Back to Journals » Journal of Inflammation Research » Volume 14
Adenosine A2A Receptor Agonist Polydeoxyribonucleotide Alleviates Interstitial Cystitis-Induced Voiding Dysfunction by Suppressing Inflammation and Apoptosis in Rats
Authors Ko IG, Jin JJ, Hwang L, Kim SH , Kim CJ , Won KY, Na YG, Kim KH, Kim SJ
Received 21 October 2020
Accepted for publication 24 December 2020
Published 15 February 2021 Volume 2021:14 Pages 367—378
DOI https://doi.org/10.2147/JIR.S287346
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Il-Gyu Ko,1 Jun-Jang Jin,1 Lakkyong Hwang,1 Sang-Hoon Kim,1 Chang-Ju Kim,1 Kyu Yeoun Won,2 Yong Gil Na,3 Khae Hawn Kim,3 Su Jin Kim4
1Department of Physiology, College of Medicine, Kyung Hee University, Seoul, 02447, Republic of Korea; 2Department of Pathology, Kyung Hee University Hospital at Gangdong, College of Medicine, Kyung Hee University, Seoul, 05278, Korea; 3Department of Urology, Chungnam National University Sejong Hospital, College of Medicine, Chungnam National University, Sejong-si, 30099, Republic of Korea; 4Department of Urology, Yonsei University Wonju College of Medicine, Wonju, 26426, Republic of Korea
Correspondence: Su Jin Kim
Department of Urology, Yonsei University Wonju College of Medicine, 20 Ilsanro, Wonju, Gangwon-do, 26426, Republic of Korea
Tel +82-10-3287-0615
Email [email protected]
Background: Interstitial cystitis (IC) is a chronic disorder that indicates bladder-related pain or discomfort. Patients with IC often experience urination problems, such as urinary frequency and urgency, along with pain or discomfort in the bladder area. Therefore, new treatments based on IC etiology are needed. Polydeoxyribonucleotide (PDRN) is a biologic agonist of the adenosine A2A receptor, and PDRN has anti-inflammatory effect and inhibits apoptosis. In the current study, the effect of PDRN on cyclophosphamide-induced IC animal model was investigated using rats.
Methodology: To induce the IC animal model, 75 mg/kg of cyclophosphamide was injected intraperitoneally once every 3 days for 10 days. The rats in the PDRN-treated groups were intraperitoneally injected with 0.5 mL physiological saline containing 8 mg/kg PDRN, once a day for 10 days after IC induction.
Results: Induction of IC by cyclophosphamide injection caused voiding dysfunction, bladder edema, and histological damage. Cyclophosphamide injection increased secretion of pro-inflammatory cytokines and enhanced apoptosis. In contrast, PDRN treatment alleviated voiding dysfunction, bladder edema, and histological damage. Secretion of pro-inflammatory cytokines and expressions of apoptotic factors were suppressed by PDRN treatment. These changes indicate that treatment with PDRN improves voiding function by ultimately promoting the repair of damaged bladder tissue.
Conclusion: The conclusion of this experiment suggests the possibility that PDRN could be used as an effective therapeutic agent for IC.
Keywords: interstitial cystitis, adenosine A2A receptor, polydeoxyribonucleotide, voiding function, inflammation, apoptosis
Introduction
Interstitial cystitis (IC) is a chronic disorder that indicates bladder-related pain or discomfort. Patients with IC often experience urination problems, such as urinary frequency and urgency, along with pain or discomfort in the bladder area.1 IC is an important disease that degrades quality of life, but nevertheless, there is no clear treatment option because the clinical phenotypes of IC are very diverse and there is insufficient information about the underlying mechanisms of IC.2 Therefore, a key priority in IC research is exploring the molecular mechanisms of this disease in order to develop new, effective, and safe treatments.
The underlying molecular processes in the pathogenesis of IC involve complex interactions between inflammation and apoptosis.3,4 Pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6, exacerbate the process of diseases, and play a particularly important role in the progression of IC.5,6 During IC progress, various damaging factors promote inflammation of bladder, increasing bladder tissue damage and contributing to the apoptosis process.7
Mitogen-activated protein kinases (MAPK), composed of extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38, involved in the development of many diseases through activation of nuclear factor-κB (NF-κB).8 MAPK is associated with inhibition of cell growth, inflammation, and with mediating apoptosis in different cell types.9 MAPK and NF-κB are phosphorylated and then activated by many stimuli such as cytokines and cell stress.10 Activation of MAPK/NF-κB can promote the expression of various pro-inflammatory cytokines, thereby regulating inflammation and apoptosis.10,11 Enhancement of MAPK/NF-κB activity has been demonstrated in urothelial cells of IC,12 and increased phosphorylation of MAPK cascade has been found in the bladder of IC patients.13 Therefore, MAPK/NF-κB signaling pathway serves as a key component in the progress of IC.14–16
Adenosine and its receptors are an essential neuromodulator that plays an important role in various pathophysiological conditions. In particular, adenosine receptors are located in the epithelium of bladder and may regulate normal bladder function.17 There are four subtypes of adenosine receptors such as A1, A2A, A2B, and A3. Among them, the adenosine A2A receptor is involved in the inflammatory reaction and, when activated, suppresses the secretion of pro-inflammatory cytokines.18 In addition, evidences suggest that A2A receptor is involved in the regulation of the voiding reflex and neuropathic pain.17,19
Polydeoxyribonucleotide (PDRN), adenosine A2A receptor agonist, has suppressing effect on the production of pro-inflammatory cytokines. PDRN inhibits inflammatory cytokines production and apoptosis in various diseases such as gastric ulcers, acute lung injury, and ischemic colitis.20–22 However, despite the excellent pharmacological efficacy of anti-inflammatory and anti-apoptotic action, few studies have evaluated the effectiveness of PDRN in urological diseases. If PDRN can inactivate the signaling pathway of MAPK/NF-κB, PDRN may have a positive effect on the treatment process of IC.
Therefore, we evaluated the effect of the MAPK/NF-κB signaling on voiding problems in the IC animal model. Moreover, the effect of A2A receptor agonist PDRN on the MAPK/NF-κB signaling pathway associated inflammation, apoptosis, and voiding dysfunction was investigated using rats.
Materials and Methods
Animals and Classification
Twelve-week-old female Sprague-Dawley rats (250 ± 10 g, n = 40) were purchased from a commercial breeder (Orient Co., Sungnam, Korea) for this research. The rats were randomly classified into four groups: control group, IC-induced group, IC-induced and PDRN-treated group, and IC-induced and PDRN with 7-dimethyl-1-propargylxanthine (DMPX) treated group 100 (n = 10 each group).
This experimental procedure was approved by the Institutional Animal Care and Use Committee of Kyung Hee University and received the following approval number (KHUASP[SE]-18-096). The experimental process was conducted in good faith in accordance with the animal care guidelines from the National Institutes of Health and the Korean Institute of Medical Sciences.
Induction of IC and Treatments
IC model was made by injection of cyclophosphamide in the same method as described below.23 To induce IC rats, the rats received intraperitoneal injection with 75 mg/kg cyclophosphamide (Sigma Chemical Co., St. Louis, MO, USA) every 3 days for 10 days (total of 3 injections). The rats in the control group received an intraperitoneal injection with the same volume of physiological saline on the same schedule. The rats in the PDRN treated groups were intraperitoneally injected with 0.5 mL physiological saline containing 8 mg/kg PDRN (Rejuvenex®, PharmaResearch Products, Pangyo, Korea), once daily for 10 days after IC induction (Figure 1). We selected PDRN concentration that was found to be most effective through previous studies20,21 and preliminary experimental result. In order to confirm that the action of PDRN occurs through the adenosine A2A receptor, 8 mg/kg DMPX (Sigma Aldrich Co.) was co-treated with PDRN.
 |
Figure 1 Experimental schedule. |
Cystometry Analysis
In the cystometry analysis, contraction pressure and contraction time were determined 19 to 20 days after IC induction in the same method as described below.23,24 After anesthesia with intraperitoneal injection with Zoletil 50® (10 mg/kg; Vibac Laboratories, Carros, France), a transperitoneal incision was made and a polyethylene catheter (PE50) was inserted into the bladder. A syringe pump (Havard Apparatus, Holliston, MA, USA) and a pressure transducer (Havard Apparatus) were connected via a three-way stopcock to inject saline into the bladder and simultaneously record the intra-bladder pressure. After emptying the bladder, 0.5 mL saline was injected, and a pressure-flow study was conducted. LabScribe (iWork System Inc., Dover, NH, USA) was used to measure the contraction pressure and contraction time of the bladder.
Tissue Preparation
Tissue preparation was conducted in the same method as described below.24 The rats were anesthetized with Zoletil 50® (10 mg/kg; Vibac Laboratories) 20 days after first administration of cyclophosphamide. The extracted bladder tissue was measured by wet-weight and the ratio to body weight was calculated. After the bladder tissue was harvested, the bladder tissue was sufficiently fixed in 4% paraformaldehyde, dehydrated with ethanol series (70%, 80%, 90%, 95%, and 100%), treated with xylene, and embedded in paraffin. Paraffin-embedded bladder tissue was cut to 5 μm thick using a microtome (Thermo Fisher Scientific, Waltham, MA, USA) and mounted on coated slides (6 sections per bladder tissue). Slides with bladder tissue were dried overnight at 37°C on a hot plate.
Hematoxylin and Eosin (H&E) Staining
To observe histological changes in the bladder tissues, H&E staining was conducted in the same method as described below.24,25 The slides were soaked in Harris hematoxylin (Young-Dong Diagnostics, Yongin, Korea) for 10 seconds and then washed with clean water. Then, the slides were dipped in eosin (Sigma Chemical Co.) for 5 seconds and washed again with distilled water. After drying the stained slides, the slides were dehydrated by dipping with ethanol and xylene, and then sealed by Permount® (Thermo Fisher Scientific).
The inflammatory score was calculated to indicate tissue damage in the same method as described below.26 Images of H&E stained slides were taken with a Leica Application Suite X Computer-Assisted Image Analysis System (Leica Microsystems, Wetzlar, Germany) attached to a light microscope (Leica DMi8, Leica Microsystems). The degree of bladder inflammation was analyzed using a 6-point scale in a double-blind manner of pathologist. Criteria for histological evaluation of bladder inflammation are presented in Table 1.
 |
Table 1 Histological Score |
Pro-Inflammatory Cytokines and cAMP Concentrations
For the ELISA analysis in bladder tissue, concentrations of TNF-α, IL-1β, and cAMP were measured by reacting with enzyme immunoassay kit (Abcam, Cambridge, UK) according to the manufacturer’s manual. For TNF-α and IL-1β measurement in the bladder tissue, standard or lysed sample was added to each well, and plates were incubated at room temperature for 2.5 hours. The prepared TNF-α and IL-1β biotin antibodies were added to each well and incubated at room temperature for 1 hour. Then, HRP-streptavidin solution was added and incubated at room temperature for 45 minutes. TMB one-step development solution was reacted at room temperature for 30 minutes in the dark on a shaker. Immediately, stop solution was added to each well and calculated at 450 nm wavelength with Multiskan Go Microplate Readers (Thermo Fisher Scientific). For cAMP calculation, prepared standard and diluted samples were added to wells. Then, prepared alkaline phosphatase-conjugate was added to wells. Subsequently, cAMP complete antibody was reacted at room temperature on a shaker for 2 hours (500 rpm). pNpp substrate solution was added to wells and incubated at room temperature for 1 hour. Then, stop solution was added and read at 405 nm wavelength with Multiskan Go Microplate Readers (Thermo Fisher Scientific).
Western Blot Analysis
Western blot analysis was conducted in the same method as described below.21 The bladder tissue was homogenized on chilled RIPA buffer (Cell Signaling Technology, Inc., Danvers, MS, USA) with 1 mM PMSF (Sigma Aldrich), and then centrifuged at 14,000 rpm for 30 minutes at 4°C. Protein contents were measured using a μ-drop reader (Thermo Fisher Scientific), and 30 μg protein was separated on SDS-PAGE gels and transferred to a nitrocellulose membrane. The primary and secondary antibodies used are as shown in Table 2.
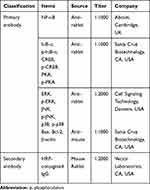 |
Table 2 Primary and Secondary Antibodies in Western Blot |
After the primary (NF-κB, IκB-α, phosphorylated (p)-IκB-α, ERK, p-ERK, JNK, p-JNK, p38, p-p38, CREB, p-CREB, PKA, p-PKA, Bax, Bcl-2, and β-actin) and secondary antibodies (mouse and rabbit horseradish peroxidase (HRP)-conjugated IgG) reactions, the blot membranes were detected by an enhanced chemiluminescence (ECL) detection kit (Bio-Rad, Hercules, CA, USA).
To compare the relative expressions of target proteins, the detected bands were calculated with Image-Pro® plus computer-assisted image analysis system (Media Cyberbetics Inc., Silver Spring, MD, USA). For the relative quantification of the band, the result of the control group was set to 1.00.
TUNEL Assay
TUNEL assay was performed using a In Situ Cell Death Detection Kit® (Roche, Mannheim, Germany) according to the manufacturer’s protocol in the same method as described below.27,28 The slides containing bladder tissue were deparaffinized using xylene, rehydrated with graded ethanol, and then rehydrated by running water for 5 minutes. The slides were boiled 10 mM sodium citrate buffer at 95°C for 2 minutes. Subsequently, slides were cooled for 30 minutes at room temperature. The slides were washed with phosphate buffered saline for 5 minutes to three times. Slides were incubated with label solution and enzyme solution mixture at 37°C for 1 hour. After wash out, slides were mounted on a coverslip using Vectashield® containing DAPI (Vector Laboratories, CA, USA). Fluorescence images were acquired by the Leica DMi8 microscope (Leica Microsystems) at excitation wavelength in the range of 450–500 nm and detection in the range of 515–565 nm (Leica Microsystems).
To identify the TUNEL-positive cells, five fields of view in the bladder mucosa were randomly selected from each sample, and more than 100 cells per field were counted under × 400 magnification. The percentage of TUNEL-positive cells was calculated by the following formula: number of TUNEL-positive cells/number of total cells × 100 (%).
Data Analysis
Multiple comparisons were conducted by SPSS software (version 23.0, IBM Corporation, Armonk, NY, USA). Statistical analysis between the groups was conducted using one-way ANOVA followed by Duncan’s post-test. The data were presented as the mean ± standard error of the mean, and P < 0.05 was found to be statistically significant.
Results
Voiding Function
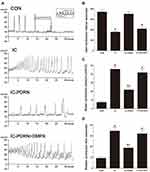
The analysis of voiding function from cystometry are presented in Figure 2A–D. Induction of IC by cyclophosphamide injection increased contraction pressure and contraction time while reducing inter-contraction interval. These changes led to impaired voiding function by induction of IC (P < 0.05). However, PDRN treatment decreased the IC-induced contraction pressure and contraction time with increased inter-contraction interval (P < 0.05). To assess the relevance of PDRN efficacy to the adenosine A2A receptor, combination treatment of DMPX with PDRN eliminated the improving effect of PDRN on voiding function such as contraction pressure, contraction time, and inter-contraction interval.
Bladder Weight and Inflammatory Score
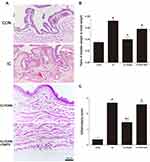
Bladder weight and histological changes of cyclophosphamide-induced IC are shown in Figure 3A–C. Induction of IC by cyclophosphamide injection caused epithelial detachment and erosion and infiltration of inflammatory cells (marked with black arrows). In addition, lamina propria showed edematous stroma and irregular ectasia vessels (marked with blue arrows).
These results increased bladder weight and inflammatory score (P < 0.05). However, PDRN treatment was observed to decrease inflammatory score and bladder weight due to the appearance of normal mucosal structure, improved inflammatory cell infiltration, and reduced swelling of the bladder tissue (P < 0.05). To assess the relevance of PDRN efficacy to the adenosine A2A receptor, combination treatment of DMPX with PDRN eliminated the reducing effect of PDRN on bladder weight and inflammation score.
Pro-Inflammatory Cytokines
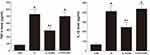
The expression levels of TNF-α and IL-1β are shown in Figure 4. Induction of IC by cyclophosphamide injection increased the expression levels of TNF-α and IL-1β in the bladder tissue (P < 0.05). In contrast, treatment with PDRN reduced the expression levels of TNF-α and IL-1β in the bladder tissue (P < 0.05). To assess the relevance of PDRN efficacy to the adenosine A2A receptor, combination treatment of DMPX with PDRN eliminated the inhibitory effect of PDRN on TNF-α and IL-1β production.
NF-κB/MAPK Signaling Pathway
Activation of NF-κB and phosphorylation of MAPK signaling pathways are shown in Figure 5. Induction of IC by cyclophosphamide injection enhanced NF-κB expression in the bladder tissue by stimulating the phosphorylation of IκB-α (P < 0.05). In contrast, PDRN treatment decreased IκB-α phosphorylation and suppressed NF-κB expression in the bladder tissue (P < 0.05), suggesting that PDRN treatment inactivated NF-κB. Induction of IC by cyclophosphamide injection enhanced phosphorylation of MAPK signaling pathway such as ERK, JNK, and p-38. PDRN treatment reduced phosphorylation of the MAPK signaling pathway in the bladder tissue (P < 0.05). To assess the relevance of PDRN efficacy to the adenosine A2A receptor, combination treatment of DMPX with PDRN eliminated the inhibitory effect of PDRN on phosphorylation of IκB-α, ERK, JNK and p38 (Supplement 1).
cAMP, p-CREB, and p-PKA Expressions
The cAMP level measured by ELISA and the ratio of p-CREB/CREB and p-PKA/PKA detected by Western blotting are shown in Figure 6A–C. Induction of IC by cyclophosphamide injection resulted in a decrease in cAMP level in the bladder (P < 0.05), whereas PDRN treatment resulted in increased cAMP level in the bladder tissue (P < 0.05). Induction of IC by cyclophosphamide injection increased the phosphorylation of CREB and PKA (P < 0.05), in contrast, PDRN treatment suppressed the phosphorylation of CREB and PKA in the bladder tissue (P < 0.05). To assess the relevance of PDRN efficacy to the adenosine A2A receptor, combination treatment of DMPX with PDRN eliminated increasing effect of PDRN on cAMP level and abolished suppressing effect of PDRN on CREB and PKA phosphorylation.
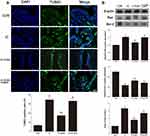
DNA Fragmentation and Bax/Bcl-2 Ratio
DNA fragmentation was detected by TUNEL staining and expressions of Bax and Bcl-2 were measured by Western blotting (Figure 7A and B). Induction of IC by cyclophosphamide injection increased DNA fragmentation and Bax/Bcl-2 ratio in the bladder (P < 0.05). However, PDRN treatment inhibited DNA fragmentation and Bax/Bcl-2 ratio in the bladder (P < 0.05). To assess the relevance of PDRN efficacy to the adenosine A2A receptor, combination treatment of DMPX with PDRN eliminated decreasing effect of PDRN on DNA fragmentation and Bax/Bcl-2 ratio.
Discussion
The symptoms of the IC patients are very diverse, and most of them show voiding problems such as urinary frequency and urgency as well as bladder associated pain or discomfort. Cyclophosphamide-induced IC model is widely used to assess the inflammatory change of the bladder and related micturition dysfunction.29 Cyclophosphamide injection also causes urothelium hyperplasia and induces inflammatory response.2 In the current study, the cystometry to measure the functional change of the bladder showed increased contraction pressure and contraction time, whereas decreased intercontraction interval. Cyclophosphamide injections caused mucosal edema and inflammatory cell infiltration, which increased bladder weight and inflammation score. Current findings were similar to previous studies showing bladder tissue damage and impaired urination due to cyclophosphamide.2,23,29
Inflammation serves to aggravate the symptoms of IC, especially, the production of pro-inflammatory cytokines by IC caused voiding dysfunction and bladder pain or discomfort.3,5,7 In the current study, the deleterious effect of cyclophosphamide on the bladder may be attributed to increased secretion of TNF-α and IL-1β in the bladder. Our experiment results showed that overproduction of pro-inflammatory cytokines aggravated the progress of IC. Clinically, because pro-inflammatory biomarkers were significantly increased in IC patients,5,30 suppression on pro-inflammatory cytokines is an important treatment strategy for IC.
Since the secretion of pro-inflammatory cytokines is inhibited by stimulation of adenosine A2A receptor in monocytes and macrophages,31,32 the adenosine A2A receptor agonist PDRN has been proposed as a treatment for inflammatory diseases.18,20,22 In the current study, PDRN treatment reduced TNF-α and IL-1β expressions in IC-induced rats, suggesting that decrease of pro-inflammatory cytokines by PDRN plays a critical step in IC treatment strategy.
Inflammation is an immune response to damage, and the MAPK signaling pathway primarily regulates the inflammation process.33,34 Phosphorylation of MAPK signaling pathway is increased in cyclophosphamide-induced IC.7,33,35 It is also well known that MAPK controls NF-κB.7 The NF-κB transcription factor plays a crucial role in the inflammatory process and is activated by the breakdown of the IκB-α protein.36 The activated NF-κB complex translocates to the nucleus to produce pro-inflammatory cytokines, increase of which is involved in the progression of inflammatory diseases such as IC.37 In the current study, administration of cyclophosphamide enhanced MAPK cascade phosphorylation in the bladder tissue, and this response eventually induces IκB-α activation, increasing the activity of NF-κB. However, PDRN treatment reduced the activation of IκB-α and NF-κB in the bladder tissue by inhibiting phosphorylation of MAPK cascade.
Stimulation-induced activation of the adenosine A2A receptor increases the concentration of cAMP in cells, thereby inhibiting inflammatory neutrophils.38 The cAMP-PKA signaling pathway is activated by the adenosine A2A receptor to accelerate phosphorylation of CREB and inhibits pro-inflammatory cytokines through regulation of NF-κB.39 In addition, increased cAMP level suppresses the phosphorylated form of the MAPK cascade in stimulated cells.38,40 In the current study, PDRN treatment increased cAMP level and adenosine A2A receptor in the bladder tissue of cyclophosphamide-induced IC rats (Supplement 2). Enhancement of cAMP level in the bladder by PDRN treatment suppressed MAPK phosphorylation and NF-κB activation.
Increased cAMP in urinary tract cells is known to induce the release of anti-apoptotic proteins to inhibit apoptosis.41 Increased cAMP concentration plays an important role in suppressing apoptotic cell death. Since apoptosis is involved as a critical process in the pathogenesis of IC,4 suppressing apoptosis is the key to IC treatment. In the current study, cyclophosphamide-induced IC rats treated with PDRN showed low expression of DNA fragmentation and a low Bax to Bcl-2 ratio.
Conclusion
In this study, PDRN treatment improved voiding dysfunction by suppressing inflammation and apoptosis in IC rats through increased cAMP concentration in the bladder tissue. On the other hand, co-treatment of adenosine A2A receptor antagonist DMPX with adenosine A2A receptor agonist PDRN did not reduce inflammation and apoptosis, resulting in no improvement in voiding dysfunction. With the current result, PDRN may be expected to be effective treatment for bladder inflammation. As a limitation of the study, pain is an important feature observed in IC patients, but was not measured in this study. For this reason, we believe that it is insufficient to demonstrate the efficacy of PDRN for pain in the IC model. Therefore, further research is needed to evaluate pain improvement.
Data Sharing Statement
All data generated in this study are included in this manuscript.
Acknowledgments
This study was supported by a grant from the National Research Foundation of Korea (NRF-2018R1A6A3A11049397)
Authorship Contributions
Ko I.G: Conceptualization, Project administration, Writing-original draft, Jin J.J: Methodology, Formal analysis, Hwang L: Methodology, Formal analysis, Kim S.H: Methodology, Investigation, Resources, Kim C.J: Supervision, Project administration, Won K.Y: Methodology, Formal analysis, Na Y.G: Supervision, Resources, Kim K.H: Investigation, Writing- review and editing, Kin S.J: Supervision, Project administration, Writing- review and editing. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest for this work to declare.
References
1. Ahn ST, Jeong HG, Park TY, et al. Differences in urodynamic para-meters according to the presence of a Hunner lesion in women with interstitial cystitis/bladder pain syndrome. Int Neurourol J. 2018;22:S55–61. doi:10.5213/inj.1835044.522
2. Akiyama Y, Luo Y, Hanno PM, et al. Interstitial cystitis/bladder pain syndrome: the evolving landscape, animal models and future perspectives. Int J Urol. 2020;27(6):491–503. doi:10.1111/iju.14229
3. Schrepf A, O’Donnell M, Luo Y, et al. Multidisciplinary approach to the study of chronic pelvic pain (MAPP) research network. Inflammation and inflammatory control in interstitial cystitis/bladder pain syndrome: associations with painful symptoms. Pain. 2014;155(9):1755–1761. doi:10.1016/j.pain.2014.05.029
4. Di Capua-Sacoto C, Sanchez-Llopis A, Oconnor JE, et al. Apoptotic effect as biomarker of disease, severity and follow-up in interstitial cystitis. Actas Urol Esp. 2018;42(4):262–266. doi:10.1016/j.acuro.2017.09.007
5. Jiang YH, Peng CH, Liu HT, et al. Increased pro-inflammatory cytokines, C-reactive protein and nerve growth factor expressions in serum of patients with interstitial cystitis/bladder pain syndrome. PLoS One. 2013;8(10):e76779. doi:10.1371/journal.pone.0076779
6. Shahid M, Lee MY, Yeon A, et al. Menthol, a unique urinary volatile compound, is associated with chronic inflammation in interstitial cystitis. Sci Rep. 2018;8(1):10859. doi:10.1038/s41598-018-29085-3
7. Luo J, Yang C, Luo X, et al. Chlorogenic acid attenuates cyclophosphamide-induced rat interstitial cystitis. Life Sci. 2020;254:117590. doi:10.1016/j.lfs.2020.117590
8. Cuschieri J, Maier RV. Mitogen-activated protein kinase (MAPK). Crit Care Med. 2005;33:S417–S419. doi:10.1097/01.CCM.0000191714.39495.A6
9. Bradley MS, Burke EE, Grenier C, et al. A genome-scale DNA methylation study in women with interstitial cystitis/bladder pain syndrome. Neurourol Urodyn. 2018;37(4):1485–1493. doi:10.1002/nau.23489
10. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. doi:10.1126/science.1072682
11. Gantke T, Sriskantharajah S, Sadowski M, et al. IκB kinase regulation of the TPL-2/ERK MAPK pathway. Immunol Rev. 2012;246(1):168–182. doi:10.1111/j.1600-065X.2012.01104.x
12. Marentette JO, Hauser PJ, Hurst RE, et al. Tryptase activation of immortalized human urothelial cell mitogen-activated protein kinase. PLoS One. 2013;8(7):e69948. doi:10.1371/journal.pone.0069948
13. Shie J-H, Liu H-T, Wang Y-S, et al. Immunohistochemical evidence suggests repeated intravesical application of botulinum toxin A injections may improve treatment efficacy of interstitial cystitis/bladder pain syndrome. BJU International. 2013;111(4):638–646. doi:10.1111/j.1464-410X.2012.11466.x
14. Abdel-Mageed AB, Bajwa A, Shenassa BB, et al. NF-κB-dependent gene expression of proinflammatory cytokines in T24 cells: possible role in interstitial cystitis. Urol Res. 2003;31(5):300–305. doi:10.1007/s00240-003-0339-9
15. Zhao J, Wang L, Dong X, et al. The c-Jun N-terminal kinase (JNK) pathway is activated in human interstitial cystitis (IC) and rat protamine sulfate induced cystitis. Sci Rep. 2016;6:19670. doi:10.1038/srep19670
16. Shie JH, Liu HT, Kuo HC. Increased cell apoptosis of urothelium mediated by inflammation in interstitial cystitis/painful bladder syndrome. Urology. 2012;79(2):
17. Yu W, Zacharia LC, Jackson EK, et al. Adenosine receptor expression and function in bladder uroepithelium. Am J Physiol Cell Physiol. 2006;291(2):C254–265. doi:10.1152/ajpcell.00025.2006
18. Guerrero A. A2A adenosine receptor agonists and their potential therapeutic applications. An update. Curr Med Chem. 2018;25(30):3597–3612. doi:10.2174/0929867325666180313110254
19. Sawynok J. Adenosine receptor targets for pain. Neuroscience. 2016;338:1–18. doi:10.1016/j.neuroscience.2015.10.031
20. Ko IG, Kim SE, Jin JJ, et al. Combination therapy with polydeoxyribonucleotide and proton pump inhibitor enhances therapeutic effectiveness for gastric ulcer in rats. Life Sci. 2018;203:12–19. doi:10.1016/j.lfs.2018.04.009
21. Ko I-G, Hwang JJ, Chang BS, et al. Polydeoxyribonucleotide ameliorates lipopolysaccharide-induced acute lung injury via modulation of the MAPK/NF-κB signaling pathway in rats. Int Immunopharmacol. 2020;83:106444. doi:10.1016/j.intimp.2020.106444
22. Kim SE, Ko IG, Jin JJ, et al. Polydeoxyribonucleotide exerts therapeutic effect by increasing VEGF and inhibiting inflammatory cytokines in ischemic colitis rats. Biomed Res Int. 2020;21:2169083. doi:10.1155/2020/2169083
23. Ko IG, Moon BM, Kim SE, et al. Effects of combination treatment of α1-adrenergic receptor antagonists on voiding dysfunction: study on target organs in overactive bladder rats. Int Neurourol J. 2016;20:S150–S158. doi:10.5213/inj.1632768.384
24. Park JS, Jung HD, Cho YS, et al. Neonatal bladder irritation is associated with vanilloid receptor TRPV1 expression in adult rats. Int Neurourol J. 2018;22(3):
25. Li M, Han T, Zhang W, et al. Simulated altitude exercise training damages small intestinal mucosa barrier in the rats. J Exerc Rehabil. 2018;14(3):341–348. doi:10.12965/jer.1835128.064
26. Starkman JS, Martinez-Ferrer M, Iturregui JM, et al. Nicotinic signaling ameliorates acute bladder inflammation induced by protamine sulfate or cyclophosphamide. J Urol. 2008;179(6):2440. doi:10.1016/j.juro.2008.01.082
27. Cheng SF, Jiang YH, Kuo HC. Urothelial dysfunction and chronic inflammation are associated with increased bladder sensation in patients with chronic renal insufficiency. Int Neurourol J. 2018;22:S46–S54. doi:10.5213/inj.1832814.407
28. Song SH, Jee YS, Ko IG, et al. Treadmill exercise and wheel exercise improve motor function by suppressing apoptotic neuronal cell death in brain inflammation rats. J Exerc Rehabil. 2018;14(6):911–919. doi:10.12965/jer.1836508.254
29. Birder L, Andersson K-E. Animal modelling of interstitial cystitis/bladder pain syndrome. Int Neurourol J. 2018;22(Suppl 1):S3–S9. doi:10.5213/inj.1835062.531
30. Song PH, Chun SY, Chung JW, et al. Comparison of 5 different rat models to establish a standard animal model for research into interstitial cystitis. Int Neurourol J. 2017;21(3):163–170. doi:10.5213/inj.1734898.449
31. Gomez G, Sitkovsky MV. Targeting G protein-coupled A2a adenosine receptors to engineer inflammation in vivo. Int J Biochem Cell Biol. 2003;35(4):410–414. doi:10.1016/s1357-2725(02)00177-2
32. Khoa ND, Montesinos MC, Williams AJ, et al. Th1 cytokines regulate adenosine receptors and their downstream signaling elements in human microvascular endothelial cells. J Immunol. 2003;171(8):3991–3998. doi:10.4049/jimmunol.171.8.3991
33. Song J, Liu L, Li L, et al. Protective effects of lipoic acid and mesna on cyclophosphamide-induced haemorrhagic cystitis in mice. Cell Biochem Funct. 2014;32(2):125–132. doi:10.1002/cbf.2978
34. Li D, Zhang X, Chen B. SIGIRR participates in negative regulation of LPS response and tolerance in human bladder epithelial cells. BMC Immunol. 2015;16:73. doi:10.1186/s12865-015-0137-5
35. Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-regulated kinases in bladder afferent pathways with cyclophosphamide-induced cystitis. Neuroscience. 2009;163(4):1353–1362. doi:10.1016/j.neuroscience.2009.07.044
36. Scheidereit C. IκB kinase complexes: gateways to NF-κB activation and transcription. Oncogene. 2006;25(51):6685–6705. doi:10.1038/sj.onc.1209934
37. Verhelst K, Carpentier I, Beyaert R. Regulation of TNF-induced NF-κB activation by different cytoplasmic ubiquitination events. Cytokine Growth Factor Rev. 2011;22(5–6):277–286. doi:10.1016/j.cytogfr.2011.11.002
38. Giambelluca MS, Pouliot M. Early tyrosine phosphorylation events following adenosine A2A receptor in human neutrophils: identification of regulated pathways. J Leukoc Biol. 2017;102(3):829–836. doi:10.1189/jlb.2VMA1216-517R
39. Haskó G, Linden J, Cronstein B, et al. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7(9):759–770. doi:10.1038/nrd2638
40. Chera S, Ghila L, Wenger Y, Galliot B. Injury-induced activation of the MAPK/CREB pathway triggers apoptosis-induced compensatory proliferation in hydra head regeneration. Dev Growth Differ. 2011;53:186–201. doi:10.1111/j.1440-169X.2011.01250.x
41. Saban MR, O’Donnell MA, Hurst RE, et al. Molecular networks discriminating mouse bladder responses to intravesical bacillus Calmette-Guerin (BCG), LPS, and TNF-α. BMC Immunol. 2008;9:4. doi:10.1186/1471-2172-9-4
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.