Back to Journals » Infection and Drug Resistance » Volume 15
Adenosine Deaminase is a Potential Molecular Marker for Diagnosis and Prognosis of Haemorrhagic Fever with Renal Syndrome
Received 20 June 2022
Accepted for publication 20 August 2022
Published 5 September 2022 Volume 2022:15 Pages 5197—5205
DOI https://doi.org/10.2147/IDR.S379228
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Xiaoli Zhu,1 Jinxi Hu2
1Department of Laboratory Medicine, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Taizhou, People’s Republic of China; 2Department of Oncological Surgery, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Taizhou, People’s Republic of China
Correspondence: Jinxi Hu, Department of Oncological Surgery, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, No. 150 Ximen Road of Linhai, Taizhou, Zhejiang Province, 317000, People’s Republic of China, Tel +86 18257689350, Email [email protected]
Objective: Haemorrhagic fever with renal syndrome (HFRS) is a serious zoonotic disease which seriously endangers physical health and mainly occurs in China. To date, there is still a lack of early and novel biomarkers to detect the severity of disease and prognosis of HFRS. This study was aimed to examine the value of the serum Adenosine deaminase (ADA) concentrations in the patients with HFRS.
Methods: The clinical and laboratory data of 124 adult patients with HFRS and 131 patients with similar clinical symptoms to HFRS were analyzed. A receiver operating characteristic (ROC) curve was used to analyze the diagnostic value of ADA in HFRS.
Results: The ADA levels in the serum of HFRS patients were significantly higher than those in control patients (P < 0.001), and ADA has a strong positive correlation with HFRS (r = 0.785, P < 0.001). The optimal cut-off value of ADA for diagnosis of HFRS was 18 U/L and the area under the curve (AUC) was 0.953 (95% CI: 0.925, 0.981). The sensitivity was 84.8%, the specificity was 93.1%, the positive predictive value was 92.2%, the negative predictive value was 86.5% and the Youden index was 77.9%. Serum ADA levels in patients with HFRS tended to decrease at discharge compared with those at admission.
Conclusion: ADA could be a potential molecular marker for diagnosis and prognosis of HFRS patients.
Keywords: haemorrhagic fever with renal syndrome, HFRS, adenosine deaminase, ADA, diagnosis, prognosis
Introduction
Haemorrhagic fever with renal syndrome (HFRS) is a rodent-borne disease that is caused by orthohantavirus infection. China is one of the most severely affected HFRS areas in the world.1 From 1950–2014, a total of 1,625,002 cases and 46,968 deaths with a death rate of 2.89% were reported.2 The HFRS-causing orthohantavirus has many variants, including Hantaan, Dobrava, Saaremaa, Seoul, Amur, Puumala and the Far East variant. The epidemiological characteristics of HFRS are related to different populations, regional differences, seasonal changes as well as host animal habitats and human production activities.3 The Hantaan and Seoul viruses were the main HFRS-causing orthohantaviruses, with the main natural hosts being Apodemus agrarius and Rattus norvegicus in China.4 The Dobrava infection is the deadliest orthohantavirus disease in Europe and its pathogenic dove host is Apodemus flavicollis.5 HFRS is characterized by thrombocytopenia, increased vascular permeability, coagulopathy and bleeding. Severe complications may involve the urinary, neurological, cardiopulmonary and cardiovascular system.6–9 Pancreatitis has also been reported to be a serious complication in patients with HFRS and has a poor prognosis.10 Therefore, early diagnosis, standardized treatment and prognostic monitoring of HFRS are very important.
The most commonly used method to diagnose HFRS is to detect orthohantavirus IgM/IgG antibodies using enzyme-linked immunosorbent assay (ELISA). Real-time RT-PCR is a sensitive tool for the early detection of orthohantavirus RNA prior to the appearance of IgM antibodies.9 Many new biomarkers such as high molecular weight Adiponectin (HMW APN), soluble CD138, platelet distribution width (PDW) and high mobility group box protein-1 (HMGB-1) have been reported to be associated with the severity of orthohantavirus infection.11–14 However, there is still a lack of effective markers for early diagnosis and monitoring of disease progression for HFRS.
Adenosine deaminase (ADA, EC 3.5.4.4) is a housekeeping enzyme for purine metabolism and there are two isoforms of ADA in the human genome, ADA1 and ADA2.15 ADA1 binds to CD16- monocytes, while ADA2 binds to neutrophils, monocytes, NK cells, B cells CD4+ T-cell and CD16+ monocytes.16 ADA is ubiquitously expressed in human tissues and the largest amounts are found in lymphoid tissues.17 ADA can inhibit adenosine accumulation and counteracts the negative effects of adenosine in immune cells, boosting the immune response.18 Alteration of ADA activity has been reported in literature due to tuberculous meningitis,19 human immunodeficiency virus (HIV),20 type 2 diabetes mellitus,21 hepatitis22 and cancers23 in the serum. These suggest that ADA can be a molecular marker in diagnosis of disease and the change in ADA can be an index to monitor disease progression and the efficacy of therapy.
Studies have shown that immune regulation plays a leading role in the pathogenesis of HFRS, including T cell response and B cell response.2 The ADA is an important immune regulatory molecule and plays an important role in the maturation and maintenance of the immune system.24,25 Therefore, we speculate that ADA can be a marker for HFRS. In this study, we analyzed the changes in serum ADA level for patients with HFRS and the correlation between ADA and HFRS. We explored the diagnostic value and prognostic effect of ADA for HFRS and found that ADA could be used as a molecular marker of HFRS.
Patients and Methods
Patients
This retrospective study cohort analysis enrolled 124 patients suffering from HFRS who were admitted to the Taizhou Hospital of Zhejiang Province from January 2012 to August 2018. Among them, 122 patients were cured and discharged from hospital while 2 patients died. The specific IgM antibody of the orthohantavirus was detected using ELISA to diagnose HFRS. 131 patients with diagnoses of fever, ascites, nephritis, nephrotic syndrome, septic shock, hypotension, hypertension, bronchitis, back pain, abdominal pain, petechiae, sepsis, renal failure, nausea and vomiting were enrolled as a control group. All of these diseases had symptoms that were similar to that shown in HFRS patients and each patient had only 1 or 2 of these symptoms. Patients who had other kidney diseases, cancer, tuberculosis, diabetes, cardiovascular disease, hematological disease, HIV, autoimmune disease, viral hepatitis, tissue damage or necrosis and other liver diseases were excluded. The study was approved by the Ethical Committee of Taizhou Hospital of Zhejiang Province (#K20220531).
Data Management
Personal features and clinical data were collected from the medical record of patients, including age, sex and laboratory results. Blood results were collected from 124 patients with HFRS and 131 control patients on the day of hospital admission (without any treatment). The blood results of the 124 HFRS patients before either being discharged, cured or dying were also collected. Laboratory parameters including white blood cells (WBC), platelet count (PLT), red blood cells (RBC) and hemoglobin (HB) were routinely tested using autoanalyzer (Sysmex XT-4000i; Sysmex, Japan). The concentration of ADA, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-glutamyl transpeptadase (GGT), total bilirubin (TB), direct bilirubin (DB), indirect bilirubin (IB), total protein (TP), albumin (ALB), total biliary acid (TBA), cholinesterase (CHE), prealbumin (PA), blood urea nitrogen (BUN), serum creatinine (Scr), uric acid (UA), glucose (GLU), triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A1 (APOA1), apolipoprotein B (APOB), lipoprotein a (LPa), creatine kinase (CK) and lactate dehydrogenase (LDH) in serum were detected using autoanalyzer (Hitachi 7600; Hitachi, Tokyo, Japan).
Statistical Analysis
For comparison of parameters between 124 patients with HFRS and 131 control patients at admission, the Independent-Sample t-test was used when the data was normally distributed, and the data was expressed as mean standard deviation. A Mann–Whitney U-test was used when the data was not normally distributed, and the data was expressed as p50% (p25%, p75%). The Spearman rank correlation coefficient was used to analyze the correlation. Diagnosis was performed using receiver operating characteristic (ROC) curves. The Paired-Sample t-test was used to analyze the comparison of each parameter for 122 patients with HFRS on admission and discharge.
Results
Comparison of Parameters Between HFRS and Control Patients
A total of 124 adult HFRS patients and 131 control patients were included in this study (Table 1). The 124 patients had a mean age of 49±14 years. Among them, 89 patients were male accounting for 71.8% while 35 patients were female accounting for 28.2%. Two HFRS patients died, with a mortality rate of 1.6%. The 131 control patients had a mean age of 51±14 years. Among them, 89 patients were male accounting for 67.9% while 42 patients were female accounting for 32.1%. There was no significant difference in the sex or age distribution between patients with HFRS and control patients (all P > 0.05). The levels of ALT, AST, GGT, TBA, ADA, Scr, BUN, UA, TG, LDH, WBC, RBC and HB in patients with HFRS were significantly higher than those in control patients (all P < 0.05). The levels of ALP, TB, IB, TP, ALB, CHE, PA, TC, HDL-C, LDL-C, APOA1, APOB and PLT in patients with HFRS were significantly lower than those in control patients (all P < 0.05). There was no significant difference in DB, GLU, LPa and CK between the two groups (all P > 0.05).
 |
Table 1 Biochemical Characteristics of Study Subjects |
Correlation of Parameters with HFRS
Among the parameters tested, many parameters exhibited significant correlation with HFRS (Table 2). ADA has a strong positive correlation with HFRS (r = 0.785, P < 0.001). In addition, there was significant positive correlation between HFRS and ALT (r = 0.506, P < 0.001), AST (r = 0.685, P < 0.001), Scr (r = 0.608, P < 0.001), TG (r = 0.504, P < 0.001) and LDH (r = 0.743, P < 0.001). There was significant negative correlation between HFRS and TP (r = −0.602, P < 0.001), ALB (r = −0.644, P < 0.001), PA (r = −0.576, P < 0.001), TC (r = −0.533, P < 0.001), HDL-C (r = −0.535, P < 0.001), LDL-C (r = −0.549, P < 0.001), APOA1 (r = −0.626, P < 0.001) and PLT (r = −0.730, P < 0.001).
 |
Table 2 Correlation of Laboratory Parameters with HFRS |
Diagnostic Value of ADA for HFRS
As described above, ADA is the parameter with the strongest correlation to HFRS. Therefore, we analyzed the diagnostic value of ADA for HFRS. A ROC curve and the area under the curve (AUC) was calculated to evaluate the diagnostic value of ADA in HFRS patients (Table 3 and Figure 1). The results indicated that the optimal cut-off value of ADA for diagnosis of HFRS was 18 U/L, with an AUC of 0.953 (95% CI: 0.925, 0.981). The sensitivity was 84.8%, the specificity was 93.1%, the positive predictive value was 92.2%, the negative predictive value was 86.5% and the Youden index was 77.9% (Table 3 and Figure 1). In addition to ADA, other parameters are also correlated with HFRS, the diagnostic value is shown in Supplementary Table 1, and the ROC curve is shown in Supplementary Figure 1. The diagnostic value of LDH and PLT for HFRS was also analyzed and the AUC was 0.928 (95% CI: 0.893, 0.964) and 0.921 (95% CI: 0.886, 0.956) respectively (Supplementary Table 1 and Supplementary Figure 1).
 |
Table 3 The Diagnostic Value of ADA for HFRS |
 |
Figure 1 ROC curve of ADA in diagnosing HFRS. |
Changes in Parameters for HFRS Patients at Admission and Discharge
Of the 124 HFRS patients, 122 patients were cured and discharged while two died. The changes for various parameters in 122 patients when they were admitted and discharged were listed in Table 4. The overall trend for ADA was a decrease in value for HRFS patients who were cured (Table 4 and Figure 2). The mean of the ADA difference between admission and discharge was 9.9±6.9 U/L (P < 0.001). Compared to levels at admission, the ALT, AST, Scr, BUN, UA, GLU, CK, LDH, WBC, RBC and HB showed a declining trend at the time of discharge, the ALP, TP, ALB, CHE, PA, TC, HDL-C, LDL-C, LPa, APOA1, APOB and PLT showed an upward trend. At the time of admission, the ADA levels of the two patients who died were 52 U/L and 28 U/L, before death, their ADA levels were 58 U/L and 26 U/L respectively.
 |
Table 4 Comparison of Each Parameter of 122 Patients with HFRS on Admission and Discharge |
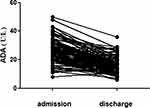 |
Figure 2 Tendency chart of ADA between admission and discharge. |
Discussion
HFRS is a relatively severe disease which has a higher mortality rate in China. Therefore, diagnosis and prognosis of HFRS is important for curing HFRS patients. In this study, we analyzed the laboratory test indicators of HFRS patients at admission and found that the levels of ALT, AST, GGT, TBA, ADA, Scr, BUN, UA, TG, LDH, WBC, RBC and HB increased compared with the control group. The levels of ALP, TB, IB, TP, ALB, CHE, PA, TC, HDL-C, LDL-C, APOA1, APOB and PLT decreased. Changes in these indicators reflected liver and kidney injury in patients. It is also suggested that HFRS may cause dyslipidemia and changes in the blood system. These findings are consistent with previous reports. HFRS is characterized by kidney damage, usually associated with a loss of kidney function.26 Other studies have shown that the liver is involved in the pathogenesis of HFRS, WBC and AST have important clinical significance in the severity and prognosis of the disease.27,28 Lipoprotein composition and metabolic changes have also been reported to occur in patients with HFRS.29
Further, we analyzed the correlation between laboratory parameters at admission and HFRS. It was found that ADA, LDH and HFRS had a strong positive correlation, while PLT and HFRS had a strong negative correlation, among which ADA and HFRS had the strongest correlation (r=0.785). Studies have shown that elevated serum LDH levels were observed in patients, indicating the presence of cellular damage.30 Another study has shown that HFRS caused by Pummula virus (PUUV) infection can lead to thrombocytopenic kidney injury. Elevated LDH and CRP values are also frequently observed31 and PLT is an independent predictor of severe HFRS.15 ADA can be involved in the regulation of various immune cells such as lymphocytes and neutrophils through the adenosine receptor pathway.24 CD8+T cell responses are essential for elimination of virus-infected cells and viral clearance in HFRS.32 A mouse model of HFRS also showed that CD8+ T cells are involved in the development of renal hemorrhaging.33 ADA is involved in T-lymphocyte differentiation and development while T cell responses contribute to virus elimination. Therefore, ADA may be involved in T cell-mediated immune response in HFRS.
In addition, we analyzed the values of ADA, LDH and PLT in the diagnosis of HFRS. The appropriate cut-off values of ADA, HDL and PLT in the diagnosis of HFRS were 18U/L, 260U/L, and 117×109/L respectively. Compared with HDL and PLT, ADA had the highest AUC (0.953 (95% CI: 0.925, 0.981)). Studies have shown that cerebrospinal fluid ADA is highly accurate in the diagnosis of tuberculous meningitis (diagnostic accuracy 71.32%).19 Abdi et al found that total activity for ADA and ADA2 isoenzymes was significantly higher in HIV-positive patients than in the serum of healthy individuals.20 Furthermore, Larijani et al found that total ADA activity was highly correlated with HbA1c (r = 0.6), with ADA activity being used as a diagnostic marker in type 2 diabetes mellitus.21 Serum ADA level was positively correlated with inflammatory response, suggesting that ADA can be a potential biomarker for predicting or monitoring autoimmune hepatitis patients.22 In our study, the Youden index, sensitivity, specificity, positive predictive value and negative predictive value of ADA in detecting HFRS were 77.9%, 84.28%, 93.1%, 92.2% and 86.5% respectively. These results strongly support that serum ADA may be a good diagnostic marker for HFRS. Compared with levels at admission, the ADA value of 122 HFRS patients showed a downward trend at discharge, with an average difference of 9.9±6.9 U/L, suggesting that ADA can be used as a potential indicator to monitor the progress of HFRS and treatment efficacy. The ADA values of the 2 patients who died in hospital were 52 U/L and 28 U/L at admission and 58 U/L and 26 U/L respectively before death. Although there were only 2 patients, a high ADA level was a predictor of poor prognosis for HFRS.
This study still has some limitations, such as the detection of total serum ADA but not ADA1 and ADA2 levels, so it is still not possible to analyze the role of ADA1 and ADA2 in disease diagnosis and monitoring. In addition, the number of patients examined was relatively small, with only 124 HFRS patients. However, we observed an increase in serum ADA level for HFRS, a phenomenon rarely reported before. We found that ADA has a strong positive correlation with HFRS and has a high diagnostic value for HFRS. In addition, ADA can monitor the disease progression of HFRS. In conclusion, ADA can be used as a marker for disease diagnosis and monitoring of HFRS.
Ethical Approval and Informed Consent
Informed consent was obtained from all patients included in this study. This research did not affect the patients’ health and privacy. All procedures performed in the studies involving human participants were in line with the ethical standards of the Medical Ethics Committee of Taizhou Hospital in Zhejiang Province affiliated with Wenzhou Medical University (#K20220531). They are also in line with the 1964 Helsinki Declaration and its later amendments or other comparable ethical standards.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Scientific Research Fund of Enze Medical Center (Group) (21EZD25).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sun L, Zou LX. Spatiotemporal analysis and forecasting model of hemorrhagic fever with renal syndrome in mainland China. Epidemiol Infect. 2018;146:1680–1688. doi:10.1017/S0950268818002030
2. Jiang H, Du H, Wang LM, et al. Hemorrhagic fever with renal syndrome: pathogenesis and clinical picture. Front Cell Infect Microbiol. 2016;6:1. doi:10.3389/fcimb.2016.00001
3. Chen JJ, Guo TC, Song SX, et al. Epidemiological characteristics and the development of spatiotemporal analysis models on hemorrhagic fever with renal syndrome in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:1735–1740. doi:10.3760/cma.j.cn112338-20191108-00794
4. Zou LX, Chen MJ, Sun L. Haemorrhagic fever with renal syndrome: literature review and distribution analysis in China. Int J Infect Dis. 2016;43:95–100. doi:10.1016/j.ijid.2016.01.003
5. Papa A. Dobrava-Belgrade virus: phylogeny, epidemiology, disease. Antiviral Res. 2012;95:104–117. doi:10.1016/j.antiviral.2012.05.011
6. Zhang S, Wang S, Yin W, et al. Epidemic characteristics of hemorrhagic fever with renal syndrome in China, 2006–2012. BMC Infect Dis. 2014;14:384. doi:10.1186/1471-2334-14-384
7. Krautkrämer E, Zeier M, Plyusnin A. Hantavirus infection: an emerging infectious disease causing acute renal failure. Kidney Int. 2013;83:23–27. doi:10.1038/ki.2012.36
8. Ferluga D, Vizjak A. Hantavirus nephropathy. J Am Soc Nephrol. 2008;19:1653–1658. doi:10.1681/ASN.2007091022
9. Vaheri A, Henttonen H, Voutilainen L, et al. Hantavirus infections in Europe and their impact on public health. Rev Med Virol. 2013;23:35–49. doi:10.1002/rmv.1722
10. Puca E, Harxhi A, Pipero P, et al. Pancreatitis in patients with hemorrhagic fever with renal syndrome: a five-year experience. J Infect Dev Ctries. 2017;11:900–903. doi:10.3855/jidc.9567
11. Tang K, Zhang C, Zhang Y, et al. Sustained high levels of both total and high molecular weight adiponectin in plasma during the convalescent phase of haemorrhagic fever with renal syndrome are associated with disease severity. J Immunol Res. 2017;2017:6468097. doi:10.1155/2017/6468097
12. Li J, Du H, Bai XF, et al. Study on expression of plasma sCD138 in patients with hemorrhagic fever with renal syndrome. BMC Infect Dis. 2018;18:100. doi:10.1186/s12879-018-3005-0
13. Fan X, Liu Z, Fu S, et al. Platelet distribution width at first day of hospital admission in patients with hemorrhagic fever with renal syndrome caused by hantaan virus may predict disease severity and critical patients’ survival. Dis Markers. 2018;2018:9701619. doi:10.1155/2018/9701619
14. Du H, Li J, Yu H, et al. HMGB-1 as a novel predictor of disease severity and prognosis in patients with hemorrhagic fever with renal syndrome. Mediators Inflamm. 2015;2015:696248. doi:10.1155/2015/696248
15. Cristalli G, Costanzi S, Lambertucci C, et al. Adenosine deaminase: functional implications and different classes of inhibitors. Med Res Rev. 2001;21:105–128. doi:10.1002/1098-1128(200103)21:2<105::aid-med1002>3.0.co;2-u
16. Kaljas Y, Liu C, Skaldin M, et al. Human adenosine deaminases ADA1 and ADA2 bind to different subsets of immune cells. Cell Mol Life Sci. 2017;74:555–570. doi:10.1007/s00018-016-2357-0
17. Sauer AV, Brigida I, Carriglio N, et al. Autoimmune dysregulation and purine metabolism in adenosine deaminase deficiency. Front Immunol. 2012;3:265. doi:10.3389/fimmu.2012.00265
18. Passos DF, Bernardes VM, da Silva JLG, et al. Adenosine signaling and adenosine deaminase regulation of immune responses: impact on the immunopathogenesis of HIV infection. Purinergic Signal. 2018;14:309–320. doi:10.1007/s11302-018-9619-2
19. Habib A, Amin ZA, Raza SH, et al. Diagnostic accuracy of cerebrospinal fluid adenosine deaminase in detecting Tuberculous Meningitis. Pak J Med Sci. 2018;34:1215–1218. doi:10.12669/pjms.345.13585
20. Abdi M, Ahmadi A, Roshany D, et al. Diagnostic value of serum adenosine deaminase activity in HIV infected patients of Kurdish population. Clin Lab. 2013;59:757–762. doi:10.7754/clin.lab.2012.120631
21. Larijani B, Heshmat R, Ebrahimi-Rad M, et al. Diagnostic value of adenosine deaminase and its isoforms in type II diabetes mellitus. Enzyme Res. 2016;2016:9526593. doi:10.1155/2016/9526593
22. Torgutalp M, Efe C, Babaoglu H, et al. Relationship between serum adenosine deaminase levels and liver histology in autoimmune hepatitis. World J Gastroenterol. 2017;23:3876–3882. doi:10.3748/wjg.v23.i21.3876
23. Kelgandre DC, Pathak J, Patel S, et al. Adenosine deaminase - A novel diagnostic and prognostic biomarker for oral squamous cell carcinoma. Asian Pac J Cancer Prev. 2016;17:1865–1868. doi:10.7314/apjcp.2016.17.4.1865
24. Antonioli L, Colucci R, La Motta C, et al. Adenosine deaminase in the modulation of immune system and its potential as a novel target for treatment of inflammatory disorders. Curr Drug Targets. 2012;13(6):842–862. doi:10.2174/138945012800564095
25. Whitmore KV, Gaspar HB. Adenosine Deaminase Deficiency - More Than Just an Immunodeficiency. Front Immunol. 2016;7:314. doi:10.3389/fimmu.2016.00314
26. Hansson M, Gustafsson R, Jacquet C, et al. Cystatin C and α-1-microglobulin predict severe acute kidney injury in patients with hemorrhagic fever with renal syndrome. Pathogens. 2020;9(8):666. doi:10.3390/pathogens9080666
27. Markotić A, Dasić G, Gagro A, et al. Role of peripheral blood mononuclear cell (PBMC) phenotype changes in the pathogenesis of haemorrhagic fever with renal syndrome (HFRS). Clin Exp Immunol. 1999;115:329–334. doi:10.1046/j.1365-2249.1999.00790.x
28. Du H, Li J, Yu HT, et al. Early indicators of severity and construction of a risk model for prognosis based upon laboratory parameters in patients with hemorrhagic fever with renal syndrome. Clin Chem Lab Med. 2014;52:1667–1675. doi:10.1515/cclm-2014-0016
29. Kim J, Park HH, Choi I, et al. Severely modified lipoprotein properties without a change in cholesteryl ester transfer protein activity in patients with acute renal failure secondary to Hantaan virus infection. BMB Rep. 2010;43:535–540. doi:10.5483/bmbrep.2010.43.8.535
30. Klingström J, Hardestam J, Stoltz M, et al. Loss of cell membrane integrity in puumala hantavirus-infected patients correlates with levels of epithelial cell apoptosis and perforin. J Virol. 2006;80:8279–8282. doi:10.1128/JVI.00742-06
31. Braun N, Haap M, Overkamp D, et al. Characterization and outcome following Puumala virus infection: a retrospective analysis of 75 cases. Nephrol Dial Transplant. 2010;25:2997–3003. doi:10.1093/ndt/gfq118
32. Terajima M, Ennis FA. T cells and pathogenesis of hantavirus cardiopulmonary syndrome and hemorrhagic fever with renal syndrome. Viruses. 2011;3:1059–1073. doi:10.3390/v3071059
33. Shimizu K, Yoshimatsu K, Taruishi M, et al. Involvement of CD8+ T cells in the development of renal hemorrhage in a mouse model of hemorrhagic fever with renal syndrome. Arch Virol. 2018;163:1577–1584. doi:10.1007/s00705-018-3786-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
