Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
Acute necrotizing encephalopathy in an adult with influenza A infection
Authors Ochi N , Takahashi K, Yamane H , Takigawa N
Received 17 December 2017
Accepted for publication 1 March 2018
Published 20 April 2018 Volume 2018:14 Pages 753—756
DOI https://doi.org/10.2147/TCRM.S160111
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Nobuaki Ochi,1 Kento Takahashi,2 Hiromichi Yamane,1 Nagio Takigawa1
1Department of General Internal Medicine 4, Kawasaki Medical School, Okayama, Japan; 2Clinical Education and Training Center, Kawasaki General Medical Center, Kawasaki Medical School, Okayama, Japan
Abstract: Acute necrotizing encephalopathy following influenza infection is a rapidly progressing disease with high morbidity. Although the neurological disorder is sometimes reported in children, it is very rare in adults. We herein describe an adult with acute necrotizing encephalopathy captured on a series of brain magnetic resonance images. A 55-year-old man had fever and impaired consciousness. He was diagnosed with influenza A (H1N1). Brain magnetic resonance imaging revealed symmetrical lesions in the cerebellum and basal nucleus, showing typical acute necrotizing encephalopathy. Physicians should know that influenza-associated acute necrotizing encephalopathy can occur even in middle-aged adults.
Keywords: acute necrotizing encephalopathy, influenza A, magnetic resonance imaging, adult
Introduction
Although neurological manifestations in children with influenza A (H1N1) are reported, it is rare in adults.1 Acute necrotizing encephalopathy, which is characterized by multifocal, symmetric brain lesions that affect the thalami bilaterally, is one of the features of influenza-associated encephalitis/encephalopathy.2 During an epidemic of encephalitis/encephalopathy in Japan in 1998–1999, 121 of 148 (81.8%) patients were aged <5 years, and the disease was rare in those aged >10 years, with acute necrotizing encephalopathy occurring in 10% of all patients.2 In a multicenter surveillance study of children and adults performed through the British Pediatric Neurology Surveillance Unit and British Neurological Surveillance Unit between February 2011 and February 2013, acute necrotizing encephalopathy was observed in 4 children and no adults among 25 patients (21 children and 4 adults) who had a neurological presentation.1
Acute necrotizing encephalopathy is a rare central nervous system complication of influenza or other viral infections causing altered mental status often with seizures, which may be rapidly progressive and with a high mortality.3,4 The prognosis varies from complete recovery to death: the mortality rate is about 30% and <10% of patients recovered completely, and neurological sequelae were frequently seen in survivors.4 Here, we describe an adult with acute necrotizing encephalopathy captured on a series of brain magnetic resonance imaging (MRI).
Case report
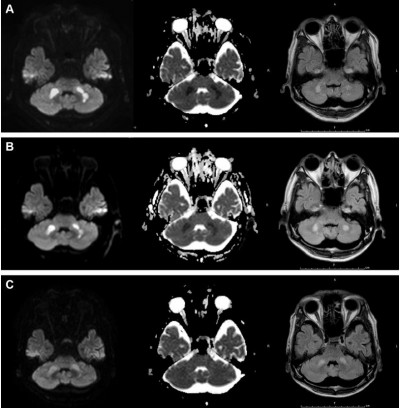
During the influenza season (February 2014), a 55-year-old man had fever of 39°C 4 hours before he presented to our hospital with impaired consciousness of Glasgow Coma Scale E4V4M5. His blood pressure was 66/42 mmHg. Physical examination did not reveal any other abnormal findings. Although impaired glucose tolerance was pointed out a few years ago, there was no other significant past medical history. There was no history of recent lower respiratory tract infection. The laboratory data were as follows: white blood cell count, 10,420/μL (reference level 3,500–9,500/μL); hemoglobin level 15.9 g/dL (13.0–16.5 g/dL); platelet count, 6.1×104/μL (15.0–35.0×104/μL), D-dimer, 13.70 μg/mL (<1.0 μg/mL); international normalized ratio of prothrombin time, 1.34 (0.85–1.13); activated partial thromboplastin time, 53.9 s (26.1–35.8 s); aspartate aminotransferase, 197 U/L (10–35 U/L); alanine aminotransferase, 123 U/L (7–42 U/L); lactase dehydrogenase, 348 U/L (120–240 U/L); γ-glutamyl transpeptidase, 643 U/L (5–60 U/L); total bilirubin, 4.8 mg/dL (0.3–1.2 mg/dL); ammonia, 3 μg/dL (12–66 μg/dL); total protein, 5.8 g/dL (6.5–8.0 g/dL); albumin, 3.2 g/dL (3.8–4.9 g/dL); creatinine 6.27 mg/dL (0.6–1.1 mg/dL); blood urea nitrogen, 41 mg/dL (8–22 mg/dL); creatinine phosphokinase, 1,039 U/L (54–324 U/L); blood glucose level, 155 mg/dL; hemoglobin A1c, 6.8% (≤6.2%); and C-reactive protein, 15.33 mg/dL (<0.3 mg/dL). Thus, multiple organ failure was suspected. He was diagnosed with influenza A by rapid antigen test using nasal swab and was treated with the antiviral drug peramivir (300 mg) once. Subsequently, influenza A H1N1 was confirmed by polymerase chain reaction using nasal secretion. Brain MRI revealed symmetrical lesions in the cerebellum (Figure 1A) and basal nucleus, showing typical acute necrotizing encephalopathy.5 Although he did not manifest a convulsion, his state of consciousness deteriorated 7 hours after the first MRI, which was consistent with the worsening MRI findings at that time (Figure 1B). Cerebrospinal fluid was not examined because his platelet count was decreased to 1.1×104/μL the day after next.
Intravenous hydration, vasopressor, gabexate mesylate, methylprednisolone (1 g/d, 3 days), and immunoglobulin (2.5 g/d, 3 days) were administered in particular. Mechanical ventilatory support and plasmapheresis, followed by continuous hemodiafiltration for acute renal failure, were also performed. On day 6 of his hospitalization, ventilator weaning was successfully done. He moved out of the intensive care unit on day 14. The treatment led to almost complete recovery, with slight disruption in daily life, and improved MRI findings 28 days after disease onset (Figure 1C).
Discussion
To the best of our knowledge, only one case of acute necrotizing encephalopathy in a patient aged >50 years was reported.6 However, brain MRI was not performed in the patient. He was an 80-year-old man, whose past and social history included diabetes for a few years, cerebral infarction at the age of 52, and a smoking history of 40 years.5 Brain MRI images of acute necrotizing encephalopathy in children have been reported.6–8 The brain MRI images in young adults with disease, such as a 23-year-old woman,9 a 27-year-old man,10 and a 27-year-old woman,11 resembled those in children. The typical images showed multiple focal lesions of edematous necrosis which are symmetrically distributed in the bilateral thalami and other brain regions such as the putamina, cerebral and cerebellar deep white matter, and brainstem tegmentum.3 Although acute necrotizing encephalopathy is usually lethal, the patient in our case fortunately recovered. Rates of mortality (31.8%) and disability (27.7%) in Japanese patients with acute necrotizing encephalopathy were found to be high.2 Although the presence of hemorrhage and localized tissue loss on MRI would predict a poor prognosis,4 there were no particular features in our case. In addition to the diagnosis by typical MRI, the prognosis could be predicted by serial MRI findings.
One might assume that the patient could have just acute encephalopathy, but not acute “necrotizing” encephalopathy. Actually, acute encephalopathy shows cytotoxic edema, producing high signal intensity on diffusion-weighted imaging asymmetrically.12,13 However, the MRI findings in the present case were compatible with those found in the previous cases of acute necrotizing encephalopathy, described earlier. Due to the decreased incidence of autopsies, the diagnosis of acute necrotizing encephalopathy was mainly based on characteristic neuroradiologic findings.4 Finally, serial brain MRI scans showed that the images were correlated with his clinical course.
The pathogenesis of influenza-associated acute necrotizing encephalopathy remains unclear. Influenza virus has been rarely identified in cerebrospinal fluid.13 It is hypothesized that the “cytokine storm” due to the systemic inflammation caused by influenza infection may be associated with the severe encephalopathy.13 Thus, immune response rather than direct viral invasion seems to be pathogenesis of the disease. Intensive care, symptomatic treatment, and empirical treatment are usually performed in patients with acute necrotizing encephalopathy, and intravenous glucocorticoids, immunoglobulin, plasmapheresis, or therapeutic hypothermia have also been tried; however, a standard treatment has not been established.4 We administered intravenous glucocorticoids and immunoglobulin and performed plasmapheresis. However, the usefulness of these remains unclear.
Conclusion
Physicians should know that influenza-associated acute necrotizing encephalopathy can occur even in middle-age adults and should be aware of the typical MRI findings of this disease.
Ethics approval and consent to participate
The authors’ institution does not require ethical approval for publication of a single case report. Written informed consent for publication of clinical details and images was obtained from the patient.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Goenka A, Michael BD, Ledger E, et al. Neurological manifestations of influenza infection in children and adults: results of a National British Surveillance Study. Clin Infect Dis. 2014;58:775–784. | ||
Morishima T, Togashi T, Yokota S, et al. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis. 2002;35:512–517. | ||
Mizuguchi M, Yamanouchi H, Ichiyama T, Shiomi M. Acute encephalopathy associated with influenza and other viral infections. Acta Neurol Scand. 2007;115(4 Suppl):45–56. | ||
Wu X, Wu W, Pan W, Wu L, Liu K, Zhang HL. Acute necrotizing encephalopathy: an underrecognized clinicoradiologic disorder. Mediators Inflamm. 2015;2015:792578. | ||
Ishii N, Mochizuki H, Moriguchi-Goto S, et al. An autopsy case of elderly-onset acute necrotizing encephalopathy secondary to influenza. J Neurol Sci. 2015;354:129–130. | ||
Zeng H, Quinet S, Huang W, et al. Clinical and MRI features of neurological complications after influenza A (H1N1) infection in critically ill children. Pediatr Radiol. 2013;43:1182–1189. | ||
Lyon JB, Remigio C, Milligan T, Deline C. Acute necrotizing encephalopathy in a child with H1N1 influenza infection. Pediatr Radiol. 2010;40:200–205. | ||
Haktanir A. MR imaging in novel influenza A(H1N1)-associated meningoencephalitis. AJNR Am J Neuroradiol. 2010;31:394–395. | ||
Narra R, Mandapalli A, Kamaraju SK. Acute necrotizing encephalopathy in an adult. J Clin Imaging Sci. 2015;5:20. | ||
Iijima H, Wakasugi K, Ayabe M, Shoji H, Abe T. A case of adult influenza A virus-associated encephalitis: magnetic resonance imaging findings. J Neuroimaging. 2002;12:273–275. | ||
Lee YJ, Smith DS, Rao VA, et al. Fatal H1N1-related acute necrotizing encephalopathy in an adult. Case Rep Crit Care. 2011;2011:562516. | ||
Tokunaga Y, Kira R, Takemoto M, et al. Diagnostic usefulness of diffusion-weighted magnetic resonance imaging in influenza-associated acute encephalopathy or encephalitis. Brain Dev. 2000;22:451–453. | ||
Akins PT, Belko J, Uyeki TM, et al. H1N1 encephalitis with malignant edema and review of neurologic complications from influenza. Neurocrit Care. 2010;13:396–406. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.