Back to Journals » Journal of Blood Medicine » Volume 13
Acute Malaria in Malawian Children and Adults is Characterized by Thrombocytopenia That Normalizes in Convalescence
Authors Mandala W , Munyenyembe A, Sulani I , Soko M, Mallewa J, Hiestand J
Received 3 June 2022
Accepted for publication 23 August 2022
Published 5 September 2022 Volume 2022:13 Pages 485—494
DOI https://doi.org/10.2147/JBM.S376476
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Wilson Mandala,1– 3 Alinane Munyenyembe,2 Innocent Sulani,2 Monica Soko,2 Jane Mallewa,4 Jasmin Hiestand4
1Basic Sciences Department, Kamuzu University of Health Sciences, Blantyre, Malawi; 2Malawi Liverpool Wellcome Trust Clinical Research Programme, Blantyre, Malawi; 3Academy of Medical Sciences, Malawi University of Science and Technology, Thyolo, Malawi; 4Medicine Department, Kamuzu University of Health Sciences, Blantyre, Malawi
Correspondence: Wilson Mandala, Tel +265 888858454, Email [email protected]
Background: Plasmodium falciparum malaria has been linked with significant perturbations of the peripheral cell-mediated immune system during acute phase. Some of these changes include lower than normal platelet counts. Although the exact mechanisms that drive thrombocytopenia in P. falciparum malaria are not fully known, a number of hypotheses have been proposed. We conducted two sets of studies with one aimed at determining platelet counts in Malawian children, and the other in adults during acute P. falciparum malaria and a month post treatment.
Materials and Methods: We recruited a total of 113 HIV-uninfected children with acute malaria [n=54 with uncomplicated malaria (UCM), n=30 with severe malarial anemia (SMA), n=29 presenting with cerebral malaria (CM)]. We also recruited 42 HIV-uninfected healthy controls. Out of the 113 participants with malaria, 73 (65%) [n=34 (63%) UCM, n=21 (70%) SMA and n=18 (62%) CM] were successfully followed-up one month after treatment. A 5mL peripheral blood sample was collected for platelet count using HMX Haematological Analyzer analysis both at baseline (acute malaria) and at follow-up a month later. Platelet counts were also determined in blood samples of 106 HIV-uninfected adults, 47 of whom presented with UCM and 29 with severe malaria (SM) and these counts were compared to those of 30 healthy controls. Of the malaria cases, platelet counts for 44 UCM and 21 SM were determined again during follow-up a month after treatment.
Results: In both children and adults, platelet counts were significantly lower during acute disease compared to the levels in the healthy controls with the lowest levels observed in CM (children) or SM (adults). These lower than normal levels increased close to normal levels a month post treatment.
Conclusion: P. falciparum malaria in Malawian children and adults was characterized by profound thrombocytopenia which recovered during convalescence.
Keywords: acute malaria, platelet counts, children and adults, convalescence
Introduction
Although remarkable achievements have been made in reducing the devastating effect of malaria globally,1,2 each year P. falciparum malaria still causes deaths of thousands of children globally, with Sub-Saharan Africa accounting for most of these deaths.3 Clinical P. falciparum malaria can arbitrarily be divided into the following main categories: uncomplicated malaria (UCM), cerebral malaria (CM), severe malarial anemia (SMA), and respiratory distress (RD), with the last three considered severe forms of the disease.4,5 Although these four groups can be detected in isolation, some children present with malaria characterized by combined features of any two or more of these syndromes and other complications.4,5 Of these four groups, CM is linked with the poorest clinical outcome with mortality observed to be highest in this group, and with some CM cases who survive suffering severe complications4 such as severe neurological sequelae and debilitating neurological impairments.6 However, the actual mechanistic basis of death in CM is yet to be fully elucidated.
Various studies have shown that P. falciparum malaria is characterized by profound perturbations of the cell-mediated immune system during acute stage,7–9 and some of the changes include significantly lower than normal peripheral platelet counts10–12. Although the exact mechanisms that drive thrombocytopenia in P. falciparum malaria are not well characterized, several hypotheses have been proposed and these include increased platelet aggregation,13 microvascular sequestration,14 and endothelial activation.13,14
A number of previous studies conducted in either children or adults presenting with acute malaria, have characterized either P. falciparum malaria or P. vivax malaria and a combination of these two with thrombocytopenia, with some15–17 even proposing that thrombocytopenia should either be used as an indicator of malaria in adults15 or be considered as one of the parameters to be used in classifying the severity of P. falciparum malaria disease.18 We conducted two sets of studies, one recruiting Malawian children and another adults, with the aim of determining absolute platelet counts at the time when they were presenting with acute P. falciparum malaria disease of different clinical types, and secondly during convalescence a month after treatment.
Materials and Methods
Study Participants
Pediatric Cohort
Study design and details of the study participants for this pediatric cohort have been reported previously.7 In summary, the study was conducted at Malawi-Liverpool-Wellcome Trust Clinical Research Programme (MLW) and Department of Paediatrics, College of Medicine (CoM), University of Malawi (UNIMA), and Blantyre Malaria Project from November 2005 to April 2006. We recruited children who acutely presented with different clinical forms of malaria at Queen Elizabeth Central Hospital (QECH) either in the outpatient department (OPD) or short-stay ward or in children's malaria admission ward. Medically well children who were attending outpatient surgical clinics at QECH and Beit Cure International Hospital (BCIH), in Blantyre, Malawi, were also recruited. Recruitment of study participants was mainly done during the malaria season which in Malawi usually coincides with the rainy season (November/December of one year to March/April of the next year).
Firstly, for each potential study participant, informed written consent was obtained from the parent or guardian, after which they were examined by a research nurse and/or a clinical officer. Once the potential participant fulfilled the preset recruitment criteria for the study, baseline demographic data were collected and recorded and a 5mL peripheral blood sample was collected. All malaria participants, regardless of the clinical form of malaria, were then assessed for level of consciousness using the Blantyre Coma Score (BCS). The consciousness assessment was done several times for each participant starting from admission and followed by two to four hourly intervals during intensive clinical care. Although in total over forty children were prospectively enrolled into each of the four groups (UCM, SMA, CM and Healthy Controls), the data for some participants from each group were excluded from the final analysis either because they tested positive for HIV, or were asymptomatically positive for malaria when included in the healthy control group, or the parents or guardians withdrew their consent after the child had already been recruited into the study.
For the purpose of this study and the other one that has already been reported7 we defined malaria as a clinical syndrome associated with febrile condition without any other apparent possible cause, which was confirmed by a thick blood film positive for P. falciparum asexual parasites on microscopy. Study participants who presented with CM had a BCS of two or less at admission and four hours later, while study participants for SMA and UCM and healthy controls had a score of five both times.7 SMA study participants had a blood hemoglobin concentration of 5 g/dl or less whereas study participants in UCM, CM and Healthy controls had a hemoglobin concentration above 5 g/dL.
All participants who tested positive for HIV infection during the screening stage were excluded from the study in order to eliminate HIV infection as a possible confounder and were immediately referred to the antiretroviral therapy clinic. Following exclusion of some study participants either because they were infected with HIV or due to detection of other infections that could be confounders, data from 113 children with malaria (54 with UCM, 30 with SMA, 29 with CM) and from 42 healthy controls were considered for analysis for this study. Out of the 113 children originally recruited with malaria, 73 (34 UCM, 21 SMA and 18 CM) were successfully followed-up a month after treatment and during the follow-up stage baseline demographic data were recorded and another 5mL peripheral blood sample was collected for analysis of platelet counts. Demographic data for all study participants whose data were used in this study are presented in Table 1.
 |
Table 1 Demographic Details, Parasitemia Levels and Platelet Counts for the Study Participants of the Pediatric Cohort. The Presented Values are Medians with the Ranges Provided in Brackets |
Adult Cohort
Similarly, demographic details for the adult cohort have previously been reported.19 Malawian adults diagnosed with either uncomplicated malaria (UCM) or severe malaria (SM) were prospectively recruited to the study after obtaining consent from the patient or a legally able guardian. Recruitment was done at the Accidents, Emergency and Trauma (AET) Centre and general medical male and female wards of QECH from July 2016 to March 2017. Of the screened 116 adults who were infected with malaria, 107 consented to participate in the study. Demographic and other related data were collected and recorded using Case Report Forms (CRFs). After recruitment, a 10mL venous blood sample was collected from each participant with a 2mL aliquot collected in EDTA tubes which was then used for confirmation of malaria parasitemia by means of thick and thin films and for the analysis of platelet counts.
For this cohort, we defined malaria as a clinical syndrome in a febrile adult who had no other possible apparent cause of disease, later confirmed by a positive malaria rapid diagnostic test (MRDT) and a positive thick blood film for P. falciparum asexual parasites using microscopy. The confirmed positive malaria cases were then divided into two clinical groups; UCM and SM. The UCM group had confirmed clinical malaria based on positive malaria slides and MRDT but did not have any unarousable coma, or lower than normal hemoglobin (Hb) levels or liver or kidney complications associated with the malaria infection. Among the SM cases were those who presented with cerebral malaria (CM), severe malarial anemia (SMA) and those with liver or renal dysfunction. In line with the WHO guidelines, we defined adult CM cases as those with a Glasgow Coma Score (GCS) of <11, with unarousable coma which could not be attributable to any other cause, and who were unable to localize stimuli and were incomprehensible to sounds. In contrast, malaria infected participants presenting with UCM or other forms of SM had a GCS score of greater than 11 at both times of consciousness assessment.
Although study participants presenting with severe SMA were included among the SM group, these had a blood Hb concentration of 7 g/dL or less or a hematocrit concentration of 20% or less together with a parasite count of more than 10,000/L, whereas the other clinical groups had an Hb concentration above this level. All malaria infected study participants were clinically and neurologically monitored during the entire period they were in the admission wards until the day of discharge or death.
Study participants were aged 18 years or more and were either out- or in-patients at QECH presenting with a temperature >37.5°C, a positive MRDT with a positive thick and thin blood smear microscopy test and with signs consistent with malaria disease according to the WHO guidelines.20 HIV-infected individuals, pregnant women, and all those who did not give consent were excluded from the study. The demographic details for the cohort are provided in Table 2. In summary, of the total 106 HIV-uninfected study participants recruited, 47 (62%) presented with UCM and 29 (38%) presented with SM, and 30 were healthy controls. Of the malaria cases, 44 UCM and 21 SM were seen again during convalescence a month after treatment.
 |
Table 2 Demographic Details of the Adult Study Participants and the Median Platelet Counts for Each Malaria Group During Acute Malaria and in Convalescence a Month After Treatment |
Investigations
Primary and confirmatory HIV tests were done using two rapid test kits; Determine (Abbott Laboratories, Japan) and Unigold (Trinity Brotch, Dublin). For confirmation of malaria parasites, both thick and thin blood smears were prepared by standard methods and screened on standard microscopy. Platelet counts were determined using a HMX Haematological Analyzer (Coulter, USA) using a blood sample collected in the EDTA tube. The HMX Haematological Analyzer performs a complete blood count and the analysis for platelet counts was conducted in accordance with a provided standard protocol and manufacturer’s instructions. All analyses were done by an experienced Laboratory Technologist who had been certified to operate this automated Haematological Analyzer. In summary, an aliquot of the EDTA blood sample was sucked into the Haematological Analyzer and once the analysis was complete for each sample, results were provided in a print-out. On each day, a standard sample was run first before the analysis of samples from patients. The analysis for some samples was repeated in the event of platelet counts that would be regarded as outliers (either too low or too high) based on the manufacturers’ standard protocol.
Ethics Review and Approval
For both the pediatric and adult studies, ethics review and approval of the study protocols were conducted by the College of Medicine Research and Ethics Committee (COMREC) of Kamuzu University of Health Sciences (KUHeS) previously known as College of Medicine. For the pediatric cohort, written informed consent was obtained from the parent or guardian of every child before the child was enrolled into the study. A 5mL venous blood sample was taken at the time of recruitment and a month after treatment. An aliquot of this blood sample was collected in EDTA tubes and it was this sample which was used for the determination of the platelet counts. For the adult study, each participant or an appropriate guardian provided informed written consent. From the 10mL venous blood sample that was collected from each participant, a 2mL EDTA aliquot was used for confirmation of malaria parasitemia using thick and thin films and for platelet count determination. Both the pediatric and adult studies were conducted in accordance with the Declaration of Helsinki.
Data Analysis
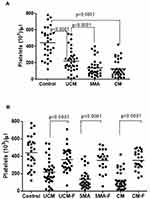
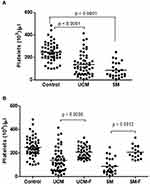
For statistical analysis, the data from the various clinical malaria groups (Healthy Controls, UCM, SMA and CM for children and healthy controls, UCM and SM for adults) were statistically analyzed using GraphPad Prism version 6.01 for Windows (GraphPad Software, San Diego California USA). For all statistical comparisons, a p value equal to 0.05 or less than 0.05 was considered statistically significant at 95% level confidence. The Kruskal–Wallis test was employed in the comparison of absolute platelet counts. Wilcoxon-matched pairs test was used to establish if the differences in platelet counts observed during acute infection and in convalescence were statistically significant for both pediatric and adult cohorts. The results in Figures 1 and 2 (and Tables 1 and 2) are medians and 10th and 90th percentiles of absolute platelet counts during acute and convalescent stages (except healthy controls) for the different study groups. For these scatter plots presented in Figures 1 and 2, the b line through the middle of the dots corresponds to the median value with the 10th percentile presented at the bottom of the scatter plot and the 90th percentile at the top).
Results
Variation of Platelet Counts in Different Malaria Types in Children
We have previously shown that the median platelet counts for children aged between 1–6 months and 3 to 7 years were 346×103 cells/L and 447×103 cells/L respectively,21 which compared well with the median platelet counts for healthy controls of the same age group of 441×103 cells/L observed in the pediatric cohort of this study. Platelet counts were markedly lower in all malaria groups during acute infection compared to the levels in controls (Figure 1A and Table 1). The counts were significantly (p<0.0001) lower in UCM (199x103 cells/L), SMA patients (98x103 cells/L) and in CM patients (85.5x103 cells/L) compared to the controls (441x103 cells/L). There was no association between parasitemia levels (median values of 52,300 parasites/L of blood in UCM, 3500 parasites/L of blood in SMA and 41,800 parasites/L of blood in CM) with the platelet counts for the three groups (199x103 cells/L for UCM, 98×103 cells/L for SMA and 85.5×103 cells/L for CM) (Table 1).
Platelet counts were observed to be significantly (p<0.0001) higher in convalescence than the values observed during acute disease for all three clinical malaria groups (328x103 cells/L versus 199×103 cells/L for UCM, 387×103 cells/L versus 98×103 cells/L for SMA and 343×103 cells/L versus 85.5×103 cells/L for CM) (Figure 1B and Table 1).
Variation of Platelet Counts in Different Malaria Types in Adults
Similar to the child cohort, we have also previously shown that in Malawian adults aged between 20 and >60 years, the median platelet counts ranged between 214×103 cells/L and 253×103 cells/L21 which also compared reasonably well with the median platelet counts for the healthy controls in this study (234x103 cells/L). Therefore, when compared to the counts in healthy children (441x103 cells/L), healthy adults had significantly (p<0.0001) lower platelet counts (234x103 cells/L). Within the adult cohort, the absolute counts of these cells were significantly (p<0.0001) lower in patients presenting with acute UCM (113x103 cells/L) and in those presenting with acute SM (60.5x103 cells/L) compared to those observed in healthy controls (234x103 cells/L) (Figure 2A and Table 2).
As was the case with the children, platelet counts were significantly (p=0.0006 for UCM versus UCM-F and p=0.0012 for SM versus SM-F) higher during convalescence compared to the values observed during acute disease for both UCM and SM (200x103 cells/L versus 113×103 cells/L for UCM and 195×103 cells/L versus 60.5×103 cells/L for SM) (Figure 2B and Table 2).
Discussion
Thrombocytopenia, defined as a platelet count of less than 150x103cells/L, is a recognized characteristic of P. falciparum malaria in adults22 which, just as our results showed, has been reported to be common in malaria infected children23 and in adults.15 Some have previously reported that platelet counts of less than 100x103cells/L were linked to a higher risk of death in a pediatric Senegalese CM cohort.24 Although the mechanisms that may contribute to thrombocytopenia are not clear, sequestration of platelets and other factors in cerebral microvasculature have been linked with death in Malawian children.14
Furthermore, it is now known that platelets can adhere to endothelial cells by means of various molecules such as CD36. In addition to various endothelial cells and monocytes, human red blood cells which express CD36 are capable of binding to platelets. As such, CD36 could act as a receptor for some parasitized red blood cells (PRBCs) and could therefore indirectly play an important role in platelet and leucocyte sequestration in blood vessels in the brain.14 Platelets are also known to play a role in facilitating the clumping of PRBCs, a feature which is also known to characterize severe malaria.25
This study’s main finding, for both children and adults, showing that the number of platelet counts significantly increased in convalescence suggests that the malaria-associated thrombocytopenia is a transient phenomenon. Previous studies investigating P. vivax malaria26 and P. falciparum malaria27 have made a similar observation with the latter study showing that the lower than normal platelet counts observed during acute malaria increased significantly in both UCM and SM, attaining normal levels within seven days post treatment. This rapid recovery therefore suggests that whether the platelets are contributing toward the pathogenesis of severe malaria14 or are involved in parasite clearance28 once the malaria infection is cleared, there is a rapid re-emergence or formation of new platelets.
These two sets of studies of ours had a few limitations, the main one being the time difference between when the pediatric study was conducted (2005/2006) and when we recruited the adult cohort (2016/2017). Secondly, overall the sample sizes for both the child and adult cohorts were not as large as was the case in other previous studies. In addition, although we were able to divide the pediatric cohort (aged between 5 months and seven years) into three different clinical malaria groups (UCM, SMA and CM), for adults we only had two clinical groups (UCM and SM with the SM group combining SMA, CM and other severe manifestations of adult malaria). Furthermore, although we were able to confirm positivity for P. falciparum for both the pediatric and adult cohorts, we did not test for P. vivax malaria.
Although the overall prevalence of P. vivax malaria in Malawi is very low, with one recent study reporting its prevalence to be as low as 0.1% in adolescents and adults across the nation,29 it is still possible that some of the adults might have been co-infected by both P. falciparum malaria parasites and P. vivax parasites at the time of recruitment. Lastly, although pseudothrombocytopenia is now recognized to be a common phenomenon in the field of blood analysis of samples collected from individuals presenting with various infectious diseases including malaria,30 we did not account for this in our analyses. Nevertheless, we feel that the inclusion of analyzing samples from healthy controls adequately addressed this problem.
Conclusion
Thrombocytopenia is now a well-known and extensively studied characteristic of severe P. falciparum and P. vivax malaria that manifests during acute disease.31–33 In this report, we have shown that the thrombocytopenia that had previously been reported in different clinical forms of severe P. falciparum malaria in subjects of different age groups in other countries where malaria is endemic, also occurs in Malawians of different ages during acute malaria infection but disappears during convalescence a month post treatment.
Acknowledgments
The authors would like to thank the children who took part in this study and their respective parents or relatives for allowing them to participate. We also thank all adult participants for consenting to take part in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Funding for the pediatric study was obtained from the Gates Malaria Partnership, which was financially supported by the Bill and Melinda Gates Foundation [Grant Number: OPP51941] whereas the adult study was a Pre-MSc grant offered by Malawi-Liverpool Wellcome Trust Clinical Programme (MLW) which is financially supported by the Wellcome Trust, UK.
Disclosure
All authors for this study declare no conflicts of interest.
References
1. Murray CJ, Ortblad KF, Guinovart C, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384(9947):1005–1070. doi:10.1016/S0140-6736(14)60844-8
2. Gething PW, Battle KE, Bhatt S, et al. Declining malaria in Africa: improving the measurement of progress. Malar J. 2014;13:39. doi:10.1186/1475-2875-13-39
3. World Malaria Report 2020. 20 years of global progress and challenges; 2020. Available from: http://www.who.int/malaria/publications/world-malaria-report-2020/report/en/.
4. Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi:10.1056/NEJM199505253322102
5. Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–732. doi:10.1038/ni.f.205
6. Newton CR, Peshu N, Kendall B, et al. Brain swelling and ischaemia in Kenyans with cerebral malaria. Arch Dis Child. 1994;70(4):281–287. doi:10.1136/adc.70.4.281
7. Mandala WL, Msefula CL, Gondwe EN, et al. Lymphocyte perturbations in malawian children with severe and uncomplicated malaria. Clin Vaccine Immunol. 2016;23:95–103. doi:10.1128/CVI.00564-15
8. Pradhan V, Ghosh K. Immunological disturbances associated with malarial infection. J Parasit Dis. 2013;37(1):11–15. doi:10.1007/s12639-012-0174-4
9. Hviid L, Kurtzhals JA, Goka BQ, Oliver-Commey JO, Nkrumah FK, Theander TG. Rapid reemergence of T cells into peripheral circulation following treatment of severe and uncomplicated Plasmodium falciparum malaria. Infect Immun. 1997;65:4090–4093. doi:10.1128/iai.65.10.4090-4093.1997
10. Gupta NK, Bansal SB, Jain UC, Sahare K. Study of thrombocytopenia in patients of malaria. Tropical Parasitol. 2013;3(1):58–61. doi:10.4103/2229-5070.113914
11. Lacerda MVG, Mourão MPG, Coelho HCC, Santos JB. Thrombocytopenia in malaria: who cares? Mem Inst Oswaldo Cruz. 2011;106(Suppl 1):52–63. doi:10.1590/S0074-02762011000900007
12. Patel U, Gandhi G, Friedman S, Niranjan S. Thrombocytopenia in malaria. J Natl Med Assoc. 2004;96(9):1212–1214.
13. De Mast Q, De Groot PG, Van Heerde WL, et al. Thrombocytopenia in early malaria is associated with GP1b shedding in absence of systemic platelet activation and consumptive coagulopathy. Brit J Haematol. 2010;151(5):495–503. doi:10.1111/j.1365-2141.2010.08399.x
14. Grau GE, Mackenzie CD, Carr RA, et al. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J Infect Dis. 2003;187:461–466. doi:10.1086/367960
15. Khan SJ, Abbass Y, Marwat MA. Thrombocytopenia as an indicator of malaria in adult population. Malar Res Treat. 2012;2012:405981. doi:10.1155/2012/405981
16. Gebreweld A, Erkihun Y, Feleke DG, Hailu G, Fiseha T. Thrombocytopenia as a diagnostic marker for malaria in patients with acute febrile illness. J Trop Med. 2021;2021:5585272. doi:10.1155/2021/5585272
17. Lampah DA, Yeo TW, Malloy M, et al. Severe malarial thrombocytopenia: a risk factor for mortality in Papua, Indonesia. J Infect Dis. 2011;211(4):623–634. doi:10.1093/infdis/jiu487
18. Hanson J, Phu NH, Hasan MU, et al. The clinical implications of thrombocytopenia in adults with severe falciparum malaria: a retrospective analysis. BMC Med. 2015;13:97. doi:10.1186/s12916-015-0324-5
19. Munyenyembe AU, Gausi K, Nyirenda TS, Hiestand J, Mallewa J, Mandala WL. HIV infection has a profound effect on hematological factors but not on electrolyte profile of Malawian adults presenting with uncomplicated malaria and severe malaria. J Blood Med. 2018;9:153–162. doi:10.2147/JBM.S172869
20. Marsh K, English M, Crawley J, Peshu N. The pathogenesis of severe malaria in African children. Ann Trop Med Parasitol. 1996;90(4):395–402. doi:10.1080/00034983.1996.11813068
21. Mandala WL, Gondwe EN, MacLennan JM, Molyneux ME, MacLennan CA. Age- and sex-related changes in hematological parameters in healthy Malawians. J Blood Med. 2017;8:123–130. doi:10.2147/JBM.S142189
22. Wilson JJ, Neame PB, Kelton JG. Infection-induced thrombocytopenia. Semin Thromb Hemost. 1982;8(3):217–233. doi:10.1055/s-2007-1005053
23. Ladhani S, Lowe B, Cole AO, Kowuondo K, Newton CR. Changes in white blood cells and platelets in children with falciparum malaria: relationship to disease outcome. Br J Haematol. 2002;119(3):839–847. doi:10.1046/j.1365-2141.2002.03904.x
24. Gérardin P, Rogier C, Ka AS, Jouvencel P, Diatta B, Imbert P. Outcome of life-threatening malaria in African children requiring endotracheal intubation. Malar J. 2007;6:51. doi:10.1186/1475-2875-6-51
25. Pain A, Ferguson DJ, Kai O, et al. Platelet-mediated clumping of Plasmodium falciparum-infected erythrocytes is a common adhesive phenotype and is associated with severe malaria. Proc Natl Acad Sci USA. 2001;98(4):1805–1810. doi:10.1073/pnas.98.4.1805
26. Kim JS, Oh JS, Chang EA, et al. Alteration of platelet counts and lipid profiles after treatment of acute. Plasmodium Vivax Acta Trop. 2008;106(1):39–43. doi:10.1016/j.actatropica.2008.01.002
27. Leowattana W, Tangpukdee N, Thar SK, et al. Changes in platelet count in uncomplicated and severe falciparum malaria. Southeast Asian J Trop Med Public Health. 2010;41(5):1035–1041.
28. Kho S, Barber BE, Johar E, et al. Platelets kill circulating parasites of all major Plasmodium species in human malaria. Blood. 2018;132(12):1332–1344. doi:10.1182/blood-2018-05-849307
29. Gumbo A, Topazian HM, Mwanza A, et al. Occurrence and distribution of non-falciparum malaria parasite species among adolescents and adults in Malawi. J Infect Dis. 2022;225(2):257–268. doi:10.1093/infdis/jiab353
30. Lardinois B, Favresse J, Chatelain B, Lippi G, Mullier F. Pseudothrombocytopenia-A review on causes, occurrence and clinical implications. J Clin Med. 2021;10(4):594. doi:10.3390/jcm10040594
31. Horstmann RD, Dietrich M, Bienzle U, Rasche H. Malaria-induced thrombocytopenia. Blut. 1981;42(3):157–164. doi:10.1007/BF01026385
32. Naing C, Whittaker MA. Severe thrombocytopaenia in patients with vivax malaria compared to falciparum malaria: a systematic review and meta-analysis. Infect Dis Poverty. 2018;7(1):10. doi:10.1186/s40249-018-0392-9
33. Ansari S, Khoharo HK, Abro A, Akhund IA, Qureshi F. Thrombocytopenia in Plasmodium falciparum malaria. J Ayub Med Coll Abbottabad. 2009;21(2):145–147.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.