Back to Journals » OncoTargets and Therapy » Volume 13
Acute Myeloid Leukemia with NUP98-RARG Gene Fusion Similar to Acute Promyelocytic Leukemia: Case Report and Literature Review
Authors Tao S, Song L, Deng Y, Chen Y, Shi Y, Gan Y, Deng Z, Ding B, He Z, Wang C, Yu L
Received 23 July 2020
Accepted for publication 9 September 2020
Published 15 October 2020 Volume 2020:13 Pages 10559—10566
DOI https://doi.org/10.2147/OTT.S273172
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Carlos E Vigil
Shandong Tao,1,2,* Lixiao Song,1,2,* Yuan Deng,1,2 Yue Chen,1,2 Yuye Shi,1,2 Yimin Gan,1,2 Zhikui Deng,1,2 Banghe Ding,1,2 Zhengmei He,1,2 Chunling Wang,1,2 Liang Yu1,2
1Department of Hematology, The Affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University, Huai’an, Jiangsu 223300, People’s Republic of China; 2Key Laboratory of Hematology of Nanjing Medical University, Nanjing 210029, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liang Yu; Chunling Wang 1 Huang River Road West, Huai’an, Jiangsu 223300, People’s Republic of China
Tel/Fax +86-517 8087 2603
Email [email protected]; [email protected]
Abstract: Retinoic acid receptor gamma (RARG) belongs to the nuclear receptor superfamily and has 90% homology to RAR alpha (RARA) and RAR beta. The promyelocytic leukemia (PML)–RARA fusion gene has been implicated in acute promyelocytic leukemia (APL). RARG gene rearrangement has been identified in a rare subtype of acute myeloid leukemia (AML) that resembles APL. To date, only 10 cases of gene rearrangements involving RARG (nucleoporin [NUP] 98–RARG, promyelocytic leukemia protein–RARG, cleavage and polyadenylation-specific factor 6–RARG, or nucleophosmin [NPM] 1–RARG–NPM1) have been reported. These patients show characteristics similar to APL, including bone marrow morphology, coagulation abnormality, and immunophenotype; however, they are resistant to all-trans retinoic acid and arsenic trioxide treatment. Moreover, there is no optimal therapeutic regimen for this subtype of AML. In this study, we report the clinical presentation and experimental findings of a case of AML with NUP98–RARG gene fusion similar to APL and review other cases of RARG gene rearrangement described in the literature.
Keywords: acute myeloid leukemia, acute promyelocytic leukemia, NUP98-RARG, RARG rearrangement
Introduction
Acute myeloid leukemia (AML) is a highly heterogeneous and malignant clonal disease of hemopoietic stem cells characterized by uncontrolled proliferation and blocked differentiation of myeloid lineage blasts.1 Acute promyelocytic leukemia (APL) is a specific type of AML, accounting for 5–15% of total AML cases.2 The fusion gene promyelocytic leukemia protein (PML)–retinoic acid receptor alpha (RARA), which is generated by the chromosomal translocation t(15;17)(q22;q21), is implicated in the pathogenesis of APL. All-trans retinoic acid (ATRA) and arsenic trioxide (ATO) have been shown to induce myeloid blast differentiation in APL;3 and it is recommended that patients with APL who have relapsed after ATRA plus chemotherapy be treated with a combined ATRA plus ATO-based regimen.4
RAR gamma (RARG) plays a key role in maintaining the self-renewal and differentiation of hematopoietic stem cells. RARG gene rearrangements have been detected in AML as a rare specific subtype with a clinical presentation resembling that of APL;5 however, these patients do not respond to treatment with ATRA and ATO. As such, the role of RARG in AML remains unclear.
The first AML patient with a nucleoporin (NUP)98–RARG gene rearrangement was identified in 2011.6 Since then, 9 additional cases of RARG rearrangement have been reported, including 2 with NUP98–RARG fusions.7–13 Here we describe a case of NUP98–RARG gene fusion in an AML patient with the classic morphologic features and immunophenotype of APL.
Materials and Methods
Case Presentation
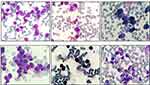
This study was approved by the institutional review board of The Affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University. On April 27, 2018, a 47-year-old female patient was admitted to our hospital presenting with skin ecchymosis and vaginal bleeding. The peripheral blood count revealed a white blood cell (WBC) count of 7.91×109/l, hemoglobin (Hg) level of 84 g/l, and platelet count of 14×109/l. Prothrombin time was 16.9 s (ref. 9.0–13.0 s), activated partial thromboplastin time was 40.7 s (ref. 20.0–40.0 s), fibrinogen level was 1.66 g/l (ref. 2.00–4.00 g/l), and
 |
Figure 4 Karyotype of an AML patient with NUP98–RARG gene fusion. G-banding revealed a karyotype of 45, X, –X, del(9)(q13q22), t(11;12)(p15; q13); arrows indicate 11p+, 12q−, and 9q−. |
Consolidation therapy with the HIAG regimen was initiated on August 13, 2018. During the BM suppression period following chemotherapy, severe pulmonary infection, septic shock, metabolic acidosis, heart failure, and other serious complications occurred; the patient was admitted to the intensive care unit for emergency treatment, and recovered after 1 month. The patient refused to undergo allogeneic hematopoietic stem cell transplantation (allo-HSCT). Since September 19, 2018, the patient has received 2 cycles of half-dose CAG regimen (aclarubicin hydrochloride [20 mg/day for 4 days], cytarabine [40 mg/day for 7 days], and GCSF [300 μg/day until WBC count was >20×109/l]) and 2 cycles of the HA regimen (homoharringtonine [4 mg/day for 3 days] and cytarabine [150 mg/day for 7 days]) as consolidation chemotherapy. The minimal residual disease detected by flow cytometry was negative during each round of consolidation chemotherapy. The treatment timeline is shown in Figure 5. The patient remains alive and was leukemia-free at the last (24-month) follow-up.
 |
Figure 5 Timeline of patient’s treatment. |
RT-PCR
NUP98–RARG mRNA was reverse transcribed into cDNA using random hexamers, and PCR was performed using the following primers: NUP98 forward, 5′-GAGTAACCCAAGCCTCACAGC-3′ and RARG reverse, 5′-CCCATAGTGGTAGCCTGAGGAC-3′. A 200-bp product was specifically amplified from the patient’s cDNA, representing the NUP98–RARG fusion product (Figure 6A).
Cytogenetic Analysis and Fluorescence in situ Hybridization (FISH)
A BM sample was processed after short-term culture (24 h) according to standard procedures. The chromosomes were stained by G-banding and the karyotype was determined according to International System for Human Cytogenetic Nomenclature recommendations. FISH was performed on 200 interphase cells using dual-color translocation probes (Figure 6B).
Results and Discussion
Nine patients harboring a NUP98–RARG, PML–RARG, CPSF6–RARG, or NPM1–RARG–NPM1 gene rearrangement have been described to date; their age, sex, and molecular and genetic characteristic are shown in Table 1. In 2011, Such et al first reported NUP98–RARG fusion in an AML patient with morphologic and immunophenotypic features resembling APL (Table 1, No.1), the patient discontinued ATRA treatment due to absence of a PML–RARA fusion gene and was switched to a standard 3+7 regimen with cytarabine and idarubicin, undergoing consolidation chemotherapy followed by autologous SCT.6 Patient 2 was a 64-year-old woman with PML–RARG fusion (Table 1, No.2); her BM smears showed atypical hypergranular promyelocytes with Auer rods, and she received idarubicin and cytarabine chemotherapy after 9 days of ATRA treatment as well as 1 cycle of high-dose cytarabine consolidation chemotherapy followed by allo-HSCT.7 In addition to NUP98–RARG fusion, CPSF6–RARG or NPM1–RARG–NPM1 fusion have been detected in AML patients who all showed the classic morphologic and immunophenotypic features of APL and were resistant to ATRA and ATO. The common characteristic of these patients was the presence of RARG rearrangement. Some partner proteins can bind to different DNA-binding domains of RARG to form fusion proteins such as PML–RARG, NUP98–RARG, CPSF6–RARG, and NPM1–RARG–NPM1. NUP98 exon 12 was fused in frame to RARG exon 4, forming the NUP98–RARG fusion gene.5 However, the role of RARG in AML has yet to be elucidated.
 |
Table 1 Acute Myeloid Leukemia Resembling Acute Promyelocytic Leukemia with RARG Rearrangements in Literatures |
The patient described herein was a 47-year-old female presenting with features of APL without a detectable PML–RARA fusion by RT-PCR. We detected the NUP98–RARG fusion gene in our patient by PCR using previously described primers (Figure 6A). The cytogenetic analysis revealed an intermediate-risk karyotype of 45, X, –X, del (9)(q13q22), t(11;12)(p15;q13), and the NUP98–RARG fusion was also detected by FISH (Figure 6B). ATRA and ATO treatment was discontinued and switched to idarubicin and cytarabine. The patient experienced serious complications during the initial IA induction chemotherapy and subsequent low-dose regimen, and had poor compliance and refused allo-HSCT; we were also concerned about the risk of treatment-related death. Therefore, the patient received 6 cycles of low-dose CAG and HA regimens as consolidation chemotherapy. At present, the patient is alive and in complete remission.
It was reported that NUP98 exon 12 is fused in-frame to RARG exon 4,5 but the mechanism of oncogenic transformation mediated by NUP98–RARG in AML is unknown. An in vitro study showed that the NUP98–RARG fusion was extremely sensitive to ATRA treatment, implying that retinoid/rexinoid signaling plays an important role in AML and is a potential therapeutic target for patients harboring this chromosomal abnormality.14 However, another study examining relapsed primary blasts of an AML patient with NUP98–RARG rearrangement found that they were resistant to ATRA.15 Consistent with other case reports, we found that leukemia cells with NUP98–RARG fusion were completely resistant to both ATRA and ATO. Abnormalities in other genes such as WT1 and enhancer of zeste homolog 2 may confer ATRA resistance.10,16 Our patient also harbored 2 WT1 mutations and IDH2, TET2, ASXL1, or TP53 mutations. AML patients with RARG rearrangement can be treated with either idarubicin or homoharringtonine and cytarabine as the induction chemotherapy regimen,6,11,17 while allo-HSCT is recommended for post-remission treatment.
In summary, previous reports and findings from our case demonstrate that NUP98–RARG rearrangement defines a novel subtype of AML with morphologic and immunologic characteristics similar to APL but showing resistance to ATRA and ATO treatment. Further studies are needed to clarify the mechanism by which NUP98–RARG fusion promotes leukemogenesis and confers ATRA resistance in this subset of AML patients.
Patient Statement
Written, informed consent for publication of the case details was obtained from the patient.
Abbreviations
allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; ASXL1, additional sex combs-like 1; ATO, arsenic trioxide; ATRA, all-trans retinoic acid; BM, bone marrow; CD, cluster of differentiation; FISH, fluorescence in situ hybridization; GCSF, granulocyte colony-stimulating factor; Hg, hemoglobin; IDH2, isocitrate dehydrogenase 2; NUP98, nucleoporin 98; RARA, retinoic acid receptor alpha; RARG, retinoic acid receptor gamma; RT-PCR, reverse transcription-polymerase chain reaction; TET2, ten-eleven translocation 2; TP53, tumor protein 53; WBC, white blood cell; WT1, Wilms’ tumor 1.
Funding
This work was supported by research grants from the Science and Technology Fund of Huai’an City (no. HAB201810) and Science and Technology Fund of Jiangsu Commission of Health (nos. H2018085 and H2019082).
Disclosure
The authors report no conflicts of interest in this work. Co-first authors: Shandong Tao, Lixiao Song.
References
1. Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392(10147):593–606. doi:10.1016/S0140-6736(18)31041-9
2. Szotkowski T, Faber E, Hubacek J, et al. Acute promyelocytic leukemia successfully treated also in elderly patients with significant comorbidities: a 20-year single-center experience. Neoplasma. 2015;62(01):146–151. doi:10.4149/neo_2015_019
3. Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–121. doi:10.1056/NEJMoa1300874
4. Sanz MA, Fenaux P, Tallman MS, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European leukemianet. Blood. 2019;133(15):1630–1643. doi:10.1182/blood-2019-01-894980
5. Conserva MR, Redavid I, Anelli L, et al. RARG gene dysregulation in acute myeloid leukemia. Front Mol Biosci. 2019;6:114.
6. Such E, Cervera J, Valencia A, et al. A novel NUP98/RARG gene fusion in acute myeloid leukemia resembling acute promyelocytic leukemia. Blood. 2011;117(1):242–245. doi:10.1182/blood-2010-06-291658
7. Ha JS, Do YR, Ki CS, et al. Identification of a novel PML-RARG fusion in acute promyelocytic leukemia. Leukemia. 2017;31(9):1992–1995. doi:10.1038/leu.2017.167
8. Miller CA, Tricarico C, Skidmore ZL, et al. A case of acute myeloid leukemia with promyelocytic features characterized by expression of a novel RARG-CPSF6 fusion. Blood Adv. 2018;2(11):1295–1299. doi:10.1182/bloodadvances.2017014183
9. Liu T, Wen L, Yuan H, et al. Identification of novel recurrent CPSF6-RARG fusions in acute myeloid leukemia resembling acute promyelocytic leukemia. Blood. 2018;131(16):1870–1873. doi:10.1182/blood-2017-11-818716
10. Qin YZ, Huang XJ, Zhu HH. Identification of a novel CPSF6-RARG fusion transcript in acute myeloid leukemia resembling acute promyelocytic leukemia. Leukemia. 2018;32(10):2285–2287. doi:10.1038/s41375-018-0095-z
11. Zhang X, Li F, Wang J, et al. RARγ-rearrangements resemble acute promyelocytic leukemia and benefit from 3 + 7 regimen. Leuk Lymphoma. 2019;60(7):1831–1834. doi:10.1080/10428194.2018.1553302
12. Luo H, Zhang S, Li K, et al. A novel entity of acute myeloid leukaemia with recurrent RARG-rearrangement resembling acute promyelocytic leukaemia. Leuk Res. 2019;77:14–16. doi:10.1016/j.leukres.2018.12.009
13. Chen X, Wang F, Zhang Y, et al. A novel NPM1-RARG-NPM1 chimeric fusion in acute myeloid leukaemia resembling acute promyelocytic leukaemia but resistant to all-trans retinoic acid and arsenic trioxide. Br J Cancer. 2019;120(11):1023–1025. doi:10.1038/s41416-019-0456-z
14. Qiu JJ, Zeisig BB, Li S, et al. Critical role of retinoid/rexinoid signaling in mediating transformation and therapeutic response of NUP98-RARG leukemia. Leukemia. 2015;29(5):1153–1162. doi:10.1038/leu.2014.334
15. Such E, Cordón L, Sempere A, et al. In vitro all-trans retinoic acid sensitivity of acute myeloid leukemia blasts with NUP98/RARG fusion gene. Ann Hematol. 2014;93(11):1931–1933. doi:10.1007/s00277-014-2073-5
16. Coccaro N, Zagaria A, Orsini P, et al. RARA and RARG gene downregulation associated with EZH2 mutation in acute promyelocytic-like morphology leukemia. Hum Pathol. 2018;80:82–86. doi:10.1016/j.humpath.2018.02.023
17. Zhang Z, Jiang M, Borthakur G, et al. Acute myeloid leukemia with a novel CPSF6-RARG variant is sensitive to homoharringtonine and cytarabine chemotherapy. Am J Hematol. 2020;95(2):E48–E51. doi:10.1002/ajh.25689
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.