Back to Journals » International Medical Case Reports Journal » Volume 14
Acute Myeloid Leukemia Transformation from Myelofibrosis Upon Remission of an Orbital Myeloid Sarcoma – A Case Report
Authors Chen N , Lin CS, Hsu YH, Huang WH, Huang CT, Lee YC
Received 9 March 2021
Accepted for publication 14 June 2021
Published 30 June 2021 Volume 2021:14 Pages 443—448
DOI https://doi.org/10.2147/IMCRJ.S310230
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Nancy Chen,1 Chia-Shuen Lin,2 Yung-Hsiang Hsu,3 Wei-Han Huang,4,5 Chin-Te Huang,6 Yuan-Chieh Lee1
1Department of Ophthalmology, Hualien Tzu Chi Hospital, Hualien, Taiwan; 2Department of Dermatology, Hualien Tzu Chi Hospital, Hualien, Taiwan; 3Department of Pathology, Hualien Tzu Chi Hospital, Hualien, Taiwan; 4Department of Clinical Pathology, Hualien Tzu Chi Hospital, Hualien, Taiwan; 5Department of Hematology and Oncology, Hualien Tzu Chi Hospital, Hualien, Taiwan; 6Department of Ophthalmology, Chung Shan Medical University Hospital, Taichung, Taiwan
Correspondence: Yuan-Chieh Lee
Department of Ophthalmology, Hualien Tzu Chi Hospital, Buddlist Tzu Chi Medical Foundation, 707 Sec. 3, Chung Yung Road, Hualien, 970, Taiwan
Tel +886 3 8561825 ext 12218
Fax +886 3 8577161
Email [email protected]
Purpose: To report a case of an orbital myeloid sarcoma concurrent with JAK2 mutation myelofibrosis, which rapidly transformed into acute myeloid leukemia upon aggressive treatment.
Results: A 51-year-old woman had progressive swelling of periorbita for one month. Magnetic resonance imaging demonstrated a well-defined, mild enhanced mass indenting the adjacent right lateral rectus muscle and the globe. Biopsy from anterior orbitotomy revealed an orbital myeloid sarcoma. Bone marrow study showed concurrent myelofibrosis. Although the orbital lesion subsided remarkably under aggressive chemotherapy and radiotherapy, the leukemic transformation was noticed in the third month following the initial presentation.
Conclusion: This case demonstrated that myeloid sarcoma should be included in the differential diagnosis of orbital diseases, with or without involvement of hematological disorders. Early diagnosis and aggressive treatment as for AML are crucial as the prognosis is usually poor for adult orbital MS.
Keywords: myeloid sarcoma, myelofibrosis, acute myeloid leukemia, JAK2 mutation
Introduction
Myeloid sarcoma, also known as chloroma or granulocytic sarcoma,1 has been found in many sites such as the lymph nodes, skin, bone, and soft tissue (including the orbit).2,3 It can also involve the retina or iridociliochoroid.4 It is characterized as extramedullary masses composed of immature myeloid precursors. It may develop de novo5,6 or concurrently with acute myeloid leukemia (AML),7,8 myeloproliferative neoplasm (MPN), or myelodysplastic syndrome (MDS).2,3,9 We herein present an adult with orbital myeloid sarcoma and concurrent JAK2-mutation myelofibrosis, which rapidly transformed into acute myeloid leukemia despite aggressive chemotherapy, irradiation, and JAK2 inhibitor treatment.
Case Presentation
A 51-year-old female patient came to our outpatient service due to progressive swelling of her right periorbita for one month. Except for microcytic anemia, she had no known systemic diseases previously. Upon ophthalmic examination, she had a painless, hard, non-movable mass occupying the inferior-lateral orbit (Figure 1). The abduction and upward gaze were limited in her right eye, but no extraocular movement pain. Her best-corrected visual acuity was 20/50 (−12.25/−3.75 x 83) in the right eye and 20/400 (−18.00/−3.50 × 150) due to an old rhegmatogenous retinal detachment in the left eye. There was also a palpable mass in the pre-auricular area of her right cheek.
 |
Figure 1 Periorbital swelling of the right eye without erythema or pain. |
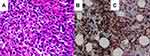
She had a white blood cell count of 5.71 × 103/μL (neutrophils: 56.9%, monocytes: 2.3%, and lymphocytes: 37.5%), a platelet count of 382 × 103/μL, and a hemoglobin of 9.7 g/dL, which were consistent with anemia. Magnetic resonance imaging demonstrated a well-defined, mild enhanced mass indenting the adjacent right lateral rectus muscle and the globe (Figures 2A and B). The lesion was iso-intense in the T1-weighted imaging and mild hyper-intense in the T2-weighted gadolinium study compared to the extraocular muscles. We performed an anterior orbitotomy and incisional biopsy via the fornix and found sclerosing, grayish lobulated tissue. The pathological examination showed medium to large myeloblast cells with round nuclei and prominent nucleoli infiltrating the adipose tissue (Figure 3A). Immunohistochemically, these neoplastic cells expressed CD34 (Figure 3B) and CD117 (Figure 3C), weak bcl-2 and CD79a, but no CD56 or myeloperoxidase. The proliferation index determined by Ki67 was 70%, which indicated a rapidly growing malignancy. The above findings suggested an orbital myeloid sarcoma. A bone marrow biopsy revealed myelofibrosis and hypocellular marrow with focal residual hematopoietic cells but no increased blast cells. Genomic DNA extracted from the bone marrow sample was further processed for an allele-specific polymerase chain reaction, which disclosed positive Val617Phe mutation of Janus Kinase 2 (JAK2) gene (Figure 4). Under the impression of orbital myeloid sarcoma with concurrent JAK2-mutation myelofibrosis, she received concurrent chemotherapy and radiotherapy (CCRT) with an I2A5 regimen containing Idarubicin 12 mg/m2 for two days, cytarabine 100 mg/m2 for five days, and local radiotherapy (20 Gy divided into ten fractions). After that, the patient was treated with ruxolitinib, a JAK2 inhibitor, 5 mg twice daily.
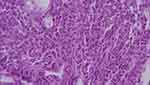
Three months after the initial presentation, the follow-up MRI displayed apparent resolving of right orbital myeloid sarcoma. However, peripheral blood film revealed 6.5% blast cells. Bone marrow biopsy showed hypercellular marrow (80% cellularity) with increased myeloid series and more than 20% blasts, which indicated an acute myeloid leukemia transformation (Figure 5). The patient received second-course chemotherapy with the same I2A5 regimen and ruxolitinib treatment and gained a hematological response. An allogeneic peripheral blood stem cell transplant from a sibling donor was scheduled. Unfortunately, she suffered from Aspergillus pneumonia after receiving initial conditioning chemotherapy with fludarabine, which progressed to sepsis and multiple organ failure. The patient passed away six months later from the initial presentation of the orbital myeloid sarcoma.
Discussion
Due to the wide variety of orbital diseases, the diagnosis of orbital lesions remained a significant challenge. Orbital masses are categorized into infection, inflammation, neoplasm, hemorrhage, metastasis, and developmental according to their pathological etiologies.10 Among the 1263 suspected orbital lesions in the study by Shields et al, 36% were malignant neoplasms with lymphoma as the most common in older patients.11 Our patient presented with swelling of periorbital tissue, eye movement limitation, and globe displacement without pain or erythema. The orbital imaging revealed well-defined mass compressing the lateral rectus muscle and the globe without infiltrating the adjacent structures, making the impression of primary neoplasm of sarcomatous origin.6,12 However, other neoplasm or inflammatory processes could not be entirely ruled out before the biopsy.10
Myeloid sarcoma (MS) of orbit, presenting as malignant myeloid precursor cells invading orbital soft tissue,13 might be the first manifestation of AML14 or signify a relapse. It often involves bones and extends into the orbital fossa.15 The common characteristics of orbital MS were proptosis, chemosis, orbital mass, and lid edema.14,16,17 Ocular MS in adult AML patients was 10 out of 3,724 (0.3%) in the study,17 whereas Cavdar et al reported 33 orbito-ocular MS in 133 Turkish children with AML.18 The Children’s Oncology Group concluded that patients with MS involving orbital and central nervous system (CNS) sites had significantly better survival than patients with non-CNS MS, with CSF leukemia, or without extramedullary leukemia.19 In contrast, orbital MS in adults is uncommon and generally carries a poor prognosis, despite early aggressive intervention.20
As for isolated MS, Dores et al reported an age-adjusted incidence rate of 0.3 per 1,000,000 person-years with a median age of 59 years, which accounted for 0.9% of all AML,21 while Lee et al reported 1.8% in all AML patients.5 The presenting symptom of the isolated MS depended on the involving sites of the body.5 MS could occur as the first manifestation of AML, concurrent with AML, or appear as the sign of a relapse after complete remission.9 It could remain as isolated MS without bone marrow disease involvement, or the MS might follow MPN and MDS.5,9 Historically, the prognosis of MS cases was considered poor, and the MS would eventually evolve into AML within 6 to 12 months if left untreated.22 However, an analysis of 345 isolated MS patients, aged 15 or older, revealed that the 3-year survival rate for MS was greater than for non-MS AML; the survival rates for isolated MS involving the pelvis/genitourinary organs, eyes/gonads, and gastrointestinal mucosa appeared to be slightly better than those involving soft tissues, lymphatic/hematopoietic tissues, or nervous system.23 In a recent analysis of 131 MS patients, there was no statistical difference in prognosis between de novo MS and MS with concomitant AML. However, one-year survival rates in de novo MS, MS with concomitant AML, and MS following MDS/MPN were 60.0%, 50.1%, and 14.3%, respectively. The underlying MDS or MPN was a poor prognostic factor.24 Yoshiki et al reported a patient with concurrent MS and myelofibrosis complicated with JAK2 V617F mutation-positive AML that relapsed rapidly.25 Although increased JAK2 V617F-mutant allele burden was associated with the risk of developing myelofibrosis, there were no definite conclusions about whether this mutation increases the risk of evolving to AML.26
MS, especially the de novo ones, were not only challenging in the clinical manifestations but also ambiguous in the pathological diagnosis. Previous MS studies had demonstrated that a proportion of cases were initially incorrectly diagnosed, mostly as malignant lymphoma, and were hence treated unsuccessfully.2,3,27 Therefore, immunohistochemical staining played a crucial role in establishing the definite diagnosis of MS. In the study by Pileri et al,3 CD68/KP1 was the most expressed marker, followed by myeloperoxidase, CD117, CD99, CD68/PG-M1, and CD34. In our case, pathological studies showed monotonous atypical blasts with positive CD117 and CD34 expression, which were diagnostic of myeloid sarcoma.
Intervals between the presentation of isolated MS and the development of systemic leukemia varied from one to forty-nine months in the study by Neiman et al,2 whereas the evolving interval was 2.4–20.1 months in the study by Lee et al.5 In the survey by Aggarwal et al,7 13 out of 18 patients with isolated orbital MS evolved to AML within 3 to 21 months with the median of 11.3 months. In the study by Tsimberidou et al,28 isolated MS patients treated with anti-AML chemotherapy (cytarabine plus idarubicin or fludarabine) had a superior complete remission rate and event-free survival than the matched AML patients. In the literature review comparing 72 isolated MS cases receiving surgical resection, irradiation, and systemic chemotherapy, the results revealed a more extended non-leukemic period in the chemotherapy group.29 Hence, the same aggressive chemotherapy regimen as for the ones associated with systemic AML is recommended for isolated MS cases.28 Among 678 pediatric AML patient data obtained from the Japanese Data Center for Hematopoietic Cell Transplantation, the presence of concurrent MS did not influence the transplant outcome.30 Allogeneic hematopoietic stem cell transplantation, which significantly improved the overall survival in a cohort of MS patients with or without concomitant AML, is nowadays regarded as the treatment of choice.31 Owing to the accumulating data in cytogenetics, target therapy could aid in the treatment according to the stratified molecular features. Ruxolitinib, a JAK1/JAK2 inhibitor, and fedratinib, a JAK2/FLT3 inhibitor, could treat myelofibrosis patients regardless of the presence of mutated JAK2. Although the JAK2 mutation allele burden was proposed as the predilection for blast transformation from myelofibrosis,10,12 there was insufficient evidence to implement anti-JAK2 medication into the treatment of JAK2 mutant-positive MS. Nevertheless, the integration of FLT3 inhibitors into the treatment algorithm had improved the clinical outcome in patients with FLT3-ITD-mutated AML.32 In the case report of Kim et al, gilteritinib, a type 1 tyrosine kinase inhibitor, was used to treat an FLT3-ITD-mutated AML patient relapse with iridociliochoroidal MS and achieved lesion regression.4
Conclusion
Although orbital MS is rare in orbital diseases among adults, clinicians should keep a high index of suspicion with or without involvement of hematological disorders. Our patient had concurrent orbital MS and JAK2 mutation myelofibrosis. Her orbital lesion regressed remarkably soon after the CCRT and JAK2 inhibitor ruxolitinib, but the myelofibrosis transformed into AML simultaneously. Early diagnosis and aggressive treatment as for AML are crucial as the prognosis is usually poor for adult orbital MS. Multi-disciplinary efforts of ophthalmologists, pathologists, and hematological specialists are mandatory for accurate diagnosis and appropriate management.
Declaration
The informed consent for publication (case details and accompanying images) was obtained from the patient’s family. Institutional approval was not required to publish the case reports.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stockl FA, Dolmetsch AM, Saornil MA, Font RL, Burnier MN. Orbital granulocytic sarcoma. Br J Ophthalmol. 1997;81(12):1084. doi:10.1136/bjo.81.12.1084
2. Neiman RS, Barcos M, Berard C, et al. Granulocytic sarcoma: a clinicopathologic study of 61 biopsied cases. Cancer. 1981;48(6):1426–1437. doi:10.1002/1097-0142(19810915)48:6<1426::AID-CNCR2820480626>3.0.CO;2-G
3. Pileri SA, Ascani S, Cox MC, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21(2):340–350. doi:10.1038/sj.leu.2404491
4. Kim RS, Yaghy A, Wilde LR, Shields CL. An Iridociliochoroidal myeloid sarcoma associated with relapsed acute myeloid leukemia with FLT3-ITD mutation, treated with gilteritinib, an FLT3 inhibitor. JAMA Ophthalmol. 2020;138(4):418–419. doi:10.1001/jamaophthalmol.2020.0110
5. Lee JY, Chung H, Cho H, et al. Clinical characteristics and treatment outcomes of isolated myeloid sarcoma without bone marrow involvement: a single-institution experience. Blood Res. 2017;52(3):184–192. doi:10.5045/br.2017.52.3.184
6. AlSemari MA, Perrotta M, Russo C, et al. Orbital myeloid sarcoma (chloroma): report of 2 cases and literature review. Am J Ophthalmol Case Rep. 2020;19:100806. doi:10.1016/j.ajoc.2020.100806
7. Aggarwal E, Mulay K, Honavar SG. Orbital extramedullary granulocytic sarcoma: clinicopathologic correlation with immunohistochemical features. Surv Ophthalmol. 2014;59(2):232–235. doi:10.1016/j.survophthal.2013.06.004
8. Ple-plakon PA, Demirci H, Cheng JX, Elner VM. Orbital myeloid sarcoma in an adult with acute myeloid leukemia, FAB M1, and 12p-deletion. Ophthalmic Plast Reconstr Surg. 2013;29(3):e73–e75. doi:10.1097/IOP.0b013e318272d497
9. Campidelli C, Agostinelli C, Stitson R, Pileri SA. Myeloid sarcoma: extramedullary manifestation of myeloid disorders. Am J Clin Pathol. 2009;132(3):426–437. doi:10.1309/AJCP1ZA7HYZKAZHS
10. Mombaerts I, Ramberg I, Coupland SE, Heegaard S. Diagnosis of orbital mass lesions: clinical, radiological, and pathological recommendations. Surv Ophthalmol. 2019;64(6):741–756. doi:10.1016/j.survophthal.2019.06.006
11. Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: the 2002 Montgomery Lecture, part 1. Ophthalmology. 2004;111(5):997–1008. doi:10.1016/j.ophtha.2003.01.002
12. Ooi GC, Chim CS, Khong PL, et al. Radiologic manifestations of granulocytic sarcoma in adult leukemia. AJR Am J Roentgenol. 2001;176(6):1427–1431. doi:10.2214/ajr.176.6.1761427
13. Zimmerman LE, Font RL. Ophthalmologic manifestations of granulocytic sarcoma (myeloid sarcoma or chloroma): the third Pan American Association of Ophthalmology and American Journal of Ophthalmology Lecture. Am J Ophthalmol. 1975;80(6):975–990. doi:10.1016/0002-9394(75)90326-8
14. Almalki AMJ, Alotaibi FA, Jabr HF, Mastan AR. Unilateral proptosis as an initial sign of acute myeloid leukemia in a child: a case report. Int Med Case Rep J. 2019;12:319–323. doi:10.2147/IMCRJ.S206596
15. Shields JA, Shields CL. Atlas of orbital tumors. Philadelphia, PA: Lippincott Wolters Kluwer; 1999.
16. Maka E, Lukats O, Toth J, Fekete S. Orbital tumour as initial manifestation of acute myeloid leukemia: granulocytic sarcoma: case report. Pathol Oncol Res. 2008;14(2):209–211. doi:10.1007/s12253-008-9028-x
17. Ohanian M, Pemmaraju N, Rozovski U, et al. Ocular extramedullary myeloid leukaemia. Br J Haematol. 2018;180(5):738–740. doi:10.1111/bjh.14430
18. Cavdar AO, Babacan E, Gözdaşoğlu S, et al. High risk subgroup of acute myelomonocytic leukemia (AMML) with orbito-ocular granulocytic sarcoma (OOGS) in Turkish children. Retrospective analysis of clinical, hematological, ultrastructural and therapeutical findings of thirty-three OOGS. Acta Haematol. 1989;81(2):80–85. doi:10.1159/000205531
19. Johnston DL, Alonzo TA, Gerbing RB, Lange BJ, Woods WG. Superior outcome of pediatric acute myeloid leukemia patients with orbital and CNS myeloid sarcoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2012;58(4):519–524. doi:10.1002/pbc.23201
20. Mangla D, Dewan M, Meyer DR. Adult orbital myeloid sarcoma (granulocytic sarcoma): two cases and review of the literature. Orbit. 2012;31(6):438–440. doi:10.3109/01676830.2012.723784
21. Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood. 2012;119(1):34–43. doi:10.1182/blood-2011-04-347872
22. Goyal G, Bartley AC, Patnaik MM, Litzow MR, Al-Kali A, Go RS. Clinical features and outcomes of extramedullary myeloid sarcoma in the United States: analysis using a national data set. Blood Cancer J. 2017;7(8):e592. doi:10.1038/bcj.2017.79
23. Movassaghian M, Brunner AM, Blonquist TM, et al. Presentation and outcomes among patients with isolated myeloid sarcoma: a surveillance, epidemiology, and end results database analysis. Leuk Lymphoma. 2015;56(6):1698–1703. doi:10.3109/10428194.2014.963080
24. Kawamoto K, Miyoshi H, Yoshida N, Takizawa J, Sone H, Ohshima K. Clinicopathological, cytogenetic, and prognostic analysis of 131 myeloid sarcoma patients. Am J Surg Pathol. 2016;40(11):1473–1483. doi:10.1097/PAS.0000000000000727
25. Yoshiki Y, Asai T, Ichikawa M, et al. A case of myeloid sarcoma with correlation to JAK2V617F mutation, complicated by myelofibrosis and secondary acute myeloid leukemia. Intern Med. 2011;50(21):2649–2652. doi:10.2169/internalmedicine.50.5783
26. Kiladjian JJ. The spectrum of JAK2-positive myeloproliferative neoplasms. Hematology Am Soc Hematol Educ Program. 2012;2012:561–566. doi:10.1182/asheducation.V2012.1.561.3807838
27. Breccia M, Mandelli F, Petti MC, et al. Clinico-pathological characteristics of myeloid sarcoma at diagnosis and during follow-up: report of 12 cases from a single institution. Leuk Res. 2004;28(11):1165–1169. doi:10.1016/j.leukres.2004.01.022
28. Tsimberidou AM, Kantarjian HM, Wen S, et al. Myeloid sarcoma is associated with superior event-free survival and overall survival compared with acute myeloid leukemia. Cancer. 2008;113(6):1370–1378. doi:10.1002/cncr.23691
29. Yamauchi K, Yasuda M. Comparison in treatments of nonleukemic granulocytic sarcoma: report of two cases and a review of 72 cases in the literature. Cancer. 2002;94(6):1739–1746. doi:10.1002/cncr.10399
30. Sakaguchi H, Miyamura T, Tomizawa D, et al. Clinical impact of extramedullary disease on allogeneic hematopoietic cell transplantation in pediatric acute myeloid leukemia: a nationwide retrospective study. Biol Blood Marrow Transplant. 2019;25(3):S105. doi:10.1016/j.bbmt.2018.12.377
31. Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2(5):309–316. doi:10.1177/2040620711410774
32. Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33(2):299–312. doi:10.1038/s41375-018-0357-9
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.