Back to Journals » Infection and Drug Resistance » Volume 16
Acute Spinal Epidural Abscess of the Cervical Spine Caused by Streptococcus constellatus Leads to Paraplegia in an Adult: A Case Report
Authors Zhang W, Lai Y, Li T, Wang X , Mu W, Jiang Z
Received 19 January 2023
Accepted for publication 11 March 2023
Published 18 March 2023 Volume 2023:16 Pages 1591—1598
DOI https://doi.org/10.2147/IDR.S405448
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Supplementary video 1 of "Acute SEA of the cervical spine" [ID 405448].
Views: 36
Wen Zhang,1,2,* Yudong Lai,2,* Tao Li,2 Xingpeng Wang,2 Weidong Mu,3,4 Zhensong Jiang2
1Department of Spine Surgery, Shandong University of Traditional Chinese Medicine, Jinan, People’s Republic of China; 2Department of spine surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, People’s Republic of China; 3Department of Traumatic Orthopaedics, Shandong University of Traditional Chinese Medicine, Jinan, People’s Republic of China; 4Department of Traumatic Orthopaedics, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhensong Jiang; Weidong Mu, Email [email protected]; [email protected]
Abstract: The incidence of a spinal epidural abscess (SEA), which can cause serious neurological complications, is low; however, the incidence of SEA caused by Streptococcus is even lower, most of which are reported in the thoracolumbar spine and lumbosacral segment. We reported a case of cervical SEA caused by Streptococcus constellatus infection, resulting in paralysis of the patient. The acute onset of SEA in a 44-year-old male led to decreased upper limb muscle strength, lower limb paralysis, and loss of bowel and bladder function, and imaging and blood tests suggested pyogenic spondylitis. Emergency decompression surgery and antibiotic therapy were given, the patient gradually recovered, and the muscle strength of the lower limbs gradually improved. This case report suggests the importance of early decompressive surgery and effective antibiotic therapy.
Keywords: Streptococcus constellatus, epidural abscess, pyogenic spondylitis, vertebral destruction, diagnosis and treatment
A Letter to the Editor has been published for this article.
Introduction
A spinal epidural abscess (SEA), also known as a spinal epidural infection, has a low incidence and is a potentially destructive entity with insidious manifestations and variable progression that can cause serious neurological complications.1 The incidence of SEA caused by Streptococcus is even lower, and SEA is an uncommon problem that accounts for 0.2 to 2 per 10,000 hospital admissions.2 Staphylococcus aureus is the most common pathogen of SEA,3 whereas SEA induced by Streptococcus constellatus, which is found in normal human flora, is very rare and has only been reported to be localized in the thoracic region, closely followed by lumbar involvement. We reported an extremely rare case of cervical spine SEA caused by Streptococcus constellatus infection causing paralysis in a patient.
Case Presentation
A 44-year-old male patient complained of “a 20-day history of neck pain with quadriplegia for 2 days”. Cervical spine CT (Figure 1A–D) at a local hospital showed abnormalities in his C6–7 cervical segment, and conservative treatment was performed with poor results. Two days before admission, he experienced numbness and weakness in his limbs, difficulty urinating, and a fever with a body temperature up to 38 °C. Cervical magnetic resonance examination found: C6/7 abnormal signals of the vertebral body and intervertebral disc, abscess formation under the anterior longitudinal ligament and spinal canal, compression of the spinal cord, and infectious lesions (Figure 2A–C). On the day of admission, the physical examination showed limited flexion and extension of the cervical spine, weak bilateral upper extremity muscle strength (bilateral triceps muscle strength class I, bilateral biceps muscle strength class II, grip strength grade 0), and loss of tendon reflexes. Both lower limb muscles had a strength level of 0 and low muscle tone. Additional physical examination findings included: bilateral Achilles tendon reflex (-), knee tendon reflex (-), Hoffmann’s sign (-), sphincter contraction without touch, bilateral ankle burn, heavy right side, and second-degree scald (Figure 3A and B). Laboratory tests, including the Brucella antibody test, tuberculosis antibody test, and Mycobacterium tuberculosis T-cell test, were negative, and serology showed inflammation (Table 1).
 |
Table 1 Laboratory Findings at Different Points in Time in This Case |
 |
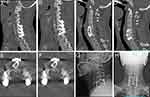
Figure 1 Preoperative CT images at admission. (A and B) Sagittal CT images at admission; (C and D) Axial CT image at admission, C6-7 vertebral body destruction. |
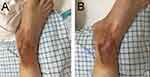 |
Figure 3 (A and B) Scalded right ankle. |
Two days after admission, the patient underwent posterior cervical spine decompression and fixation under general anesthesia. Preoperative X-ray examination showed C6-7 cervical vertebral body abnormalities and cervical spine degeneration (Figure 4A and B), and the operation went smoothly and the decompression was adequate. Postoperative examination of the internal fixation position was good, as seen in perspective (Figure 4C and D), but there was no significant improvement in limb mobility immediately after the operation. On the first day after surgery, the patient indicated a significant recovery in the strength of both upper limbs, and the physical examination showed that the grip strength of both hands was restored to grade 3 and the sensation of both lower limbs was restored, yet the muscle strength was not significantly improved. On the second day after surgery, the patient complained of an increase in body temperature at night, up to 38.5 °C, and an increase in his inflammatory factors compared to preoperatively. Blood culture findings showed Streptococcus constellatus infection, and antimicrobial susceptibility test results indicated that blood isolates were sensitive to vancomycin, penicillin G, cefepime, cefotaxime, levofloxacin, chloramphenicol, and linezolid. Considering that the patient had severe symptoms, the patient was given systemic antibiotic therapy (vancomycin injection 1000 mg, q12h, IV drip; Cefzoxime sodium 1 g, q8h, IV drip) and symptomatic treatments such as fluid replacement, maintenance of water-electrolyte balance, and nutritional support.
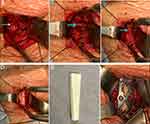
After 2 weeks of vancomycin and cephalosporin treatment, the patient’s blood culture reexamination results were negative, the serological inflammatory indicators showed a downward trend (Table 1), the body temperature remained stable at 38 °C, and the sensory and muscle strength of both lower limbs recovered more than before, the left side was obvious and the right side was weak (Supplementary Video 1); however, there was a neck rash. The dermatology department and clinical pharmacy department considered that the neck rash was related to the previous use of vancomycin, so we changed the antibiotic (linezolid 0.6 g, q12h, IV drip) for symptomatic treatment. After 3 weeks of systemic antibiotic treatment, the patient’s condition tended to be stable, and the muscle strength and sensory motor function of both lower limbs were significantly improved compared with those before surgery (Supplementary Video 2). The muscle strength of the left lower limb was grade 4, and that of the right lower limb was grade 3. Inflammatory indicators showed a good downward trend (Table 1). The MRI reexamination results showed that the cervical spine infection was relieved compared with the previous observation (Figure 5A–D). CT reexamination of the cervical spine showed that the C6/7 vertebral body was severely damaged and the bone defect was obvious (Figure 6A–F). Therefore, the patient underwent a second operation under general anesthesia for an anterior infection lesion removal and internal fixation of the cervical spine. During the operation, it was found that the lower edge of the C6 vertebral body, the upper edge of the C7 vertebral body, and the C6/7 intervertebral space were obviously damaged, cavities were formed, and some blood gushed out of the cavity (Figure 7A–C). The infection lesion was carefully removed, and the tissue was removed for routine pathological culture. A fibular allogeneic bone of appropriate length (Figure 7D and E) was selected and placed between the C5-7 vertebral bodies, and an anterior cervical titanium plate was fixed in front of C5 and C7 (Figure 7F). The results of pathological culture during the operation showed acute and chronic inflammatory cell infiltration and granulation tissue formation, and the clinical results were consistent with inflammatory lesions (Figure 8A and B). According to all the test results, tuberculosis infection and Brucella infection were excluded, and the patient was finally diagnosed with SEA caused by Streptococcus constellatus.
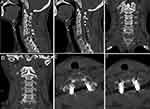 |
Figure 6 Postoperative CT. (A and B) Sagittal CT image; (C and D) Coronal CT image; (E) C6 upper edge of the vertebral body axial CT image; (F) Lower edge of the C6 vertebral body axial CT image. |
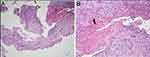 |
Figure 8 (A and B) H&E staining of C6–7 disc space tissues shows acute and chronic inflammatory cell infiltration and granulation tissue formation. |
Within a week of the second operation, the patient continued to receive systemic antibiotics, and the serological inflammatory index gradually decreased to normal. Bacterial culture and anaerobic culture of the puncture fluid were both negative (Table 1), indicating that systemic antibiotic therapy was effective. The patient’s CT (Figure 9A–D) and X-ray (Figure 9G and H) review images showed that the internal fixation position was good, the intervertebral space and C6-7 vertebral bone mass were filled on the CT review image (Figure 9E and F), and the patient was discharged. Two weeks after discharge, the patient’s serological examination showed that the inflammatory indicators tended to be normal, and there was no significant change (Table 1).
Discussion
Streptococcus constellatus belongs to the group of Streptococcus anginosus (SAG) and tends to cause purulent infections at different sites,4 such as liver abscess, empyema, endocarditis, and odontogenic infections.5 The incidence of SEA caused by streptococci is low, reported mainly in the thoracolumbar spine and lumbosacral segment, and the causative bacterium is usually Staphylococcus aureus. Cervical spine SEA induced by Streptococcus constellatus infection is extremely rare, such as the case we reported, in which patients are characterized by quadriplegia and rapid progression of infection.
SEA, also known as a spinal epidural infection, is a rare but serious condition that carries with it a high rate of mortality and associated factors, including immunocompromise, bacteremia, and adjacent infection,6 characterized by insidious manifestations and variable progression that can cause serious neurological complications. Streptococcus constellatus is an extremely rare SEA pathogen with only a few cases. For instance, Dai et al reported that a 58-year-old female had SEA caused by Streptococcus constellatus infection,7 Korugan et al reported invasive suppurative spondylitis caused by Streptococcus constellatus infection,8 Jin et al reported another obese patient with acute suppurative spondylitis caused by Streptococcus constellatus,3 and Takada et al reported a rare case of suppurative thrombophlebitis of the posterior neck caused by Streptococcus constellatus.9 All of these patients recovered after surgery and 6–8 weeks of systemic antibiotics.3
The diagnosis of SEA needs to be made carefully, and a correct diagnosis is crucial to the treatment of SEA. As we reported in this case, his clinical symptoms (high fever, limb paraplegia, motor sensory dysfunction, urination and defecation dysfunction) were not specific, and there are similar manifestations in other diseases, such as cervical spondylotic myelopathy.10 Imaging manifestations such as bone defects and bone destruction can also be seen in spinal tuberculosis disease. The laboratory test results (increased inflammatory indicators such as CRP and ESR) are similar to some infections commonly seen, such as Brucella infection.11 Therefore, the diagnosis of SEA should be based on the patient’s comprehensive conditions, combining clinical symptoms, laboratory examination results, imaging examination results, blood culture, bacterial culture, tissue culture, etc.
The first choice for SEA treatment is surgery combined with systemic antibiotic treatment. Surgical treatment is indicated in patients with spinal cord or cauda equina compression with progressive neurological deficits.12 In our case, after the first surgical treatment, the muscle strength and sensorimotor functions of the extremities, and stool and urine functions gradually improved, indicating the importance of timely surgical intervention. Systemic antibiotic treatment can effectively inhibit the progression of SEA. After 2–3 weeks of systemic antibiotic treatment, the patient’s routine blood test, blood culture, bacterial culture and other examination results improved significantly. The choice of antibiotics should be based on the results of the bacterial culture drug sensitivity test. During systemic antibiotic treatment, antibiotics should be appropriately changed according to the patient’s laboratory examination results and clinical manifestations. Relevant studies show that systemic antibiotic treatment should be maintained for at least 6 weeks.13 The specific stop time should be based on the patient’s condition and laboratory examination results.
In contrast to the previously reported SEA in the thoracolumbar and lumbosacral segments, in this case, we reported SEA in the cervical spine, with more severe neurological deficits and almost zero muscle strength in the extremities, and the patient also had some liver dysfunction. Bone destruction is not uncommon in SEA.14 In this case, through two CT examinations before and after surgery, it was found that bone destruction progressed rapidly within 3 weeks. One operation was not enough to solve the problem, so a second operation was needed. In this case, it was found that the C6 vertebral body was severely damaged during the second operation, and the remaining upper edge was smaller, leading to difficulty in placing nails. The C6-7 intervertebral space and C7 upper edge were also severely damaged, so we finally adopted the surgical method of implanting allogeneic bone between C5-7 and fixing a titanium plate on C5 and C7.
Through this case, we learned that correct diagnosis and timely treatment are the top priority when patients have SEA caused by streptococcal infection, and systemic antibiotic treatment is essential. Patients with neurological defects should be immediately decompressed by surgery because it is not clear at what time the neurological defects become irreversible.15 In the following month of follow-up, the patient’s limbs recovered well and had the ability of autonomous movement (Supplementary Videos 3 and 4).
Conclusion
In this report, we present a rare case of SEA caused by Streptococcus constellatus. The uniqueness of this case is that the infection site occurred in the cervical vertebra, the bone destruction progressed rapidly within 3 weeks, and the patient underwent two operations. After emergency decompression operation and antibiotic treatment, the patient’s feelings gradually recovered, and lower limb muscle strength gradually improved. This case report demonstrates the importance of early decompression surgery and effective antibiotic treatment.
Patient Consent and Ethics Statement
Compliance with ethical standards. The patient provided informed consent for publication of the case, which included the publication of the patient’s own clinically relevant images No institutional approval was required for the publishing of this case report.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The present study was supported by the Natural Science Foundation of Shandong Province (grant nos. ZR2021QH045 and ZR2021MH169).
Disclosure
Wen Zhang and Yudong Lai are co-first authors for this study. The authors declare that they have no conflicts of interest.
References
1. Schwab JH, Shah AA. Spinal epidural abscess: diagnosis, management, and outcomes. J Am Acad Orthop Surg. 2020;28(21):e929–e938. doi:10.5435/JAAOS-D-19-00685
2. Grewal S, Hocking G, Wildsmith JA. Epidural abscesses. Br J Anaesth. 2006;96(3):292–302. doi:10.1093/bja/ael006
3. Jin Y, Yin X. Acute pyogenic spondylitis caused by Streptococcus constellatus in an obese patient: a case report. Infect Drug Resist. 2022;15:4361–4367. doi:10.2147/IDR.S371411
4. Xia J, Xia L, Zhou H, Lin X, Xu F. Empyema caused by Streptococcus constellatus: a case report and literature review. BMC Infect Dis. 2021;21(1):1267. doi:10.1186/s12879-021-06955-2
5. Al Asaadi Z, Srinivasan B, Melchers LJ, Brennan PA. Streptococcus constellatus causing bony destruction secondary to odontogenic infection: three rare cases. Br J Oral Maxillofac Surg. 2019;57(6):594–596. doi:10.1016/j.bjoms.2019.05.004
6. Long B, Carlson J, Montrief T, Koyfman A. High risk and low prevalence diseases: spinal epidural abscess. Am J Emerg Med. 2022;53:168–172. doi:10.1016/j.ajem.2022.01.008
7. Dai G, Li S, Yin C, et al. Studies on 11 cases of spinal epidural abscess and literature review. Infect Drug Resist. 2020;13:3325–3334. doi:10.2147/IDR.S257398
8. Koruga N, Roncevic A, Soldo Koruga A, et al. Aggressive pyogenic spondylitis caused by S. constellatus: a case report. Diagnostics. 2022;12(11):2686. doi:10.3390/diagnostics12112686
9. Takada K, Nakamura M, Samura M, et al. Suppurative thrombophlebitis of the posterior neck caused by Streptococcus constellatus: a case report and literature review. Yakugaku Zasshi. 2022;142(2):189–193. Japanese. doi:10.1248/yakushi.21-00179
10. McCormick JR, Sama AJ, Schiller NC, Butler AJ, Donnally CJ. Cervical spondylotic myelopathy: a guide to diagnosis and management. J Am Board Fam Med. 2020;33(2):303–313. doi:10.3122/jabfm.2020.02.190195
11. Zhang W, Wang J, Zhang Y, Ma R, Zhang Q. Salmonella enteritis spondylitis with Brucella melitensis infection: a rare case of mixed infections of spine. Infect Drug Resist. 2022;15:6525–6531. doi:10.2147/IDR.S385759
12. Sato K, Yamada K, Yokosuka K, et al. Pyogenic spondylitis: clinical features, diagnosis and treatment. Kurume Med J. 2019;65(3):83–89. doi:10.2739/kurumemedj.MS653001
13. Spennato P, Renedo D, Cascone D, et al. Spinal epidural abscess in children: a case-based review. Childs Nerv Syst. 2020;36(7):1385–1392. doi:10.1007/s00381-020-04609-3
14. Long B, Carlson J, Montrief T, Koyfman A. Further considerations regarding spinal epidural abscess in the ED setting. Am J Emerg Med. 2022;57:162–163. doi:10.1016/j.ajem.2022.04.045
15. Babic M, Simpfendorfer CS, Berbari EF. Update on spinal epidural abscess. Curr Opin Infect Dis. 2019;32(3):265–271. doi:10.1097/QCO.0000000000000544
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.