Back to Journals » Medical Devices: Evidence and Research » Volume 12
Addition Of A Sleeve To The Etanercept Autoinjector (Enbrel® MyClic®) Improves Ease Of Use In Patients With Rheumatoid Arthritis
Authors Rekaya N, Pickersgill A , Harvey L
Received 23 May 2019
Accepted for publication 9 October 2019
Published 25 October 2019 Volume 2019:12 Pages 443—450
DOI https://doi.org/10.2147/MDER.S216649
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Naceur Rekaya,1 Alexandra Pickersgill,1 Louisa Harvey2
1Pfizer R&D Ltd, Device Centre of Excellence, Cambridge, UK; 2Harvey Medical Consulting Ltd, Cambridge, UK
Correspondence: Naceur Rekaya
Pfizer R&D Ltd, Device Centre of Excellence, Granta Park, Great Abington, Cambridge CB21 6GP, UK
Tel +44 1304 616 161
Email [email protected]
Purpose: A novel device (sleeve) has been developed that attaches to compatible autoinjectors and is designed to improve the patient experience, particularly for those with limited manual dexterity. This study was designed to explore whether user experience is improved when using the Enbrel® MyClic® autoinjector in conjunction with the sleeve. The Enbrel MyClic autoinjector contains etanercept (Enbrel®), a biologic drug that patients self-administer subcutaneously for the treatment of rheumatoid arthritis (RA).
Patients and methods: Twenty-four adult patients (16 female) with RA and varying degrees of manual dexterity impairment took part in this user study. Written informed consent was supplied by each patient prior to performing the study. They each performed two simulated injections into skin pads, one with the Enbrel MyClic alone and the other with the Enbrel MyClic + sleeve. Following the simulated injections, participants answered questions about their experience of using the sleeve and rated the following on a scale of 1 (poor experience) to 7 (optimal experience): overall use; ease of preparation; ease of administration; ease of learning to use; look, feel and size of the device; overall experience.
Results: Participants rated the Enbrel MyClic + sleeve more highly than the Enbrel MyClic alone for overall use, ease of administration, feel, size and overall experience. Participants with severe dexterity impairment (n = 12) were more likely to rate these features as better with the sleeve in place than those with mild dexterity impairment (n = 10). Three-quarters of participants said they would request the sleeve if it became available and all said that they would recommend it to others. The main benefits cited by participants were better grip and a better feeling of control.
Conclusion: Addition of the sleeve improved patients’ experience of using the Enbrel MyClic. The benefits of the sleeve outweighed any inconvenience associated with the additional steps needed to prepare the device.
Keywords: human factors, usability, user study
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease that primarily affects the joints. The hallmark swelling causes pain and stiffness; when this occurs in the hands, patients’ manual dexterity can be severely impaired. Conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), such as methotrexate, are used first-line to inhibit joint damage and improve symptoms with the aim of reducing functional disability and improving quality of life.1 Patients who do not respond to csDMARDs may be treated with either biological DMARDs (bDMARDs) or targeted synthetic DMARDs.1 One of the most commonly used bDMARDs is the tumor necrosis factor inhibitor, etanercept (Enbrel®; Pfizer Ltd, Sandwich, UK).
bDMARDs are frequently self-administered by patients and different subcutaneous injection methods are available: vials with a syringe, pre-filled syringes, and autoinjector devices (pens). Studies have shown that patients with RA prefer autoinjectors to pre-filled syringes as they are easier to use, less painful, and more convenient.2–4 As RA affects manual dexterity, which can make self-injection difficult, ease of use is an important consideration for autoinjectors.5 Features such as size and shape of the autoinjector, the nature of any dose delivery indicators, and the activation mechanism for the injection are all important factors in patient satisfaction.6 In other disease states, use of an autoinjector has helped patients overcome needle anxiety.7
The Enbrel® MyClic® (Pfizer Ltd, Sandwich, UK) is a single-dose, single-use autoinjector. After removal of a protective cap, patients press the device onto the skin to retract the needle shield and then press an activation button with their thumb to start the injection. When pressing the button, a click is heard. A second click indicates that the dose has been delivered and the patient can remove the device from the skin. Patients can check that the entire dose has been injected via a dose delivery window on the side of the device.
A sleeve has been developed that attaches to compatible autoinjectors such as the Enbrel MyClic and is designed to make use of the autoinjector easier. The sleeve attaches to the autoinjector using a twist-lock mechanism. For the locking mechanism to work, patients must align the sleeve and autoinjector correctly, as indicated by features (dots) on both components. With the sleeve locked in place, the injection is initiated by sliding the sleeve down the autoinjector, causing the sleeve to push onto the button until the first click is heard. This removes the need for the patient to push the activation button with their thumb, allowing them to perform the injection in one easy motion. Here, we describe the results of a study to assess the impact of the sleeve on user experience with the Enbrel MyClic in patients with RA.
Materials And Methods
Objectives
The aim of the study was to determine whether user experience is improved by using the sleeve with the Enbrel MyClic. Specifically, the study explored: i) whether patients experience a benefit when using the sleeve, and if so, whether any particular type of patient benefits the most; ii) whether patients have any difficulties using the sleeve.
Study Devices
The study used off-the-shelf stock of the Enbrel MyClic autoinjector containing active drug. Participants were instructed only to inject into the skin pads provided. The sleeves used in this study were production devices with production packaging and instructions for use.
Participants
The study included male and female patients aged ≥18 years with RA who were currently treated with either Enbrel MyClic, another biologic administered via an autoinjector, or an oral synthetic DMARD. The aim was to recruit at least eight participants in each treatment group. To reflect the prevalence of RA among females, participants were recruited at a ratio of seven females to three males. Participants were screened before recruitment for their level of dexterity impairment (mild, moderate or severe). All participants signed a consent form and were recompensed for their time. Ethics committee approval was not necessary for this study, which was carried out in accordance with the International Organization for Standardization’s guidance on studying usability of medical devices, and FDA guidance on human factors in medical devices. All data were anonymized: each participant was assigned a unique identifier (P1, P2, etc) that was used on all documentation.
Procedure
The study was conducted by Harvey Medical Consulting Ltd, a human factors research company based near Cambridge, UK. Participants were interviewed one-to-one by a moderator, with an observer taking notes. After a short introduction to explain the format of the interview, the moderator measured each participant’s dominant hand and tested the grip strength of both hands. Participants were also asked to complete the Purdue Peg Board test8 to check their fine motor skills. This test consisted of a board with two parallel columns of holes, into which the participants were asked to place as many pairs of metal pegs as possible in one minute.
Participants were shown how to use the Enbrel MyClic autoinjector by a general practitioner, before being asked to prepare the device themselves and administer the injection into a skin pad. They were then given the sleeve (in its packaging) with a copy of the instructions for use and were asked to administer an injection into a skin pad using the autoinjector with the sleeve in place. For both injections, the moderator noted the device number, and whether the two clicks were heard during administration.
Following the simulations, the moderator asked a series of subjective questions to assess the participant’s experience of using the autoinjector, both with and without the sleeve. Participants were asked if there were any circumstances under which they would find the sleeve more useful, or whether they would envisage using the sleeve for every injection. They were also asked to describe what benefits they experienced when using the sleeve, whether the sleeve reduced any anxiety they may have when using the autoinjector, how they would describe the sleeve to a fellow patient, and whether they would choose to do anything differently when injecting with the autoinjector + sleeve. The moderator also asked whether the participants felt they were able to maintain a consistent, firm pressure when injecting, and whether they could still see the injection window and hear the second click during the injection process with the sleeve in place.
Participants rated overall use and ease of use (ie, how easy they found learning to use the device, preparing it, and administering the dose) on a scale of 1 to 7, where 1 = not at all easy and 7 = extremely easy. They also rated the look, feel and size of the device, and their overall experience of using it on a scale of 1 to 7, where 1 = not at all positive and 7 = extremely positive. Finally, they rated how likely they were to recommend the sleeve to other patients and to request the sleeve for themselves on a scale of 1 to 7, where 1 = not at all likely and 7 = extremely likely.
Data Analysis
During the interviews, the observer typed notes into a data capture matrix. Qualitative and quantitative data were then coded in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) and analyzed to identify emergent patterns, trends and themes.
Results
Participants
Twenty-four participants were recruited; Table 1 shows their characteristics. Sixteen (67%) were female and 15 (63%) were currently using an autoinjector to administer their medication. All had some degree of dexterity impairment; in 12 participants (50%) this was severe (see Table S1 in Supplementary materials).
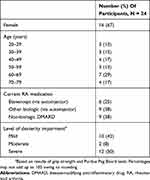 |
Table 1 Patient Characteristics |
Ratings Scales
Overall, the sleeve was well received by participants, who rated the autoinjector + sleeve more highly than the autoinjector alone for five out of the eight features they were asked about (overall use, ease of administration, feel, size and overall experience). The autoinjector + sleeve received a marginally lower average rating for ease of preparation and ease of learning to use than the autoinjector alone. Both the autoinjector alone and autoinjector + sleeve were rated the same in terms of look. Figure 1 shows the average scores given by participants for each feature; further details of participants’ ratings are given below.
There were no significant differences between ratings given by those patients who were using Enbrel MyClic as their current medication and those who were using other autoinjectors (see Figure S1 in Supplementary materials).
Usability
All participants successfully managed to deliver the full dose of medication both with and without the sleeve in place. Fifteen participants (63%) gave the autoinjector + sleeve a score of 7 for overall use and 23 (96%) rated it as the same or better than the autoinjector alone (Figure 2). Eleven of the 12 participants with severe dexterity impairment (92%) rated overall use of the autoinjector + sleeve as better than the autoinjector alone, compared with four of the 10 (40%) with mild dexterity impairment. Interestingly, one participant with severe dexterity impairment who was currently using the Enbrel MyClic, having previously been prescribed a biosimilar etanercept autoinjector, stated that she would rather use the Enbrel MyClic + sleeve than the biosimilar etanercept autoinjector.
 |
Figure 2 Comparison of the Enbrel® MyClic® autoinjector with and without the sleeve attached. |
Ten participants (42%) gave the autoinjector + sleeve a score of 7 for ease of preparation and 16 (67%) rated it as the same or better than the autoinjector alone (Figure 2). Six (25%) experienced initial difficulty locking the sleeve into place and five (21%) initially put the sleeve onto the wrong end of the autoinjector. Two participants tried to remove the protective cap from the autoinjector before inserting it into the sleeve; both were advised by the moderator not to do this as it could increase the risk of needle-stick injury. A number of participants experienced initial confusion when lining up the dots on the autoinjector with the dots on the sleeve. Despite this, they still managed to successfully assemble the device and deliver the injection into the skin pad.
Participants found administration easier with the addition of the sleeve. Seventeen participants (71%) gave the autoinjector + sleeve a score of 7 and 23 (96%) rated it as the same or better than the autoinjector alone (Figure 2). Ten participants (83%) with severe dexterity impairment rated ease of administration as better with the autoinjector + sleeve than with the autoinjector alone, compared with six (60%) of those with mild dexterity impairment.
Participants rated the autoinjector + sleeve as being slightly more difficult to learn how to use than the autoinjector alone (Figure 1). Seven participants (29%) gave the autoinjector + sleeve a score of 7 and 14 (58%) rated it the same or better than the autoinjector alone (Figure 2).
Eleven participants (46%) gave the autoinjector + sleeve a score of 7 out of 7 for intuitiveness. Twenty participants (83%) found it moderately, very, or extremely intuitive to use.
The sleeve did not interfere with the dose delivery indicators: 23 participants (96%) said they could still see the viewing window when the sleeve was attached. The remaining participant did not look at the viewing window, preferring instead to listen for the two clicks. Similarly, 23 participants (96%) said they could easily hear the second click when the sleeve was attached. The remaining participant was unsure about whether or not he had heard the click.
Twenty-three participants (96%) said they were able to maintain a consistent, firm pressure when injecting with the autoinjector + sleeve. Eighteen participants (75%) said they found this easier when using the autoinjector + sleeve compared with the autoinjector alone.
Look, Feel And Size Of The Device
There was no difference in participants’ opinion of the look of the autoinjector with and without the sleeve in place (Figure 1). Fourteen participants (58%) gave the autoinjector + sleeve a score of 7 and 22 (92%) rated it the same or better than the autoinjector alone (Figure 2).
Participants preferred the feel of the autoinjector + sleeve (Figure 1). Fourteen participants (58%) gave the autoinjector + sleeve a score of 7 and 22 (92%) rated it the same or better than the autoinjector alone (Figure 2). Eleven participants (92%) with severe dexterity impairment rated the feel of the autoinjector + sleeve as better than the autoinjector alone, compared with five (50%) of those with mild dexterity impairment. When asked how they would describe the sleeve to others, 11 participants (46%) commented on the good grip afforded by the sleeve and three (13%) said that the sleeve was “comfortable”.
Participants preferred the size of the autoinjector + sleeve (Figure 1). Fifteen participants (63%) gave the autoinjector + sleeve a score of 7 and 22 (92%) rated it the same or better than the autoinjector alone (Figure 2). Eleven participants (92%) with severe dexterity impairment rated the size of the autoinjector + sleeve as better than the autoinjector alone, compared with four (40%) of those with mild dexterity impairment. Three participants (13%) mentioned the extra bulk that the sleeve adds, but none specifically suggested that the sleeve should be smaller or lighter.
Overall Experience
Participants rated their overall experience of using the autoinjector as better with the sleeve in place (Figure 1). Thirteen participants (54%) gave the autoinjector + sleeve a score of 7 and 19 (79%) rated it as the same or better than the autoinjector alone (Figure 2). Of the five participants who rated the autoinjector alone as better than the autoinjector + sleeve, four had mild dexterity impairment; the other participant had moderate dexterity impairment, but this was related to grip strength rather than fine motor skills (in other words, it is likely that this participant did not have any issues with pressing the activation button with his thumb and therefore did not necessarily see removal of the need to do this as a particular benefit). Ten participants (83%) with severe dexterity impairment rated their overall experience with the autoinjector + sleeve as better than the autoinjector alone, compared with four (40%) of those with mild dexterity impairment. Only four participants (17%) reported feeling anxious about using the autoinjector, and all four said that their anxiety was reduced when they used the autoinjector + sleeve. Reasons given were removal of the need to press a button, a quicker injection process and the device being sturdier with the sleeve in place.
Requesting The Sleeve For Personal Use
Sixteen participants (67%) said they would be extremely likely to request the sleeve, should it become available. Two participants (8%) were very likely to request it. Of these 18 patients, 16 had severe dexterity impairment. Conversely, the six participants (25%) who said they would not be likely to request the sleeve had mild (n = 5) or moderate (n = 1) dexterity impairment. Again, it is interesting to note that the participant with moderate dexterity impairment had reduced grip strength, but fine motor skills within the normal range.
Recommending The Sleeve To Others
All participants said they were likely to recommend the sleeve to others, with 16 (67%) being extremely likely and eight (33%) being likely or very likely to recommend it. When asked how they would describe the sleeve to a fellow patient, participants were overwhelmingly positive. The most common attributes that participants said they would mention were the good grip afforded by the sleeve, ease of use, and comfort.
Benefits Of The Sleeve
Table 2 shows the benefits experienced by participants when using the autoinjector + sleeve compared with the autoinjector alone. The most common benefit was better grip, which was cited by approximately 80% of participants. More than half of participants cited a better feeling of control as a benefit. Only three participants (all of whom had mild dexterity impairment) stated that they did not experience any benefit from addition of the sleeve.
 |
Table 2 Benefits Of Using The Sleeve With The Enbrel® MyClic® |
Patients were also asked their opinion on not having to press an activation button when the sleeve is attached to the autoinjector. Seventeen participants (71%) said they found it “easier/better” or “much easier/much better”. Conversely, two participants preferred using the button, with one noting that the button gave them more control.
Discussion
Convenient, user-friendly drug delivery systems are important for those patients with RA who self-inject their medication. The addition of the sleeve to the Enbrel MyClic offers a new option that could improve the patient experience. Overall, the sleeve was well received by this group of patients, who rated the autoinjector + sleeve more highly than the autoinjector alone for five out of the eight features they were asked about (overall use, ease of administration, feel, size and overall experience). The similarity in responses between participants who were using Enbrel MyClic as their current medication and those who were using other autoinjectors suggests that preferences were not driven by familiarity with the Enbrel MyClic device.
The main benefit for participants in this study was grip. Unlike the Enbrel MyClic, the sleeve is rubberized to prevent patients’ hands slipping while administering the injection. Addition of the sleeve to the Enbrel MyClic also makes the device considerably larger in diameter (45.5 mm compared with 18.1 mm without the sleeve), making it easier and more comfortable to hold for patients with limited hand function and reduced grip. All except one participant were able to maintain a consistent firm pressure when injecting with the autoinjector + sleeve, and most found it easier to maintain this pressure with the sleeve in place. This aligns with an analysis carried out during development of the sleeve, which showed that patients would not need to use extra force when using the Enbrel MyClic with the sleeve in place.
Use of the sleeve requires an additional step in the assembly process; however, this did not affect the ratings for ease of preparation as much as we had anticipated. Most participants did not identify the extra assembly step as a significant difficulty; only one-third gave the autoinjector + sleeve a lower score than the autoinjector alone.
Although the participants rated the autoinjector + sleeve marginally harder to learn to use than the autoinjector alone, more than 80% found it intuitive to use, suggesting that it would not take them long to feel comfortable with using the sleeve as part of their routine.
The combination of the activation button, size of the device and lack of grip may make injection challenging with the Enbrel MyClic for patients with limited hand function. While the addition of the sleeve benefitted participants regardless of their degree of dexterity impairment, those with severe impairment (half of the participants) appeared to benefit the most.
Potential limitations of our study are that participants injected into skin pads and only performed two injections (one with the sleeve and one without). It is possible that patient preference patterns could be different if they are injecting into themselves over a period of time.5 Another potential limitation is that participants were from a restricted geographical area (London and eastern counties of England); however, there is no reason to suggest that they were not representative of the wider RA population.
Conclusion
Addition of the sleeve enhanced the use of the Enbrel MyClic. The benefits of the sleeve outweighed any inconvenience associated with the additional steps needed to prepare the device. The sleeve offers a new option that could improve patients’ experience of self-injection with the Enbrel MyClic.
Abbreviations
bDMARD, biological disease-modifying antirheumatic drug; csDMARD, conventional synthetic disease-modifying antirheumatic drug; RA, rheumatoid arthritis.
Acknowledgments
Healthcare Fieldwork Ltd (a sister company of Harvey Medical Consulting Ltd) were responsible for participant recruitment and data analysis. Dr Sarah Ray, a general practitioner based in Cambridge, UK, instructed participants on use of the Enbrel MyClic. Dr Joanna Todd (Stellar Medical Communications Limited, Ely, UK) provided medical writing and editorial assistance; this was funded by Pfizer R&D UK Ltd.
Disclosure
Naceur Rekaya and Alexandra Pickersgill are employees of Pfizer R&D Ltd, UK. Naceur Rekaya reports a patent (application number: 62789460) pending to Noble/Genia. Louisa Harvey is an employee of Harvey Medical Ltd, who was engaged by Pfizer to conduct the study. The authors report no other conflicts of interest in this work.
References
1. Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. doi:10.1136/annrheumdis-2016-210715
2. Kivitz A, Cohen S, Dowd JE, et al. Clinical assessment of pain, tolerability, and preference of an autoinjector pen versus a prefilled syringe for patient self-administration of the fully human, monoclonal antibody adalimumab: the TOUCH trial. Clin Ther. 2006;28(10):1619–1629. doi:10.1016/j.clinthera.2006.10.006
3. Borrás-Blasco J, Gracia-Pérez A, Rosique-Robles JD, Casterá MD, Abad FJ. Acceptability of switching adalimumab form a prefilled syringe to an autoinjection pen. Expert Opin Biol Ther. 2010;10(3):301–307. doi:10.1517/14712590903530633
4. Demary W, Schwenke H, Rockwitz K, et al. Subcutaneously administered methotrexate for rheumatoid arthritis, by prefilled syringes versus prefilled pens: patient preference and comparison of the self-injection experience. Patient Prefer Adherence. 2014;8:1061–1071. doi:10.2147/PPA.S64111
5. Egeth M, Soosaar J, Nash P, et al. Patient and healthcare professionals preference for Brenzys vs. Enbrel autoinjector for rheumatoid arthritis: a randomized crossover simulated-use study. Adv Ther. 2017;34:1157–1172. doi:10.1007/s12325-017-0523-x
6. Tischer B, Mehl A. Patients’ and nurses’ preferences for autoinjectors for rheumatoid arthritis: results of a European survey. Patient Prefer Adherence. 2018;12:1413–1424. doi:10.2147/PPA.S169339
7. Phillips JT, Fox E, Grainger W, Tuccillo D, Liu S, Deykin A. An open-label, multicenter study to evaluate the safe and effective use of the single-use autoinjector with an Avonex® prefilled syringe in multiple sclerosis subjects. BMC Neurol. 2011;11:126. doi:10.1186/1471-2377-11-126
8. Tiffin J, Asher EJ. The purdue pegboard: norms and studies of reliability and validity. J Appl Psychol. 1948;32(3):234–247. doi:10.1037/h0061266
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

