipstein-Grobusch K. Determinants of adherence to antiretroviral therapy among HIV-positive adults in sub-Saharan Africa: a systematic review. BMJ Glob Health. 2016;1(4):e000125. doi:10.1136/bmjgh-2016-000125
6. Nigusso FT, Mavhandu-Mudzusi AH. Magnitude of non-adherence to antiretroviral therapy and associated factors among adult people living with HIV/AIDS in Benishangul-Gumuz Regional State, Ethiopia. PeerJ. 2020;8:e8558. doi:10.7717/peerj.8558
7. Desta AA, Kidane KM, Woldegebriel AG, et al. Level of adherence and associated factors among HIV-infected patients on antiretroviral therapy in Northern Ethiopia: retrospective analysis. Patient Prefer Adherence. 2020;14:1585–1594. doi:10.2147/PPA.S268395
8. Ejigu M, Desalegn Z, Mulatu B, Mosisa G. Adherence to combined antiretroviral therapy and associated factors among people living with HIV attending Nekemte Specialized Hospital, Oromia, Ethiopia: a cross-sectional study. HIV AIDS (Auckl). 2020;12:97–106. doi:10.2147/HIV.S239995
9. Morowatisharifabad MA, Movahed E, Farokhzadian J, et al. Antiretroviral therapy adherence based on information, motivation, and behavioral skills model and its association with depression among HIV-positive patients: health promotion strategy towards the 909090 target. J Educ Health Promot. 2019;8:192. doi:10.4103/jehp.jehp_42_19
10. Wagner G, Miller LG. Is the influence of social desirability on patients’ self-reported adherence overrated? JAIDS. 2004;35(2):203–204. doi:10.1097/00126334-200402010-00016
11. Arnsten JH, Demas PA, Farzadegan H, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33(8):1417–1423. doi:10.1086/323201
12. Simoni JM, Huh D, Wang Y, et al. The validity of self-reported medication adherence as an outcome in clinical trials of adherence-promotion interventions: findings from the MACH14 study. AIDS Behav. 2014;18(12):2285–2290. doi:10.1007/s10461-014-0905-x
13. Mekuria LA, Prins JM, Yalew AW, Sprangers MAG, Nieuwkerk PT. Which adherence measure – self‐report, clinician recorded or pharmacy refill – is best able to predict detectable viral load in a public ART programme without routine plasma viral load monitoring? Trop Med Int Health. 2016;21(7):856–869. doi:10.1111/tmi.12709
14. Nieuwkerk PT, Oort FJ. Self-reported adherence to antiretroviral therapy for HIV-1 infection and virologic treatment response: a meta-analysis. J Acquir Immune Defic Syndr. 2005;38(4):445–448. doi:10.1097/01.qai.0000147522.34369.12
15. Damtie Y, Tadese F. Antiretroviral therapy adherence among patients enrolled after the initiation of the Universal Test and Treat strategy in Dessie town: a cross-sectional study. Int J STD AIDS. 2020;31(9):886–893. doi:10.1177/0956462420927205
16. Stafford KA, Odafe SF, Lo J, et al. Evaluation of the clinical outcomes of the test and treat strategy to implement treat all in Nigeria: results from the Nigeria multi-center ART study. PLoS One. 2019;14(7):e0218555. doi:10.1371/journal.pone.0218555
17. Puttkammer N, Parrish C, Desir Y, et al. Toward universal HIV treatment in haiti: time trends in ART retention after expanded ART eligibility in a national cohort from 2011 to 2017. J Acquir Immune Defic Syndr. 2020;84(2):153–161. doi:10.1097/qai.0000000000002329
18. Lilian RR, Rees K, McIntyre JA, Struthers HE, Peters RPH. Same-day antiretroviral therapy initiation for HIV-infected adults in South Africa: analysis of routine data. PLoS One. 2020;15(1):e0227572. doi:10.1371/journal.pone.0227572
19. Rosen S, Maskew M, Larson B, et al. Simplified clinical algorithm for identifying patients eligible for same-day HIV treatment initiation (SLATE): results from an individually randomized trial in South Africa and Kenya. PLoS Med. 2019;16:e1002912. doi:10.1371/journal.pmed.1002912
20. Ahmed I, Demissie M, Worku A, Gugsa S, Berhane Y. Effectiveness of same-day antiretroviral therapy initiation in retention outcomes among people living with human immunodeficiency virus in Ethiopia: empirical evidence. BMC Public Health. 2020;20(1):1802. doi:10.1186/s12889-020-09887-9
21. Federal Ministry of Health of Ethiopia. National consolidated guidelines for comprehensive HIV prevention, care and treatment. WHO | Regional Office for Africa; 2018. Available from: https://www.afro.who.int/publications/national-consolidated-guidelines-comprehensive-hiv-prevention-care-and-treatment.
22. Mekuria L, Prins J, Yalew A, Spanglers M, Nieuwkerk P. Sub-optimal adherence to combination antiretroviral therapy and its associated factors according to self-report, clinician-recorded and pharmacy-refill assessment methods among HIV-infected adults in Addis Ababa. AIDS Care. 2016. doi:10.1080/09540121.2016.1234681
23. Molla AA, Gelagay AA, Mekonnen HS, Teshome DF. Adherence to antiretroviral therapy and associated factors among HIV positive adults attending care and treatment in University of Gondar Referral Hospital, Northwest Ethiopia. BMC Infect Dis. 2018;18(1):266. doi:10.1186/s12879-018-3176-8
24. Boneya D, Asmare M, Aychiluhem M, Ayana M. Level of ART adherence and associated factors among adult sero-positive HIV patients on highly active antiretroviral therapy in Debre Markos Referral Hospital, Northwest Ethiopia; 2016. Available from: https://www.longdom.org/proceedings/level-of-art-adherence-and-associated-factors-among-adult-seropositive-hiv-patients-on-highly-active-antiretroviral-ther-6104.html.
25. Mekuria LA, Prins JM, Yalew AW, Sprangers MAG, Nieuwkerk PT. Sub-optimal adherence to combination anti-retroviral therapy and its associated factors according to self-report, clinician-recorded and pharmacy-refill assessment methods among HIV-infected adults in Addis Ababa. AIDS Care. 2017;29(4):428–435. doi:10.1080/09540121.2016.1234681
26. Abdissa A, Yilma D, Fonager J, et al. Drug resistance in HIV patients with virological failure or slow virological response to antiretroviral therapy in Ethiopia. BMC Infect Dis. 2014;14:181. doi:10.1186/1471-2334-14-181
27. Melaku Z, Lamb MR, Wang C, et al. Characteristics and outcomes of adult Ethiopian patients enrolled in HIV care and treatment: a multi-clinic observational study. BMC Public Health. 2015;15:462. doi:10.1186/s12889-015-1776-4
28. Li C. Little’s test of missing completely at random. Stata J. 2013;13(4):795–809. doi:10.1177/1536867X1301300407
29. Elze MC, Gregson J, Baber U, et al. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol. 2017;69(3):345–357. doi:10.1016/j.jacc.2016.10.060
30. Golinelli D, Ridgeway G, Rhoades H, Tucker J, Wenzel S. Bias and variance trade-offs when combining propensity score weighting and regression: with an application to HIV status and homeless men. Health Serv Outcomes Res Methodol. 2012;12(2–3):104–118. doi:10.1007/s10742-012-0090-1
31. MacKinnon JG, Webb MD. When and how to deal with clustered errors in regression models; 2019. Available from: http://qed.econ.queensu.ca/pub/faculty/mackinnon/working-papers/qed_wp_1421.pdf.
32. Jayatillake RV, Sooriyarachchi MR, Senarathna DLP. Adjusting for a cluster effect in the logistic regression model: an illustration of theory and its application. J Natl Sci Found Sri Lanka. 2011;39(3):201–218. doi:10.4038/jnsfsr.v39i3.3624
33. Hosmer D, Lemeshow S. Applied Logistic Regression.
34. Norton EC, Miller MM, Kleinman LC. Computing adjusted risk ratios and risk differences in Stata. Stata J. 2013;13(3):492–509. doi:10.1177/1536867X1301300304
35. Ahmed I, Demissie M, Worku A, Gugsa S, Berhane Y. Virologic outcomes of people living with human immunodeficiency virus who started antiretroviral treatment on the same-day of diagnosis in Ethiopia: a multicenter observational study. PLoS One. 2021;16(9):e0257059. doi:10.1371/journal.pone.0257059
36. San Lio MM, Carbini R, Germano P, et al. Evaluating adherence to highly active antiretroviral therapy with use of pill counts and viral load measurement in the drug resources enhancement against AIDS and malnutrition program in Mozambique. Clin Infect Dis. 2008;46(10):1609–1616. doi:10.1086/587659
37. WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach; 2016. Available from: https://apps.who.int/iris/handle/10665/208825.
38. Magaço A, Dovel K, Cataldo F, et al. “Good health” as a barrier and facilitator to ART initiation: a qualitative study in the era of test-and-treat in Mozambique. Cult Health Sex. 2019;21(9):1059–1073. doi:10.1080/13691058.2018.1535091
39. Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM, Bimirew MA, Kassie DM. Barriers and facilitators of adherence to antiretroviral drug therapy and retention in care among adult HIV-positive Patients: a qualitative study from Ethiopia. PLoS One. 2014;9(5):e97353. doi:10.1371/journal.pone.0097353
40. Azia IN, Mukumbang FC, van Wyk B. Barriers to adherence to antiretroviral treatment in a regional hospital in Vredenburg, Western Cape, South Africa. South Afr J HIV Med. 2016;17(1). doi:10.4102/sajhivmed.v17i1.476
41. Letta S, Demissie A, Oljira L, Dessie Y. Factors associated with adherence to Antiretroviral Therapy (ART) among adult people living with HIV and attending their clinical care, Eastern Ethiopia. BMC Int Health Hum Rights. 2015;15(1):1–7. doi:10.1186/s12914-015-0071-x
42. Penn AW, Azman H, Horvath H, et al. Supportive interventions to improve retention on ART in people with HIV in low- and middle-income countries: a systematic review. PLoS One. 2018;13(12):e0208814. doi:10.1371/journal.pone.0208814
43. Iwuji C, McGrath N, Calmy A, et al. Universal test and treat is not associated with sub-optimal antiretroviral therapy adherence in rural South Africa: the ANRS 12249 TasP trial. J Int AIDS Soc. 2018;21(6):e25112. doi:10.1002/jia2.25112
44. Ghate M, Zirpe S, Gurav N, Paranjape R, Rewari B, Gangakhedkar R. Transfer out patients receiving antiretroviral therapy from programme clinic: a potential “leak” in the HIV treatment cascade. Sci Res Publ. 2014;4:382–386.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
