Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Addressing the Continuum of Dysglycaemia and Vascular Complications in Prediabetes and Type 2 Diabetes: Need for Early and Intensive Treatment
Authors Ghannam N, Alahmed S , Aldahash R , Aljohani N, Alshammary A, Amir A, Kamal A, Khader S, Salah M, Shalabi H, Abdallah A , Elboghdady A
Received 8 November 2022
Accepted for publication 23 December 2022
Published 11 January 2023 Volume 2023:16 Pages 105—115
DOI https://doi.org/10.2147/DMSO.S396621
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Nadia Ghannam,1 Saleh Alahmed,2 Raed Aldahash,3 Naji Aljohani,4 Afaf Alshammary,5 Ashraf Amir,6 Abdullah Kamal,7 Said Khader,8 Mohammed Salah,9 Hani Shalabi,10,11 Ahmed Abdallah,12 Ahmed Elboghdady12
1Ghannam Clinic, Jeddah, Saudi Arabia; 2Almana Group of Hospitals, Dammam, Saudi Arabia; 3Ministry of National Guard (Health Affairs) and King Abdullah International Medical Research Center, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia; 4King Fahad Medical City, Riyadh, Saudi Arabia; 5Ministry of National Guard (Health Affairs), King Abdulaziz Medical City, Riyadh and King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia; 6Family Medicine International Medical Center, Jeddah, Saudi Arabia; 7Dallah Hospital, Riyadh, Saudi Arabia; 8Dr. Sulaiman Al Habib Medical Group, Riyadh, Saudi Arabia; 9Cairo University, Cairo, Egypt and GNP Hospital, Jeddah, Saudi Arabia; 10University of Jeddah, Jeddah, Saudi Arabia; 11Dr. Soliman Fakeeh Hospital, Jeddah, Saudi Arabia; 12Merck Serono Middle East FZ-Ltd, Jeddah, Saudi Arabia
Correspondence: Nadia Ghannam, Ghannam Clinic, King Abdulaziz Road, Jeddah, 21411, Saudi Arabia, Email [email protected]
Abstract: The onset of type 2 diabetes increases the risk of vascular complications and death. We know now that that this risk begins long before the diabetes diagnosis. Prediabetes and type 2 diabetes are not separate entities in practice and exist within a continuum of dysglycaemia and vascular risk that increases in severity over time. This excess risk requires early intervention with lifestyle therapy supported with pharmacologic antidiabetic therapy, intensified promptly where necessary throughout the duration of the diabetes continuum. Metformin is an evidence-based treatment for preventing prediabetes and improves cardiovascular outcomes in people with type 2 diabetes from diagnosis onwards. Newer agents (SGLT2 inhibitors and GLP-1 agonists) are appropriate for people presenting with type 2 diabetes and significant cardiovascular comorbidity. Additional therapies should be used without delay to achieve patients’ individualised HbA1c goals and to minimise cardiovascular risk.
Keywords: prediabetes, type 2 diabetes, diabetes complications, antidiabetic therapy
Introduction
The publication of the Diabetes Control and Complications Trial in 1993 proved conclusively that long-term hyperglycaemia in the setting of type 1 diabetes was associated with cardiorenal complications with the potential to reduce greatly both the quantity and quality of patients’ lives.1 This was also the first randomised trial to prove that intensive vs standard control of blood glucose reduced the risk of diabetes complications. The results of the UK Prospective diabetes Study (UKPDS) in 1998 showed that intensive glucose management per se (compared with diet-based treatment only) delivered significant reductions in long-term microvascular complications in people with type 2 diabetes,2 with significant cardiovascular outcome benefits observed in patients randomised to metformin vs the diet intervention.3 These landmark trials, together with epidemiological associations of hyperglycaemia and adverse clinical outcomes, underpinned the design of diabetes management algorithms intended to preserve long-term health among people with diabetes.
Current management recommendations for type 2 diabetes include individualised HbA1c goals based on age, comorbidities, patient preferences, risk of adverse effects of treatment, and other factors.4,5 A general recommendation to reduce HbA1c to <7.0% for most non-pregnant adults reflects observations that the risk of diabetes complications begins to increase more steeply above this level of HbA1c, although there is no lower cut-off for HbA1c that negates the risk of complications.6,7 Concepts of “prediabetes”, “intermediate hyperglycaemia”, or “non-diabetic hyperglycaemia” have evolved to characterise the substantial population with markers of blood glucose that are elevated, but insufficiently to trigger the diagnosis of type 2 diabetes. In practice, there is growing recognition that the boundary between prediabetic states and clinical type 2 diabetes is arbitrary, in that it does not mark a starting point for increased risk of diabetes-associated complications.8 In this article, we review the evidence that prediabetes and diagnosed type 2 diabetes represent a continuum of vascular risk that should be managed early and continuously.
Clinical Relevance of the Diabetes Continuum
Glycaemic Control
Individuals at risk of developing type 2 diabetes demonstrate a progressive increase in indices of glycaemia. The development of insulin resistance at this time prompts increased secretion of insulin to maintain blood glucose near normal levels, despite a concurrent progressive decline in pancreatic β-cell function.9 Increases in fasting glucose and post-load glucose and HbA1c occur during this period that can prompt a diagnosis of prediabetes according to criteria provided by expert societies (the diagnostic cut-offs used to diagnose prediabetes and type 2 diabetes are summarised in Table 1).10,11 Interestingly, there is evidence that men tend to present with prediabetes driven by elevation of fasting glucose (impaired fasting glucose; IFG), driven by increased hepatic glucose output and blunted first-phase insulin secretion, while women tend to present with elevation of post-load glucose (impaired glucose tolerance; IGT), driven by insulin resistance in the periphery.12 Systematic reviews have associated changes in the gut microbiome with insulin resistance, prediabetes and type 2 diabetes, with some evaluations of probiotics demonstrating improvements in glycaemic control.13–15 These associations are variable, however, and this approach remains in the arena of research. Further study will be needed in this area before targeted modulation of the gut microbiome joins the evidence-based interventions for managing the diabetes continuum that are described later in this article.
 |
Table 1 Diagnosis of Prediabetes and Type 2 Diabetes |
Any form of prediabetes markedly increases the risk of developing subsequent type 2 diabetes. For example, up to 8 years of longitudinal follow-up of a cohort in Sweden showed that IFG and impaired glucose tolerance (IGT) increased the risk of developing type 2 diabetes by about 2-fold and 5-fold, respectively, compared with normoglycemic subjects, with a higher risk still for subjects with combined IFG and IGT.16 A meta-analysis of observational studies showed that IGT and IFG (American Diabetes Association [ADA] criteria) increased the 5-year risk of type 2 diabetes by about 3–5-fold, with a higher risk of about 7–8-fold associated with elevated HbA1c.17
Eventually, β-cell function declines to a point where it is no longer possible to maintain the level of insulin secretion needed to overcome insulin resistance: blood glucose increases further and clinical type 2 diabetes becomes established.9 It is important to note that these diagnoses are categorical, in that only a small increase in the level of hyperglycaemia is required to progress from any category of prediabetes to clinical type 2 diabetes. In addition, the impairments in insulin sensitivity and β-cell function begin long before diabetes is diagnosed: for example, data from the UKPDS suggested that β-cell function had been declining for as much as 12 years before the initiation of the trial, on average, in this population with newly-diagnosed type 2 diabetes.18 Finally, the magnitude of increases in blood glucose in people with prediabetes or early type 2 diabetes is not sufficient to produce symptoms of hyperglycaemia and prediabetes and type 2 diabetes often go undiagnosed. For example, more than 8/10 people with prediabetes in the USA do not know they have the condition19 and surveys of community-based populations have discovered substantial proportions of people with previously undiagnosed diabetes.20–23
Vascular Complications
The increase in the risk of major adverse cardiovascular events in people with vs without type 2 diabetes has been understood for decades.6 Although mortality rates in people with diabetes have been falling in recent years, likely due to increased medical management of cardiovascular risk factors and cardiac events, the magnitude of the excess mortality in people with vs without diabetes has remained constant over time.24 This section will therefore focus on the less well-understood relationships between prediabetes and adverse vascular outcomes.
Observational studies25–28 and systematic reviews29–31 have demonstrated an increased risk of adverse macrovascular complications, including all-cause mortality, reminiscent of type 2 diabetes in populations with IFG and/or IGT, although it should be noted that such associations have not been seen in all studies.32 Figure 1 shows an example of the associations of prediabetes diagnosed as IFG, IGT and elevated HbA1c with a range of adverse macrovascular outcomes from a recent systematic review: prediabetes, however diagnosed, increased the risks of all-cause and cardiovascular death, cardiovascular events, coronary heart disease events, and stroke.30
 |
Figure 1 Associations between different definitions of prediabetic states and adverse macrovascular outcomes from a systematic review. Abbreviations: CHD, coronary heart disease; CVD, cardiovascular disease; IFG, impaired fasting glucose; IGT, impaired glucose tolerance. Notes: Definitions of prediabetic states shown here were according to American Diabetes Association criteria. Data from Gujral et al.30 |
To some extent, the excess cardiovascular risk associated with prediabetes may be mediated by classical cardiovascular risk factors associated with insulin resistance and the metabolic syndrome.33–36 Women tend to demonstrate more adverse classical cardiovascular risk profiles during conversion from prediabetes to type 2 diabetes, compared with men.37 Endothelial dysfunction has been observed in people with prediabetes and this increases the risk of subsequent conversion to type 2 diabetes.38 Although endothelial dysfunction has been associated with insulin resistance,39,40 evaluations of interventions designed to ameliorate insulin resistance, such as diet and exercise, have been mixed.41–43 Treatment with metformin has been shown to improve coronary endothelial function in people with prediabetes and pre-existing coronary artery disease.44 Accelerated atherosclerosis has also been observed in people with prediabetes, as measured by carotid intima-media-thickness,45,46 a validated surrogate measure of the overall burden of atherosclerosis47 and a powerful predictor of cardiovascular risk.48 Heart failure is now recognised as the most commonly occurring cardiorenal complication of type 2 diabetes.49,50 Observational studies or systematic reviews have51,52 or have not30,32 demonstrated strong associations of incident heart failure with prediabetes. Other analyses suggested more adverse outcomes from pre-existing heart failure in populations with vs without prediabetes.53–55 The excess risk of cardiovascular disease associated with type 2 diabetes is markedly greater for women vs men; although current data suggest that women with IGT, but not IFG, may be at higher risk of adverse cardiovascular outcomes.37
A number of studies have demonstrated a higher risk of microvascular changes in people with vs without prediabetes. Adverse microvascular changes in the eye have included abnormalities in retinal arteriolar structure or function,56–59 macular thinning,60 or impaired retinal function.61 A diagnosis of prediabetes was associated with a higher risk of diagnosis of retinopathy in a retrospective study in a primary care population.62 Again, it should be noted that not all studies have associated significant adverse microvascular findings in the eye with prediabetes.63 Similarly, prediabetes has been associated with dysfunction of the kidney.30,62,64–72 Evidence of neuropathy or microvascular dysfunction has been found in people with vs without prediabetes in skin,56 the heart,73,74 and in sensory nerves.75 Other studies showed trends to adverse changes in nerve function, rather than overt neuropathy, in people with prediabetes.76–79 Prediabetes has also been associated with loss of brain volume and other potentially adverse ultrastructural changes.80,81 In general, the severity of these adverse changes in people with prediabetes was intermediate between those with normoglycaemia and type 2 diabetes.82
Intervening in the Diabetes Continuum
The data summarised above show that the insidious and largely undiagnosed and untreated progression of hyperglycaemia in the setting of prediabetes (and undiagnosed type 2 diabetes) can persist for a decade or more, leaving the vascular system exposed to an increased risk of vascular and cardiac complications similar to those observed in people with established type 2 diabetes. Figure 2 summarises key principles in the management of the diabetes continuum, which are described below.
 |
Figure 2 Schematic representation of key goals in managing the diabetes continuum. Abbreviation: AD, antidiabetic drug. Notes: aUse with caution in combination with SGLT2 inhibitor or sulfonylurea to minimise risk of hypoglycaemia; bpatients with established cardiovascular disease or heart failure at presentation (see text); cfor selected people with prediabetes (see text94) and/or at diagnosis of type 2 diabetes consistent with joint guidance from the American Diabetes Association/European Association for the study of Diabetes.4 |
Intervene Early to Optimise Long-Term Outcomes – Prediabetes
Early intervention is important for interrupting this process and optimising long-term outcomes. Lifestyle intervention remains the foundation treatment for all stages of the diabetes continuum and all subjects with prediabetes or established diabetes should be encouraged to improve their diets and increase their level of physical activity. Two landmark randomised studies in subjects with IGT, the Diabetes Prevention Program (DPP, USA)83 and the Diabetes Prevention Study (DPS, Finland)84 showed that an intensive lifestyle intervention (≥150 min/week of moderate exercise and improved diet aimed at facilitating weight loss) reduced the risk of developing diabetes by 58% over 3 years, compared with standard lifestyle advice. The DaQing study, a cluster randomised trial in subjects with IGT in China, showed that, either exercise or diet plus exercise reduced significantly the 6-year risk of diabetes.85 The benefit for diabetes prevention persisted long after the end of the randomised trials, with lower incidences of diabetes for former intervention vs former control groups after 25 years (DPP),86–88 13 years (DPS),89 and 30 years (DaQing).90 Importantly, the diabetes prevention intervention in the DaQing study (for pooled diet and exercise groups vs control) resulted in a significantly reduced risk of cardiovascular events, microvascular events, and mortality after 30 years of follow-up.90 Long-term follow up to the DPP did not produce sufficient events to demonstrate significant outcomes benefits for individual study interventions, but diabetes prevention per se in the overall population was associated with improved cardiovascular and microvascular outcomes.86 Finally, we focused on these three studies here because of their long-term follow-up programmes; many other studies have demonstrated the benefits of lifestyle interventions in people with prediabetes, and these are reviewed elsewhere.91
Many patients cannot, or will not, comply with lifestyle interventions, however, and pharmacologic interventions also have the potential to prevent/delay the onset of type 2 diabetes.91 Metformin has the largest clinical evidence base for use in prediabetes and had been granted a therapeutic indication for this purpose in 67 countries in 2017.92,93 Metformin reduced the incidence of diabetes by 31% vs standard lifestyle advice in the DPP, but was similarly effective to the intensive lifestyle intervention in this trial (see above) in younger, heavier, and more hyperglycaemic subjects.83 Guidelines or expert opinion in Europe and the USA support the use of metformin in this population, and also in women at risk of type 2 diabetes through prior gestational diabetes (Table 2).94–96
 |
Table 2 European and US Guidance on the Use of Metformin to Prevent or Delay the Onset of Type 2 Diabetes |
The DPP did not demonstrate clinical outcomes benefits for any individual study arm, as described above. However, a real-world analysis has shown that the use of metformin in people with prediabetes was associated with a significantly lower (p < 0.001) incidence of new cardiovascular disease for people with BMI ≥35 kg/m2 (21%) vs <35 kg/m2 (28%), which is consistent with current guidance in this area.97
Intervene Early to Optimise Long-Term Outcomes – Type 2 Diabetes
The primary randomisation of the UKPDS included allocation of 753 people with newly diagnosed type 2 diabetes to receive 10 years of intensive glycaemic management with metformin or to the diet control arm.3 Randomisation to metformin was associated with clinically and statistically significant reductions in mortality and endpoints related to cardiovascular disease that were greater than those expected from improved glycaemic control alone.3 Intensive glycaemic management with sulfonylurea of insulin in the UKPDS significantly reduced microvascular endpoints in a larger population of 3867 patients, without significant cardiovascular benefit.2
Patients returned to the care of their usual physicians at the end of the randomised phase of the UKPDS, and average HbA1c quickly became similar between the two groups.98 A further 10 years of epidemiological follow-up of these patients showed that the cardiovascular benefit of metformin was still evident for patients who did vs did not receive randomised treatment with metformin, with fewer macrovascular events and lower mortality.98 Cardiovascular benefits were also evident at this time for people who had formerly been randomised to sulfonylurea/insulin vs those who did not, including significantly lower rates of all-cause mortality and myocardial infarction.98 Thus, early and intensive intervention to control glycaemia can provide long-term reductions in mortality and cardiovascular events over and above those seen during initial short-term treatment. These “legacy benefits” of intensive glycaemic control applied early were not observed in populations with more advanced diabetes after intensification of blood glucose control.99
The cardiovascular protection observed with metformin in newly diagnosed type 2 diabetes patients in the UKPDS supports initiation of pharmacologic antidiabetic pharmacotherapy with this agent, and this remains consistent with the recommendation of the joint guideline from the ADA and European Association for the Study of Diabetes where patients do not have pre-existing heart failure or cardiovascular or renal dysfunction.4 European Society of Cardiology guidance includes metformin as a first-line management option.100 Metformin can be combined with any other glucose-lowering treatment. Both guidelines agree that patients with new type 2 diabetes and established cardiovascular disease should be considered for treatment with a GLP-1 agonist, and patients with heart failure or chronic kidney disease should be considered for a SGLT2 inhibitor.
Long Term Diabetes Management
Starting with Metformin
Antidiabetic treatments must also be given at an effective dose. For example, the DPP employed a target dose of metformin of 1750 mg/day for diabetes prevention83 and the median dose of metformin, the most common first pharmacologic antidiabetic therapy, in the UKPDS was 2550 mg/day.1 Randomised trials and many observational studies indicate cardiovascular benefit with metformin at all stages of type 2 diabetes (reviewed elsewhere).101,102 These are important considerations, because in the clinical experience of the authors, metformin is often under dosed, especially in prediabetes. A range of tablet strengths facilitates titration of metformin, and in the authors’ experience, use of a 750 mg metformin tablet facilitates achievement of a dose of 1500 mg for prediabetes, and the 1000 mg XR tablet facilitates achievement of the maximum dosage of 2000 mg/day.
Metformin is generally safe and well tolerated by most patients if initiated and titrated appropriately, including in people with prediabetes, and an extended-release formulation has been shown to have better gastrointestinal tolerability than the older, immediate-release version.103,104 It is important to monitor patients carefully for the existence or appearance of contraindications to metformin that might increase the risk of lactic acidosis. In addition, the presence of significant cardiovascular comorbidities at diagnosis may require additional use of a GLP-1 agonist or SGLT2 inhibitor, as described above.
Countering Clinical Inertia Over the Longer Term
“Clinical inertia” describes the situation where a patient who requires initiation or intensification of therapy does not receive it, or receives it only after an unnecessary delay.105–107 People who are at all stages of the diabetes continuum are at risk from therapeutic inertia, from under treatment of prediabetes, via delayed antidiabetes treatment on type 2 diabetes diagnosis, to reluctance to initiate insulin late in the course of diabetes.105–107 The result is unnecessary exposure of people with prediabetes or diabetes to long-term hyperglycaemia and increased risk of diabetes complications.
Three key sources of clinical inertia have been identified, relating to the patient (estimated as contributing 30% of the problem), health-care providers (50% of the problem) and the health-care system itself (30% of the problem).105–107 Multiple factors are at play within each of these areas and these are summarised in Figure 3.105 Clearly, different factors are likely to impact the care of each individual patient. This, in turn, emphasises the key importance of individualised patient care in order to understand and overcome barriers to effective and timely delivery of care. Therapeutic inertia has been described as “the enemy of therapeutic success in the management of diabetes and its complications”.107 Avoiding clinical inertia by prompt application of required treatments (or intensification of treatments) holds the key to improving long-term outcomes at all stages of the continuum of dysglycaemia.
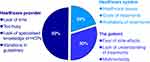 |
Figure 3 Principal sources of therapeutic inertia: contributions from the healthcare system, healthcare providers, and the patients themselves. Notes: Data from Khunti S, Khunti K, Seidu S.105 Percentages shown are the relative contributions of each domain to the overall problem of clinical inertia in the opinion of the authors of reference.105 |
Conclusions
Prediabetes and type 2 diabetes are not separate entities: in practice, they exist as a continuum of dysglycaemia and increasing vascular risk. Indeed, multiple studies have now demonstrated that the increased risk of vascular complications usually associated with type 2 diabetes is evident in people with early forms of dysglycaemia that are commonly described as prediabetes. This adverse process may continue for more than a decade before a formal diagnosis of type 2 diabetes is reached. There is an opportunity here to intervene early and intensively with lifestyle intervention and evidence-based pharmacologic therapies. Metformin is supported evidence-based treatment for prediabetes in a defined subgroup of subjects and has been shown to improve long-term outcomes at all stages of diabetes. GLP-1 agonists and SGLT2 inhibitors are also available for people presenting with type 2 diabetes and established cardiovascular comorbidities. The same approach must be maintained later in the course of the continuum, with prompt and effective intensification of treatment, without undue delay and unnecessary exposure of the patient to hyperglycaemia.
Funding
Merck Serono Middle East FZ-Ltd funded open access publication and editorial support (see below). No other funding applied. Authors remained in complete control of the content of the article throughout. A medical writer (Dr Mike Gwilt, GT Communications) provided editorial support, funded by Merck Serono Middle East FZ-Ltd.
Disclosure
AAb and AE are employees of Merck Serono Middle East FZ-Ltd, an affiliate of Merck KGaA, Darmstadt, Germany. Other authors of this manuscript have acted as consultants for Merck Serono Middle East FZ-Ltd, and declared no additional duality of interest.
References
1. DCCT Research Group. The association between glycemic exposure and long-term diabetes complications in the diabetes control and complications trial. Diabetes. 1995;44:968–983. doi:10.2337/diab.44.8.968
2. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1988;352:837–853. doi:10.1016/s0140-6736(98)07019-6
3. UK Prospective Diabetes Study Group. Effect of intensive blood glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1988;352:854–865. doi:10.1016S0140-6736(98)07037-8
4. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65:1925–1966. doi:10.1007/s00125-022-05787-2
5. Draznin B; American Diabetes Association Professional Practice Committee; American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl1):S83–S96. doi:10.2337/dc22-S006
6. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi:10.1136/bmj.321.7258.405
7. Consensus Committee. Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: the American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care. 2007;30(9):2399–2400. doi:10.2337/dc07-9925
8. Garcia-Moll X, Barrios V, Franch-Nadal J. Moving from the stratification of primary and secondary prevention of cardiovascular risk in diabetes towards a continuum of risk: need for a new paradigm. Drugs Context. 2021;10. doi:10.7573/dic.2021-6-3
9. Wysham C, Shubrook J. Beta-cell failure in type 2 diabetes: mechanisms, markers, and clinical implications. Postgrad Med. 2020;132:676–686. doi:10.1080/00325481.2020.1771047
10. Draznin B; American Diabetes Association Professional Practice Committee; American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl1):S17–S38. doi:10.2337/dc22-S002
11. World Health Organization, International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Report of a WHO/IDF consultation. Available from: www.who.int/diabetes/publications/Definitionanddiagnosisofdiabetes_new.pdf.
12. Ciarambino T, Crispino P, Leto G, Mastrolorenzo E, Para O, Giordano M. Influence of gender in diabetes mellitus and its complication. Int J Mol Sci. 2022;23:8850. doi:10.3390/ijms23168850
13. Zeighamy Alamdary S, Afifirad R, Asgharzadeh S, et al. The influence of probiotics consumption on management of prediabetic state: a systematic review of clinical trials. Int J Clin Pract. 2022;2022:5963679. doi:10.1155/2022/5963679
14. Naseri K, Saadati S, Ashtary-Larky D, et al. Probiotics and synbiotics supplementation improve glycemic control parameters in subjects with prediabetes and type 2 diabetes mellitus: a GRADE-assessed systematic review, meta-analysis, and meta-regression of randomized clinical trials. Pharmacol Res. 2022;184:106399. doi:10.1016/j.phrs.2022.106399
15. Letchumanan G, Abdullah N, Marlini M, et al. Gut microbiota composition in prediabetes and newly diagnosed type 2 diabetes: a systematic review of observational studies. Front Cell Infect Microbiol. 2022;12:943427. doi:10.3389/fcimb.2022.943427
16. Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes Care. 2009;32:281–286. doi:10.2337/dc08-1264
17. Lee CMY, Colagiuri S, Woodward M, et al. Comparing different definitions of prediabetes with subsequent risk of diabetes: an individual participant data meta-analysis involving 76 513 individuals and 8208 cases of incident diabetes. BMJ Open Diabetes Res Care. 2019;7:2019. doi:10.1136/bmjdrc-2019-000794
18. U.K. Prospective Diabetes Study Group. U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249–1258. doi:10.2337/diab.44.11.1249
19. Centers for Disease Control. Prediabetes – your chance to prevent type 2 diabetes. Available from: https://www.cdc.gov/diabetes/basics/prediabetes.html.
20. Xia PF, Pan XF, Li Y, et al. Trends in diagnosed and undiagnosed diabetes among adults in the U.S., 2005–2016. Diabetes Care. 2021;44:e175–e177. doi:10.2337/dc21-1156
21. Leahy S, O’ Halloran AM, O’ Leary N, et al. Prevalence and correlates of diagnosed and undiagnosed type 2 diabetes mellitus and pre–diabetes in older adults: findings from the Irish Longitudinal Study on Ageing (TILDA). Diabetes Res Clin Pract. 2015;110:241–249. doi:10.1016/j.diabres.2015.10.015
22. Moody A, Cowley G, Ng Fat L, Mindell JS. Social inequalities in prevalence of diagnosed and undiagnosed diabetes and impaired glucose regulation in participants in the Health Surveys for England series. BMJ Open. 2016;6:e010155. doi:10.1136/bmjopen-2015-010155
23. Bener A, Zirie M, Janahi IM, Al–Hamaq AO, Musallam M, Wareham NJ. Prevalence of diagnosed and undiagnosed diabetes mellitus and its risk factors in a population–based study of Qatar. Diabetes Res Clin Pract. 2009;84:99–106. doi:10.1016/j.diabres.2009.02.003
24. Pearson–Stuttard J, Bennett J, Cheng YJ, et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2021;9:165–173. doi:10.1016/S2213-8587(20)30431-9
25. Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all–cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation. 2007;116:151–157. doi:10.1161/CIRCULATIONAHA.106.685628
26. Wen CP, Cheng TY, Tsai SP, Hsu HL, Wang SL. Increased mortality risks of pre–diabetes (impaired fasting glucose) in Taiwan. Diabetes Care. 2005;28:2756–2761. doi:10.2337/diacare.28.11.2756
27. Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Subclinical states of glucose intolerance and risk of death in the U. S. Diabetes Care. 2001;24:447–453. doi:10.2337/diacare.24.3.447
28. Sorkin JD, Muller DC, Fleg JL, Andres R. The relation of fasting and 2–h postchallenge plasma glucose concentrations to mortality: data from the Baltimore Longitudinal Study of Aging with a critical review of the literature. Diabetes Care. 2005;28:2626–2632. doi:10.2337/diacare.28.11.2626
29. Mutie PM, Pomares–Millan H, Atabaki–Pasdar N, et al. An investigation of causal relationships between prediabetes and vascular complications. Nat Commun. 2020;11:4592. doi:10.1038/s41467-020-18386-9
30. Gujral UP, Jagannathan R, He S, et al. Association between varying cut–points of intermediate hyperglycemia and risk of mortality, cardiovascular events and chronic kidney disease: a systematic review and meta–analysis. BMJ Open Diabetes Res Care. 2021;9:e001776. doi:10.1136/bmjdrc-2020-001776
31. Ford ES, Zhao G, Li C. Pre–diabetes the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55:1310–1317. doi:10.1016/j.jacc.2009.10.060
32. Deedwania P, Patel K, Fonarow GC, et al. Prediabetes is not an independent risk factor for incident heart failure, other cardiovascular events or mortality in older adults: findings from a population–based cohort study. Int J Cardiol. 2013;168:3616–3622. doi:10.1016/j.ijcard.2013.05.038
33. Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59:635–643. doi:10.1016/j.jacc.2011.08.080
34. Welsh C, Welsh P, Celis-Morales CA, et al. Glycated hemoglobin, prediabetes, and the links to cardiovascular disease: data from UK Biobank. Diabetes Care. 2020;43:440–445. doi:10.2337/dc19-1683
35. Vistisen D, Witte DR, Brunner EJ, et al. Risk of cardiovascular disease and death in individuals with prediabetes defined by different criteria: the Whitehall II Study. Diabetes Care. 2018;41:899–906. doi:10.2337/dc17-2530
36. De Oliveira CM, Tureck LV, Alvares D, et al. Cardiometabolic risk factors correlated with the incidence of dysglycaemia in a Brazilian normoglycaemic sample: the Baependi Heart Study cohort. Diabetol Metab Syndr. 2020;12:6. doi:10.1186/s13098-019-0512-0
37. Beulens J, Rutters F, Rydén L, et al. Risk and management of pre-diabetes. Eur J Prev Cardiol. 2019;26(2_suppl):47–54. doi:10.1177/2047487319880041
38. Huemer MT, Huth C, Schederecker F, et al. Association of endothelial dysfunction with incident prediabetes, type 2 diabetes and related traits: the KORA F4/FF4 study. BMJ Open Diabetes Res Care. 2020;8:e001321. doi:10.1136/bmjdrc-2020-001321
39. Parrinello CM, Hua S, Carnethon MR, et al. Associations of hyperglycemia and insulin resistance with biomarkers of endothelial dysfunction in Hispanic/Latino youths: results from the Hispanic Community Children’s Health Study/Study of Latino Youth (SOL Youth). J Diabetes Complications. 2017;31:836–842. doi:10.1016/j.jdiacomp.2017.01.019
40. Walther G, Obert P, Dutheil F, et al. Metabolic syndrome individuals with and without type 2 diabetes mellitus present generalized vascular dysfunction: cross-sectional study. Arterioscler Thromb Vasc Biol. 2015;35:1022–1029. doi:10.1161/ATVBAHA.114.304591
41. Malin SK, Gilbertson NM, Eichner NZM, Heiston E, Miller S, Weltman A. Impact of Short-term continuous and interval exercise training on endothelial function and glucose metabolism in prediabetes. J Diabetes Res. 2019;2019:4912174. doi:10.1155/2019/4912174
42. Torres-Peña JD, Garcia-Rios A, Delgado-Casado N, et al. Mediterranean diet improves endothelial function in patients with diabetes and prediabetes: a report from the CORDIOPREV study. Atherosclerosis. 2018;269:50–56. doi:10.1016/j.atherosclerosis.2017.12.012
43. Desch S, Sonnabend M, Niebauer J, et al. Effects of physical exercise versus rosiglitazone on endothelial function in coronary artery disease patients with prediabetes. Diabetes Obes Metab. 2010;12:825–828. doi:10.1111/j.1463-1326.2010.01234.x
44. Sardu C, Paolisso P, Sacra C, et al. Effects of metformin therapy on coronary endothelial dysfunction in patients with prediabetes with stable angina and nonobstructive coronary artery stenosis: the CODYCE Multicenter Prospective Study. Diabetes Care. 2019;42:1946–1955. doi:10.2337/dc18-2356
45. Bulut A, Avci B. Carotid intima–media thickness values are significantly higher in patients with prediabetes compared to normal glucose metabolism. Medicine. 2019;98:e17805. doi:10.1097/MD.0000000000017805
46. Xing FY, Neeland IJ, Gore MO, et al. Association of prediabetes by fasting glucose and/or haemoglobin A1c levels with subclinical atherosclerosis and impaired renal function: observations from the Dallas Heart Study. Diab Vasc Dis Res. 2014;11:11–18. doi:10.1177/1479164113514239
47. Sibal L, Agarwal SC, Home PD. Carotid intima-media thickness as a surrogate marker of cardiovascular disease in diabetes. Diabetes Metab Syndr Obes. 2011;4:23–34. doi:10.2147/DMSO.S8540
48. Willeit P, Tschiderer L, Allara E, et al. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: meta-analysis of 119 clinical trials involving 100 667 patients. Circulation. 2020;142:621–642. doi:10.1161/CIRCULATIONAHA.120.046361
49. Dunlay SM, Givertz MM, Aguilar D, et al. Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140:e294–e324. doi:10.1161/CIR.0000000000000691
50. Ceriello A, Catrinoiu D, Chandramouli C, et al. Heart failure in type 2 diabetes: current perspectives on screening, diagnosis and management. Cardiovasc Diabetol. 2021;20:218. doi:10.1186/s12933-021-01408-1
51. Cai X, Liu X, Sun L, et al. Prediabetes and the risk of heart failure: a meta–analysis. Diabetes Obes Metab. 2021;23:1746–1753. doi:10.1111/dom.14388
52. Sinha A, Ning H, Ahmad FS, et al. Association of fasting glucose with lifetime risk of incident heart failure: the Lifetime Risk Pooling Project. Cardiovasc Diabetol. 2021;20:66. doi:10.1186/s12933-021-01265-y
53. Kristensen SL, Preiss D, Jhund PS, et al. Risk related to pre–diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI With ACEI to determine impact on global mortality and morbidity in Heart Failure Trial. Circ Heart Fail. 2016;9:e002560. doi:10.1161/CIRCHEARTFAILURE.115.002560
54. Mai L, Wen W, Qiu M, et al. Association between prediabetes and adverse outcomes in heart failure. Diabetes Obes Metab. 2021;23:2476–2483. doi:10.1111/dom.14490
55. Pavlović A, Polovina M, Ristić A, et al. Long-term mortality is increased in patients with undetected prediabetes and type-2 diabetes hospitalized for worsening heart failure and reduced ejection fraction. Eur J Prev Cardiol. 2019;26:72–82. doi:10.1177/2047487318807767
56. Sörensen BM, Houben AJ, Berendschot TT, et al. Prediabetes and type 2 diabetes are associated with generalized microvascular dysfunction the Maastricht Study. Circulation. 2016;134:1339–1352. doi:10.1161/CIRCULATIONAHA.116.023446
57. Zaleska–żmijewska A, Piątkiewicz P, Śmigielska B, et al. Retinal photoreceptors and microvascular changes in prediabetes measured with adaptive optics (rtx1™): a case–control study. J Diabetes Res. 2017;2017:4174292. doi:10.1155/2017/4174292
58. Li W, Schram MT, Berendschot TTJM, et al. Type 2 diabetes and HbA1c are independently associated with wider retinal arterioles: the Maastricht study. Diabetologia. 2020;63:1408–1417. doi:10.1007/s00125-020-05146-z
59. Lott ME, Slocomb JE, Shivkumar V, et al. Impaired retinal vasodilator responses in prediabetes and type 2 diabetes. Acta Ophthalmol. 2013;91:e462–e469. doi:10.1111/aos.12129
60. De Clerck EEB, Schouten JSAG, Berendschot TTJM, et al. Macular thinning in prediabetes or type 2 diabetes without diabetic retinopathy: the Maastricht Study. Acta Ophthalmol. 2018;96:174–182. doi:10.1111/aos.13570
61. Chande PK, Raman R, John P, Srinivasan S. Contrast–sensitivity function and photo stress–recovery time in prediabetes. Clin Optom. 2020;12:151–155. doi:10.2147/OPTO.S259397
62. Palladino R, Tabak AG, Khunti K, et al. Association between pre–diabetes and microvascular and macrovascular disease in newly diagnosed type 2 diabetes. BMJ Open Diabetes Res Care. 2020;8:e001061. doi:10.1136/bmjdrc-2019-001061
63. Li Rudvan AL, Can ME, Efe FK, Keskin M, Beyan E. Evaluation of retinal microvascular changes in patients with prediabetes. Niger J Clin Pract. 2021;24:911–918. doi:10.4103/njcp.njcp_193_20
64. Plantinga LC, Crews DC, Coresh J, et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010;5:673–682. doi:10.2215/CJN.07891109
65. Kim GS, Oh HH, Kim SH, Kim BO, Byun YS. Association between prediabetes (defined by HbA1C, fasting plasma glucose, and impaired glucose tolerance) and the development of chronic kidney disease: a 9–year prospective cohort study. BMC Nephrol. 2019;20:130. doi:10.1186/s12882-019-1307-0
66. Markus MRP, Ittermann T, Baumeister SE, et al. Prediabetes is associated with microalbuminuria, reduced kidney function and chronic kidney disease in the general population: the KORA (Cooperative Health Research in the Augsburg Region) F4–Study. Nutr Metab Cardiovasc Dis. 2018;28:234–242. doi:10.1016/j.numecd.2017.12.005
67. Neves JS, Correa S, Baeta Baptista R, Bigotte Vieira M, Waikar SS, Mc Causland FR. Association of prediabetes with CKD progression and adverse cardiovascular outcomes. An analysis of the CRIC Study. J Clin Endocrinol Metab. 2020;105:e1772–e1780. doi:10.1210/clinem/dgaa017
68. Echouffo–Tcheugui JB, Narayan KM, Weisman D, Golden SH, Jaar BG. Association between prediabetes and risk of chronic kidney disease. A systematic review and meta–analysis. Diabet Med. 2016;33:1615–1624. doi:10.1111/dme.13113
69. Shilpasree AS, Patil VS, Revanasiddappa M, Patil VP, Ireshnavar D. Renal Dysfunction in Prediabetes: confirmed by Glomerular Hyperfiltration and Albuminuria. J Lab Physicians. 2021;13:257–262. doi:10.1055/s-0041-1731107
70. Rodriguez–Poncelas A, Coll–de–Tuero G, Blanch J, Comas–Cufí M, Saez M, Barceló MA. Prediabetes is associated with glomerular hyperfiltration in a European Mediterranean cohort study. J Nephrol. 2018;31:743–749. doi:10.1007/s40620-018-0524-0
71. Rodríguez–Poncelas A, Franch–Nadal J, Coll–de Tuero G, et al. High levels of fasting glucose and glycosylated hemoglobin values are associated with hyperfiltration in a Spanish prediabetes cohort. The PREDAPS Study. PLoS One. 2019;14:e0222848. doi:10.1371/journal.pone.0222848
72. Okada R, Wakai K, Naito M, et al. Renal hyperfiltration in prediabetes confirmed by fasting plasma glucose and hemoglobin A1c. Ren Fail. 2012;34:1084–1090. doi:10.3109/0886022X.2012.717516
73. Coopmans C, Zhou TL, Henry RMA, et al. Both prediabetes and type 2 diabetes are associated with lower heart rate variability: the Maastricht Study. Diabetes Care. 2020;43:1126–1133. doi:10.2337/dc19-2367
74. Ziegler D, Voss A, Rathmann W, et al. Increased prevalence of cardiac autonomic dysfunction at different degrees of glucose intolerance in the general population: the KORA S4 survey. Diabetologia. 2015;58:1118–1128. doi:10.1007/s00125-015-3534-7
75. Lin YC, Lin CS, Chang TS, et al. Early sensory neurophysiological changes in prediabetes. J Diabetes Investig. 2020;11:458–465. doi:10.1111/jdi.13151
76. Katon JG, Reiber GE, Nelson KM. Peripheral neuropathy defined by monofilament insensitivity and diabetes status: NHANES 1999–2004. Diabetes Care. 2013;36:1604–1606. doi:10.2337/dc12-1102
77. Bongaerts BW, Rathmann W, Kowall B, et al. Postchallenge hyperglycemia is positively associated with diabetic polyneuropathy: the KORA F4 study. Diabetes Care. 2012;35:1891–1893. doi:10.2337/dc11-2028
78. Dyck PJ, Clark VM, Overland CJ, et al. Impaired glycemia and diabetic polyneuropathy: the OC IG Survey. Diabetes Care. 2012;35:584–591. doi:10.2337/dc11-1421
79. Thaisetthawatkul P, Lyden E, Americo Fernandes J, Herrmann DN. Prediabetes, diabetes, metabolic syndrome, and small fiber neuropathy. Muscle Nerve. 2020;61:475–479. doi:10.1002/mus.26825
80. Marseglia A, Fratiglioni L, Kalpouzos G, Wang R, Bäckman L, Xu W. Prediabetes and diabetes accelerate cognitive decline and predict microvascular lesions: a population–based cohort study. Alzheimers Dement. 2019;15:25–33. doi:10.1016/j.jalz.2018.06.3060
81. van Agtmaal MJM, Houben AJHM, de Wit V, et al. Prediabetes is associated with structural brain abnormalities: the Maastricht Study. Diabetes Care. 2018;41:2535–2543. doi:10.2337/dc18-1132
82. Gottwald-Hostalek U, Gwilt M. Vascular complications in prediabetes and type 2 diabetes: a continuous process arising from a common pathology. Curr Med Res Opin. 2022;38:1841–1851. doi:10.1080/03007995.2022.2101805
83. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi:10.1056/NEJMoa012512
84. Lindström J, Louheranta A, Mannelin M, et al. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3–year results on diet and physical activity. Diabetes Care. 2003;26:3230–3236. doi:10.2337/diacare.26.12.3230
85. Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi:10.2337/diacare.20.4.537
86. Diabetes Prevention Program Group and Diabetes Prevention Program Outcome Study Research Group. New data on clinical outcomes from the Diabetes Prevention Program Outcomes Study.
87. American Diabetes Association. New data from Diabetes Prevention Program Outcomes Study shows persistent reduction of type 2 diabetes development over 22–year average follow–up; 2020. Available from: https://diabetes.org/newsroom/press-releases/2020/new-data-from-diabetes-prevention-program-outcomes-study-shows-persistent-reduction-of-t2d-development-over-22-year-average-follow-up.
88. Busko M. DPPOS at 22 years: “Diabetes prevention is possible” long term. Medscape Diabetes Endocrinol. 2020; Available from: https://www.medscape.com/viewarticle/932876.
89. Lindström J, Peltonen M, Eriksson JG, et al. Improved lifestyle and decreased diabetes risk over 13 years: long–term follow–up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia. 2013;56:284–293. doi:10.1007/s00125-012-2752-5
90. Gong Q, Zhang P, Wang J, et al.; Da Qing Diabetes Prevention Study Group. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30–year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 7;2019:452–461. doi:10.1016/S2213-8587(19)30093-2
91. Echouffo–Tcheugui JB, Selvin E. Prediabetes and what it means: the epidemiological evidence. Annu Rev Public Health. 2021;42:59–77. doi:10.1146/annurev-publhealth-090419-102644
92. Hostalek U, Gwilt M, Hildemann S. Therapeutic use of metformin in prediabetes and diabetes prevention. Drugs. 2015;75:1071–1094. doi:10.1007/s40265-015-0416-8
93. Hostalek U, Campbell I. Metformin for diabetes prevention: update of the evidence base. Curr Med Res Opin. 2021;37:1705–1717. doi:10.1080/03007995.2021.1955667
94. ElSayed NA, Aleppo G, Aroda VR, et al. 3. Prevention or delay of type 2 diabetes and associated comorbidities: standards of care in diabetes-2023. Diabetes Care. 2023;46(Supplement_1):S41–S48. doi:10.2337/dc23-S003
95. Paulweber B, Valensi P, Lindström J, et al. A European evidence–based guideline for the prevention of type 2 diabetes. Horm Metab Res. 2010;42(Suppl 1):S3–S36. doi:10.1055/s-0029-1240928
96. National Institute for Health and Care Excellence. Group and individual–level interventions to prevent type 2 diabetes among people at high risk. Available from: https://www.nice.org.uk/guidance/PH38/chapter/Recommendations#metformin.
97. Murillo JE, McNeil C, Van Nest K, et al. Abstract 9819: real world data: off–label metformin in patients with prediabetes is associated with improved cardiovascular outcomes. Circulation. 2021;144(Suppl_1):A9819.
98. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10–year follow–up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi:10.1056/NEJMoa0806470
99. Folz R, Laiteerapong N. The legacy effect in diabetes: are there long–term benefits? Diabetologia. 2021;64:2131–2137. doi:10.1007/s00125-021-05539-8
100. Cosentino F, Grant PJ, Aboyans V, et al. ESC Guidelines on diabetes, pre–diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2019;2020(41):255–323.
101. Petrie JR, Rossing PR, Campbell IW. Metformin and cardiorenal outcomes in diabetes: a reappraisal. Diabetes Obes Metab. 2020;22:904–915. doi:10.1111/dom.13984
102. Schernthaner G, Brand K, Bailey CJ. Metformin and the heart: update on mechanisms of cardiovascular protection with special reference to comorbid type 2 diabetes and heart failure. Metabolism. 2022;130:155160. doi:10.1016/j.metabol.2022.155160
103. Diabetes Prevention Program Research Group. Long–term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737. doi:10.2337/dc11-1299
104. Feher MD, Al–Mrayat M, Brake J, Leong KS. Tolerability of prolonged–release metformin (Glucophage® SR) in individuals intolerant to standard metformin — results from four UK centres. Br J Diabetes Vasc Dis. 2007;7:225–228. doi:10.1177/14746514070070050501
105. Khunti S, Khunti K, Seidu S. Therapeutic inertia in type 2 diabetes: prevalence, causes, consequences and methods to overcome inertia. Ther Adv Endocrinol Metab. 2019;10:2042018819844694. doi:10.1177/2042018819844694
106. Okemah J, Peng J, Quiñones M. Addressing clinical inertia in type 2 diabetes mellitus: a review. Adv Ther. 2018;35:1735–1745.
107. Andreozzi F, Candido R, Corrao S, et al. Clinical inertia is the enemy of therapeutic success in the management of diabetes and its complications: a narrative literature review. Diabetol Metab Syndr. 2020;12:52. doi:10.1186/s13098-020-00559-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
