Back to Journals » Infection and Drug Resistance » Volume 15
Acute Post-Cataract Endophthalmitis Due to Phialemoniopsis curvata: A Rare Case Report
Authors Tang M, Li J, Xia F , Min C, Liu Z, Hu Y, Wang H, Xu H, Zou M
Received 23 January 2022
Accepted for publication 26 March 2022
Published 8 April 2022 Volume 2022:15 Pages 1651—1657
DOI https://doi.org/10.2147/IDR.S359481
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Mengli Tang,1,* Jun Li,1,2,* Fengjun Xia,1 Changhang Min,1 Zhaojun Liu,1 Yongmei Hu,1,2 Haichen Wang,1,2 Heping Xu,3 Mingxiang Zou1,2
1Department of Clinical Laboratory, Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China; 2National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China; 3Department of Clinical Laboratory, the First Affiliated Hospital of Xiamen University, Xiamen, Fujian, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mingxiang Zou, Department of Clinical Laboratory, Xiangya Hospital, Central South University, No. 87, Xiangya Road, Changsha, 410008, Hunan, People’s Republic of China, Tel +86 13907496278, Email [email protected]
Abstract: Phialemonium species are a class of opportunistic pathogenic fungi widely present in the environment that cause invasive diseases in hosts with normal or weak immune functions. Common infections include peritonitis, endocarditis, osteomyelitis, and skin infections of wounds after burns, whereas endophthalmitis is rarely reported. Here, we report acute post-cataract endophthalmitis caused by Phialemoniopsis curvata in China. The isolated pathogen was identified using microscopy, culture, and sequencing. After vitrectomy, intraocular lens removal surgery, voriconazole injection, and topical voriconazole treatment, the patient’s symptoms were alleviated.
Keywords: Phialemoniopsis curvata, fungal endophthalmitis, cataract surgery, voriconazole
Introduction
Acute post-cataract endophthalmitis is the most serious complication of cataract surgery and may cause permanent vision loss in the affected eye. In culture-positive cases, Gram-positive cocci, accounting for approximately 95% of the isolates, and coagulase-negative staphylococci are the main pathogens (70% of the cases).1 Phialemoniopsis curvata is a dematiaceous fungus that is rarely diagnosed as a pathogenic fungus of endophthalmitis.2 The pathogenicity of the pathogen may be ignored in some cases because of difficulties in its identification and detection.3
Although rare, fungal endophthalmitis can seriously affect the vision of patients.4 However, because of the rarity of P. curvata infection, there are no accurate data to base a recommendation for the treatment of choice.5 Herein, we report the first case of acute post-cataract endophthalmitis caused by P. curvata in China, which may provide a reference for the diagnosis and treatment of this rare pathogen.
Case Report
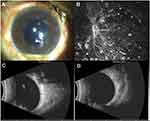
A healthy 38-year-old man was admitted to our hospital with purulent endophthalmitis of the left eye. A month ago, the patient underwent cataract surgery, corneal debridement, and suture surgery due to the left eye being stabbed by a rusty wire. Ten days after surgery, the patient complained of blurred vision and received intraocular injections of ceftazidime, vancomycin, dexamethasone, and fluconazole (FLC) (usage was unclear); however, there was no significant improvement. His best-corrected visual acuity (BCVA) in the left eye was counting fingers at 0.2 m on admission. There were a slightly congested conjunctiva, an unclear iris, a slightly edematous corneal epithelium, and a slightly dilated pupil (5 mm) with no light reflex. A 2-mm hypopyon was seen and purulent discharge was observed in the vitreum, the bottom of the eye could not be seen clearly (Figure 1A). Eye B-ultrasound showed a large number of low and medium echo spots in the vitreous cavity (Figure 1C). Subsequently, vitrectomy and intraocular lens removal were performed. In vivo confocal microscopy showed partially defective corneal epithelium and inflammatory cells with filamentous high-reflective clusters (consistent with fungal hyphae, invading approximately 85 μm), suggesting a fungal infection (Figure 1B).
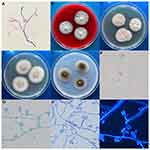
To detect the pathogen, intraocular lens specimens were collected, stained with Gram staining, and inoculated on blood agar (BA) and Sabouraud’s dextrose agar (SDA) at 35°C and 28°C. Direct Gram staining of the specimen microscopically showed oval fungal spores and hyphae, which were similar to those of Fusarium (Figure 2A). Fungal growth was visible on both the BA and SDA after 2 days of incubation. The fungus was inoculated on potato dextrose agar and Czapek Dox agar at 28°C. After 7 days of culture, the colonies became wet, flat, and yellow-white, without aerial hyphae, and the back was brown-yellow (Figure 2B–E). The conidiophore was rooted in irregularly swollen hyphae, and conidia were smooth, hyaline, thick-walled, single, or clumped at the mucoid pseudohead (Figure 2F–I). DNA was extracted using the TIANamp Yeast DNA Kit (Tiangen, Beijing, China). The internal transcribed spacer (ITS) gene was amplified and sequenced to identify the species using the primers ITS-F (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS-R (5′-TCCTCCGCTTATTGATATGC-3′).6 Polymerase chain reaction (PCR) was performed using an Applied Biosystems thermal cycler (Applied Biosystems, Foster City, CA, USA) as follows: denaturation for 5 min at 94°C; 30 cycles of 30 s at 95°C, 45 s at 55°C, and 45 s at 72°C; and a final extension for 10 min at 72°C. The amplified DNA fragments were 540 bp in size, and similarity to the P. curvata sequence (GenBank KC771519.1) was as high as 100.0%. In vitro antifungal susceptibility was determined by broth dilution, according to CLSI M38-A.7 The minimum inhibitory concentrations (MICs) of posaconazole (POS), voriconazole (VRC), itraconazole (ITR), anidulafungin (AND), micafungin (MCF), caspofungin (CAS), amphotericin B (AMB), FLC, and flucytosine (5-FC) were 0.03, 0.06, 0.03, 0.03, 0.03, 0.06, 0.25, 8, and >64 μg/mL, respectively.
The antifungal regimen was based on the results of drug susceptibility and treatment advice for dematiaceous fungi.8 The patient was treated with VRC (200 μg) injections into the vitreous cavity every 3 or 4 days and six times in total, FLC (0.2 g) intravenously infused once a day, and 1% VRC eye drops six times a day. BCVA was 0.01 at discharge. The patient continued taking VRC eye drops six times a day and VRC oral tablets (0.2 g) twice a day. Three weeks later, conjunctival hyperemia of the left eye was relieved, vitreous opacity was reduced according to eye B-ultrasound (Figure 1D), and no purulent substance could be seen.
Discussion
Phialemoniopsis was originally named Phialemonium and includes two species, P. obovatum and P. curvatum (named P. curvata now). Fungal infections due to Phialemoniopsis have rarely been reported in medical literature. In this case, we first reported a patient diagnosed with acute post-cataract endophthalmitis due to P. curvata in China, which was based on the combination of intraocular lens specimen Gram staining, culturing, gene sequencing, clinical features, and efficacy after adjustment of the treatment regimen for the fungus.
In our case, the patient was previously healthy and immunocompetent. P. curvata-induced endophthalmitis occurred due to the left eye being stabbed by a rusty wire. Presently, there are only four reports of endophthalmitis caused by P. curvata in the PubMed database (Table 1). Two of the previous cases were secondary to self-injection of drugs contaminated with P. curvata and were treated with AMB.9–11 The third was corneal infection after phacoemulsification for cataract, taking combination therapy with AMB, natamycin, and ketoconazole.12 The fourth was a corneal infection combined with P. curvata and Acanthamoeba, treated with polyhexamethylene biguanide and VRC against the two pathogens.13 Phialemoniopsis infection often occurs in patients undergoing hemodialysis for end-stage renal failure, hematological malignancies, kidney transplantation, and other immunocompromised diseases, and endophthalmitis caused by P. curvata mainly affects the elderly.10–12 However, referring to the study by Roszkowska et al13 and this case, it can also occur in immunocompetent and younger patients. For patients with underlying diseases or complications, endophthalmitis caused by P. curvata can be secondary to bloodstream infection,10,11 while for patients who underwent cataract surgery or were previously healthy, P. curvata infections were mostly exogenous and confined to the eyes.12,13
 |
Table 1 Reported Cases of Endophthalmitis Caused by Phialemoniopsis curvata |
Initial fungal endophthalmitis may be difficult to diagnose because of the difficulty in obtaining satisfactory intraocular specimens and prior use of antibiotics. Pigmentation of Phialemoniopsis hyphae and spores is not always obvious, and the yellow-white and cottony colonies are similar to those of Fusarium. In our case, the result of the direct smear microscopic examination of P. curvata was thought to be Fusarium, and the microscopic image after culture was similar to the poorly sporulating Fusarium and Acremonium.10,14,15 In some cases, histological staining, such as Fontana–Masson silver staining, can demonstrate Phialemoniopsis hyphae pigmentation, which helps distinguish these fungi from other species, especially Aspergillus. The diagnosis of P. curvata depends on molecular biology methods, which can be clearly identified through amplification and sequencing comparison of 28S rRNA and ITS gene.16 In cases of rapid disease progression and difficulty in culturing, the use of specific primer pairs for quick molecular detection of Phialemoniopsis is expected to be therapeutically effective.
As it is a rare clinical isolate, there are no exact data to recommend the best treatment for P. curvata. Previous reports have shown that these three patients received surgical treatment and intravitreal injection of AMB after developing P. curvata causing endophthalmitis, but the effect was not good10–12—two of the patients had to have their eyes removed,10,12 and one patient’s postoperative vision was limited to 20/2500.11 In our case, the in vitro drug susceptibility test showed that the MICs of POS, VRC, ITR, AND, MCF, and CAS were low (≤0.06 μg/mL), whereas the MICs of FLC (8 μg/mL) and 5-FC (>64 μg/mL) were high. Compared with most strains reported in the literature, the MICs of triazole drugs (ITR, FLC, VRC, and POS) and 5-FC reported in our case were consistent, whereas the MICs of MCF and CAS were lower.2,17–19 Treatment with local intravitreal injections (200 μg/every 3 or 4 days) and 1% VRC (six times/day) eye drops was effective. More than 70% of patients with Phialemoniopsis infection used AMB, as previously reported.18,19 However, in vitro drug susceptibility test results showed that the MIC of AMB was ≥2 mg/L in more than 50% of Phialemoniopsis strains, whereas the MIC of VRC was ≤1 mg/L in 85% of strains.2,17–19 VRC may be the preferred empirical drug because of its broad activity, accumulated clinical experience, fewer side effects, and availability of intravitreal injection.
Conclusion
To our knowledge, this is the first reported case of P. curvata-induced endophthalmitis in China. P. curvata caused acute post-cataract endophthalmitis in a healthy middle-aged patient, and treatment with VRC intraocular injections proved to be effective. Fungal-induced endophthalmitis is uncommon and difficult to discern based on clinical presentation. Molecular detection can effectively help identify rare fungi whose morphological staining and biochemical characteristics are difficult to ascertain.
Abbreviations
BCVA, best-corrected visual acuity; BA, blood agar; SDA, Sabouraud’s dextrose agar; ITS, internal transcribed spacer; MICs, minimum inhibitory concentrations; POS, posaconazole; VRC, voriconazole; ITR, itraconazole; AND, anidulafungin; MCF, micafungin; CAS, caspofungin; AMB, amphotericin B; FLC, fluconazole; 5-FC, flucytosine.
Data Sharing Statement
All the data are available from the corresponding author on reasonable request.
Ethical Approval and Informed Consent
This research was carried out in compliance with the recommendations of the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Central South University Ethics Committee (Changsha, Hunan Province, People’s Republic of China) with ID 201703302.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report.
Acknowledgments
We would like to express our gratitude for the support in data and sample collection provided by doctors from ophthalmology department and clinical laboratory department of Xiangya hospital, Central South University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities of Central South University (No. 2021zzts1043), the National Natural Science Foundation of China (No. 81702068), the Natural Science Foundation of Hunan Province (No. 2020JJ4886), and the Science Foundation of Hunan Health Commission in Hunan province (No. 202111000066).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Durand ML. Bacterial and fungal endophthalmitis. Clin Microbiol Rev. 2017;30(3):597–613. doi:10.1128/CMR.00113-16
2. Proia LA, Hayden MK, Kammeyer PL, et al. Phialemonium: an emerging mold pathogen that caused 4 cases of hemodialysis-associated endovascular infection. Clin Infect Dis. 2004;39(3):373–379. doi:10.1086/422320
3. Perdomo H, Sutton DA, García D, et al. Molecular and phenotypic characterization of Phialemonium and Lecythophora isolates from clinical samples. J Clin Microbiol. 2011;49(4):1209–1216. doi:10.1128/JCM.01979-10
4. Narang S, Gupta A, Gupta V, et al. Fungal endophthalmitis following cataract surgery: clinical presentation, microbiological spectrum, and outcome. Am J Ophthalmol. 2001;132(5):609–617. doi:10.1016/S0002-9394(01)01180-1
5. Arcobello JT, Revankar SG. Phaeohyphomycosis. Semin Respir Crit Care Med. 2020;41(1):131–140. doi:10.1055/s-0039-3400957
6. Zou Y, Bi Y, Bu H, He Y, Guo L, Shi D. Infective meningitis caused by Phialemonium curvatum. J Clin Microbiol. 2014;52(8):3111–3113. doi:10.1128/JCM.00419-14
7. Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi.
8. Revankar SG. Dematiaceous fungi. Mycoses. 2007;50(2):91–101. doi:10.1111/j.1439-0507.2006.01331.x
9. Strahilevitz J, Rahav G, Schroers HJ, et al. An outbreak of Phialemonium infective endocarditis linked to intracavernous penile injections for the treatment of impotence. Clin Infect Dis. 2005;40(6):781–786. doi:10.1086/428045
10. Weinberger M, Mahrshak I, Keller N, et al. Isolated endogenous endophthalmitis due to a sporodochial-forming Phialemonium curvatum acquired through intracavernous autoinjections. Med Mycol. 2006;44(3):253–259. doi:10.1080/13693780500411097
11. Zayit-Soudry S, Neudorfer M, Barak A, Loewenstein A, Bash E, Siegman-Igra Y. Endogenous Phialemonium curvatum endophthalmitis. Am J Ophthalmol. 2005;140(4):755–757. doi:10.1016/j.ajo.2005.04.037
12. Freda R, Dal Pizzol MM, Fortes Filho JB. Phialemonium curvatum infection after phacoemulsification: a case report. Eur J Ophthalmol. 2011;21(6):834–836. doi:10.5301/EJO.2011.8360
13. Roszkowska AM, Severo AA, Biondo C, Postorino EI, Inferrera L, Aragona P. Combined Phialemonium curvatum and Acanthamoeba keratitis: the importance of early diagnosis and specific therapy. Cornea. 2021;40(10):1340–1343. doi:10.1097/ICO.0000000000002660
14. Dan M, Yossepowitch O, Hendel D, Shwartz O, Sutton DA. Phialemonium curvatum arthritis of the knee following intra-articular injection of a corticosteroid. Med Mycol. 2006;44(6):571–574. doi:10.1080/13693780600631883
15. Gavin PJ, Sutton DA, Katz BZ. Fatal endocarditis in a neonate caused by the dematiaceous fungus Phialemonium obovatum: case report and review of the literature. J Clin Microbiol. 2002;40(6):2207–2212. doi:10.1128/JCM.40.6.2207-2212.2002
16. Perdomo H, García D, Gené J, et al. Phialemoniopsis, a new genus of Sordariomycetes, and new species of Phialemonium and Lecythophora. Mycologia. 2013;105(2):398–421. doi:10.3852/12-137
17. Aydın M, Özçelik Ü, Çevik H, Çınar Ö, Evren E, Demirağ A. Multiple brain abscesses due to phialemonium in a renal transplant recipient: first case report in the literature. Exp Clin Transplant. 2015;13(Suppl 3):77–80. doi:10.6002/ect.tdtd2015.P31
18. Rivero M, Hidalgo A, Alastruey-Izquierdo A, Cía M, Torroba L, Rodríguez-Tudela JL. Infections due to Phialemonium species: case report and review. Med Mycol. 2009;47(7):766–774. doi:10.3109/13693780902822800
19. Singh AK, Chandra A, Islahi S, Das A, Malhotra K, Rao N. Phialemonium obovatum infection of the renal allograft: case report and review of the literature. Exp Clin Transplant. 2021;19(8):871–876. doi:10.6002/ect.2020.0313
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.