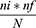Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 16
Acute Kidney Injury Among Admitted COVID-19 Patients in Addis Ababa, Ethiopia
Authors Goffe TK, Alemu ZA, Niguss Derese T , Bayou Tilahun Y, Bayou Tilahun R
Received 28 January 2023
Accepted for publication 15 March 2023
Published 22 March 2023 Volume 2023:16 Pages 83—92
DOI https://doi.org/10.2147/IJNRD.S402946
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pravin Singhal
Tigist Kefyalew Goffe,1,* Zewdie Aderaw Alemu,1,* Tadios Niguss Derese,2,* Yohannes Bayou Tilahun,3 Robel Bayou Tilahun4
1Department of Public Health, Gamby Medical and Business College, Addis Ababa, Ethiopia; 2Department of Research and Training, Eka Kotebe General Hospital, Addis Ababa, Ethiopia; 3General Practitioner at Health Hub Specialty Clinics by Al-Futtaim, Dubai, United Arab Emirates; 4General Practitioner, Uniteam Medical Assistant, Abu Dhabi, United Arab Emirates
*These authors contributed equally to this work
Correspondence: Tigist Kefyalew Goffe, Tel +251960291564, Email [email protected]
Background: Although diffuse alveolar damage and respiratory failure are the most common symptoms of coronavirus disease 2019, other organ involvement, such as the kidney, has been reported. The incidence of acute kidney injury in COVID-19 patients has been reported to vary greatly. In this study, we look at the magnitude and risk factors for acute kidney injury in COVID-19 patients in Ethiopia, a developing country.
Methods: A hospital-based retrospective cross-sectional study design was conducted among admitted COVID-19 patients at Eka Kotebe general hospital and Saint Peter COVID-19 treatment center by reviewing data from September 2020 to September 2021. A random sampling technique with proportional size allocation was used to select a total sample of 402 patients (225 from Eka Kotebe and 177 from St. Peter). Secondary data was collected from patient medical records using a standard, pre-tested data collection checklist using the Kobo toolbox, which was then exported to SPSS version 25.0 for analysis. The association between dependent and independent variables was analyzed using binary logistic regression. A statistical significance test was declared at a p value of <=0.05 with a 95% confidence interval.
Results: A total of 402 patient charts were reviewed, and the proportion of patients with acute kidney injury was found to be 18.9%. After adjusting for potential confounders, age<=35 years (AOR = 0.23, 95% CI = 0.07– 0.72), female gender (AOR = 0.51, 95% CI = 0.28– 0.94), and isolation type ICU (AOR = 5.11, 95% CI = 1.44– 18.06) were significantly associated with acute kidney injury.
Conclusion: Acute kidney injury is a common complication in hospitalized COVID-19 patients. The prevalence of acute kidney injury in this study was 18.9%. Age, gender, and type of isolation were the factors that had a significant association with acute kidney injury. Clinicians and other concerned parties should provide more care to ICU patients and COVID-19 patients who are older.
Keywords: acute kidney injury, COVID-19 patients, Addis Ababa
Introduction
Corona virus disease 2019 is a contagious infection caused by the Corona virus type 2 that causes severe acute respiratory syndrome.1 The disease was first identified in 2019 in Wuhan, China’s capital, and has since spread globally, resulting in the 2019 Corona virus pandemic.2
The most common symptoms of COVID-19 are fever, dry cough, and difficulty breathing, but other symptoms such as muscle pain, fatigue, sputum production, diarrhea, and a sore throat may also occur.3
The reverse transcription polymerase chain reaction (rRT-PCR) from a nasopharyngeal swab is the standard method of diagnosis. A combination of symptoms, risk factors, and a chest CT scan showing features of pneumonia can also be used to diagnose the infection.4
Although COVID-19 is most commonly associated with acute respiratory illness, it can also affect the kidney, heart, digestive tract, and blood.5 The mechanism of kidney involvement is unknown, but kidney injury in SARS-CoV-2 infected patients is a major concern.6 Because angiotensin converting enzyme-2 (ACE-2) is highly expressed in proximal tubule cells and podocytes, the kidney may be a target for organ injury in COVID-19.7
AKI, the primary end point, was defined as per Kidney Disease Improving Global Outcomes (KDIGO) criteria: a change in the serum creatinine of 0.3 mg/dl over a 48-hour period or a 50% increase in baseline creatinine. For patients with a previous serum creatinine in the 7–365 days prior to admission, the most recent serum creatinine value was considered the baseline creatinine. For patients without a baseline creatinine in the 7–365 days prior to admission, the admission creatinine was imputed on the basis of a Modification of Diet in Renal Disease (MDRD) eGFR of 75 mL/min per 1.73 m as per the KDIGO AKI guidelines.8
Despite initial reports of a low incidence, acute kidney injury (AKI) in COVID-19 patients is now recognized as a serious disease complication.9 The kidneys are the second most frequently affected organ by SARS-CoV-2, after the lungs.9 COVID-19 has affected millions of individuals worldwide, with considerable morbidity and mortality, and it imposes a serious public health pandemic affecting the whole world. Besides its high infectivity, SARS-CoV-2 causes multiple serious derangements.10
The prevalence of AKI in COVID-19 was found to be 33.9% in Africa. In contrast, hemodialysis was required in 76% of extreme ICU COVID-19 patients who developed AKI. Whereas the mortality rate in COVID-19 patients with or without AKI was 15.4% and 4.8%, respectively.7
Overall AKI affects the prognosis of patients with COVID-19 especially in developing countries which increases the morbidity and mortality rates and imposing a further burden on both patients and the healthcare system.5 The impact of AKI on patient outcomes may differ due to factors such as geographical area, differences of health-care systems or hospital capacities.8 Despite this fact there are no adequate studies done in Ethiopia assessing the prevalence of AKI and its factors associated with it among COVID-19 patients.
Methods
Study Area
The study was conducted at Eka Kotebe general hospital COVID-19 treatment center and St. Peter COVID-19 treatment center.
Study Design
A hospital based retrospective cross-sectional study was conducted.
Source Population
All confirmed COVID-19 cases with hospital admissions in Addis Ababa, Ethiopia.
Study Population
All confirmed COVID-19 positive patients who were admitted to Eka Kotebe General Hospital and Saint Peter COVID-19 treatment center from September 1, 2020 to September 30, 2021.
Sample Size Determination
The sample size was calculated using the single population proportion formula for magnitude and the Open-epi software for factors.
After adding 10% for chart loss, the final sample size was 402, which was the sample size calculated by the single proportion formula.
Sampling Technique
There are a total of 4 COVID-19 treatment centers in Addis Ababa. A simple random sampling method was used to select two hospitals, which were Eka Kotebe general hospital and St. Peter COVID treatment center. Then the number of study units to be sampled from each COVID-19 treatment center was determined using a proportional size allocation formula.
Where ni= number of COVID-19 patients in each COVID-19 treatment center.
nf= Final sample size of the study
N= total number of COVID-19 patients in the selected treatment centers.
So for Eka Kotebe General Hospital, from a total of 3450 COVID-19 patients, 225 patients were selected, and for St. Peter COVID-19 Treatment Center, from a total of 2700 COVID-19 patients, 177 patients were selected.
A simple random sampling technique was used to select COVID-19 patients from each COVID-19 treatment center.
Inclusion and Exclusion Criteria
Patients above 18 years old, a laboratory confirmed SARS-COV-2 infection, and patients admitted to the selected COVID-19 treatment centers were the inclusion criteria for this study.
Patients, who developed CKD before admission, underwent maintenance dialysis or kidney transplantation before admission, had an incomplete medical chart, had end-stage kidney disease (ESKD), and pregnant COVID-19 patients were excluded from the study.
Data Collection Technique
A pre-developed data collection checklist was used to collect data from the patient’s medical record retrospectively, which was developed after thoroughly reviewing previous relevant literature and modified after an intensive literature review. Three data collectors, all Bsc nurses by profession, and one supervisor, an Msc nurse by profession, collected the data. The checklist was filled out and collected by the data collectors from the patient’s medical record.
Study Variable
The dependent variable for this study was acute kidney injury in COVID-19 patients.
Socio-demographic (age, gender), comorbidities (DM, HTN, cardiac disease, asthma, liver disease, malignancy), patients’ presentation (fever, cough, SOB, sore throat, fatigue, GI symptom), laboratory results (WBC, Hgb, Plt, CRP, BUN, and serum Cr), medications taken (diuretics, glucosteriods, antivirals, penicillin, 1st and 2nd generation cephalosporin, NSIADS), and disease severity of COVID-19 were the independent variables.
Operational Definition
AKI: according to the KDIGO, AKI is defined as any of the following criteria’s:
- Increase in serum creatinine (SCr) by > or= 0.3mg/dl within 48 hours OR
- Increase in serum creatinine (SCr) ≥ 1.5 times from the baseline, which is known or presumed to have occurred within the prior 7 days OR
- Urine volume < 0.5mL/kg/h for 6 hours.
But for this study we only use the serum creatinine as a measuring unit because we collected a secondary data, we could not find the documented urine output.
A confirmed case of COVID-19: Is defined by a positive RT-PCR assay of a specimen collected through a nasopharyngeal swab.
Ward isolated COVID-19 patient: - Includes all patients who had COVID-19 pneumonia diagnosed by a typical chest computed tomography and a positive PCR of a nasopharyngeal sample for SARS-CoV-2 with mild symptoms and did not need for supplementary oxygen.
HDU isolated COVID-19 patient:- Includes all patients who had COVID-19 pneumonia diagnosed by a typical chest computed tomography and a positive PCR of a nasopharyngeal sample for SARS-CoV-2 with requirement for supplementary oxygen, furthermore, met any of the following criteria: (A) respiratory distress, defined as the respiratory rate ≥30 times/min, with cyanosis; (B) arterial digital oxygen saturation ≤93% (at room air); (C) the ratio of the partial pressure of oxygen to the fraction of inspired oxygen (PaO2/FiO2) ≤300 mmHg (1 mmHg = 0.133 kPa).
ICU COVID-19 patient: - Includes all patients of hospitalized group admitted to ICU stay and met any of the following criteria: (1) respiratory failure that requires mechanical ventilation; (2) shock; and (3) multiple organ failure that requires ICU life support.
Data Quality Control and Analysis
A half-day training was given to the data collectors and supervisor to ensure the validity and reliability of the data collection tool, and a pre-test was done on 5% of the total sample size outside the selected COVID-19 treatment centers before the actual data collection.
The data was collected and filled out on a software tool called Kobo Tool Box, checked for quality, and then exported to SPSS V-25 for analysis. A binary logistic regression model was used to assess the association between the independent variables and acute kidney injury. Odds ratio along with 95% confidence interval was estimated to assess the strength of association and variables with p- value of < =0.05 was considered to declare statistically significant association in the multivariable analysis.
Ethical Consideration
The study was conducted after obtaining ethical clearance from Gamby medical and business college review board. A medical record number was used for the data collection, and patient names were not used in collecting data from the medical files. Access to the collected information was limited to the principal investigator, and confidentiality was maintained throughout the project.
Result
Socio-Demographic Characteristics of Study Participants
A total of 402 study participants’ charts were reviewed in this study. The mean age of the participants was 52.66 years (SD = 17.60 years).
Majority 176 (43.8%) of the study participants were 35–60 years old, with ≥60 years 145 (35.1%) and <=35 years 85 (21.1%) following. Two hundred thirty-six (58.7%) of the study participants were male, and the rest, 166 (41.3%), were female (Table 1).
 |
Table 1 Socio-Demographic Characteristics of the Study Participants (n=402) |
Comorbidity Status of Study Participants
The majority of the study participants, 279 (69.4%), did not have diabetes mellitus, while 123 (30.6%) did. One hundred thirty-five (33.6%) of the study participants had hypertension, while the rest, 267 (66.4%), did not. The majority of 357 (88.8%) study participants did not have cardio-vascular disease, while only 45 (11.2%) did. Three hundred ninety-five (98.3%) of the study participants did not have chronic obstructive pulmonary disease, while only seven (1.7%) of the study participants had chronic obstructive pulmonary disease (Table 2).
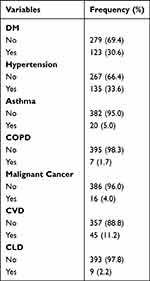 |
Table 2 Comorbidity Status of the Study Participant’s (n=402) |
Clinical Presentation of the Study Participants
Two hundred sixty-seven (66.4%) of the study participants had fever during their presentation to the hospitals, and the rest 135 (33.6%) did not have fever during their presentation. Almost all of the study participants coughed during their presentation, with the exception of two (0.5%) who did not. Two hundred and eighty-one (69.9%) of the study participants did not have sore throats, and the remaining 121 (30.1%) of them had sore throats during their presentations. Almost all 398 (99.0%) of the study participants had fatigue during their presentation, while only four (1.0%) of them did not have fatigue during their presentation (Table 3).
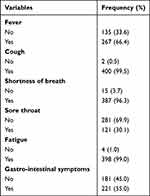 |
Table 3 Clinical Presentation of Study Participants’ (n=402) |
Medication Status of the Study Participants
Of the study participants, 26 (93.5%) took diuretics during their hospital stay, while the rest, 376 (93.5%), did not take diuretics during their hospital stay. Glucocorticoids were taken by almost all 409 (99.8%) of the study participants during their hospital stay, with only 1 (0.2%) not taking them. Of the study participants 14 (3.5%) took anti-viral medication during their hospital stay and 388 (96.5%) of the study participants did not took anti-viral medication during their hospital stay. The majority, 371 (92.3%) of the study participants, took cephalosporin during their hospital stay, while only 31 (7.7%) of them did not take cephalosporin during their hospital stay (Table 4).
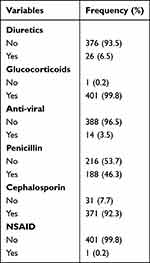 |
Table 4 Medication Status of the Study Participants (n=402) |
Laboratory Findings and Severity Status of Study Participants
The mean hemoglobin count of the study participants was 14.23 g/dl with a standard deviation of 2.34 g/dl. The mean WBC count of the study participants was 9404 per microliter with a standard deviation of 5480 per microliter. The majority of the study participants (72.1%) had a normal platelet count; followed by those with a low platelet count (24.1%) and a high platelet count (15.7%).
Forty-six (11.5%) of the study participants had critical COVID-19 status, while 356 (88.5%) of them had moderate or severe COVID-19 status. Of the study participants 31 (7.7%) were dead, while the rest 371 (92.3%) of study participants were cured (Table 5).
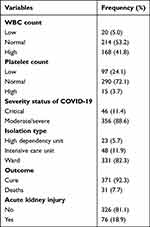 |
Table 5 Laboratory Findings, Level of AKI, and Severity Status of Study Participants (n=402) |
Seventy-six (18.9%) of the study participants had acute kidney injury, whereas 326 (81.1%) of the study participants did not have acute kidney injury.
Associated Factors
After adjustment for possible confounders in multivariable analysis, age, gender, and isolation type had significant associations with the outcome variable in multivariable analysis at 95% CI (p < 0.05) (Table 6).
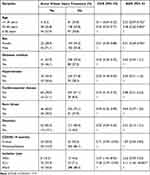 |
Table 6 Logistic Regression Analysis for Associated Factors (n=402) |
Discussion
The magnitude of acute kidney injury in this study was found to be 18.9%. Comparable findings were observed in a study conducted in Romania at two university hospitals (19.0%),11 Modena, Italy (22.4%)12 and Tehran, Iran (24.0%).13 The finding of this study is lower than a study conducted at London, United Kingdom (76.7%),14 Brazil (55.9%),15 Mount Sinai, Egypt (46.0%).16 This difference may be due to the difference in the study population (ICU and critically ill patients), but the study participants for the current study are not only ICU patients or critically ill patients but also moderate and severe COVID-19 patients. The finding of this study is also higher than a study conducted in Somalia, Mogadishu (12.7%).17
COVID-19 patients under the age of 35 were 77% less likely to develop acute kidney injury than COVID-19 patients over the age of 60 (AOR = 0.23, 95% CI = (0.07–0.72)). Similar findings were also observed in a systematic review, which is conducted based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).10 The possible explanation for this is that as we age, the rate of cellular apoptosis in the kidney increases, resulting in a lower number of functional nephrons, which contributes to a decrease in GFR and creatinine clearance ratio, which induces a decrease in renal functional reserve, making the kidney more susceptible to AKI.
Another significant variable that showed a significant association with acute kidney injury was gender: female COVID-19 patients were 49% less likely than male patients to have acute kidney injury (AOR = 0.51, 95% CI = (0.28–0.94)). This could be because most of the study participant in this study were male (58.7%) and only 16.1% of those male study participants were below 35 years.
A contrary finding was also observed in a study conducted in Somalia at Mogadishu, which revealed that female COVID-19 patients were 17.05 times more likely to have acute kidney injury than male COVID-19 patients. The difference in sample size and study population could explain this difference.17
COVID-19 patients who were isolated in the ICU were 5.11 times more likely to have acute kidney injury than those COVID-19 patients who were isolated in the ward (AOR = 5.11, 95% CI = (1.4418.06)). This could be because the patients who were isolated in the intensive care unit (ICU) had a critical COVID-19 severity status. Similar findings were observed in a study conducted in Somalia, Mogadishu.17 This similarity may be explained by the two populations’ similar lifestyles and economic status.
Conclusion and Recommendation
AKI is a common complication in hospitalized COVID-19 positive patients. Acute kidney injury was found in 18.9% of COVID-19 patients at Eka Kotebe General Hospital and Saint Peter Specialized Hospital COVID-19 treatment centers. The factors that had a significant association with acute kidney injury among COVID-19 patients were age, gender, and isolation type. Clinicians and other concerned parties should provide more care to ICU patients, and elderly COVID-19 patients and clinicians should raise their awareness of acute kidney injury in patients with severe COVID-19 and prioritize primary prevention and community education in order to implement COVID-19 preventive measures. More research studies need to be conducted, which can provide more detailed data which were not included in this study, ie, urine output as a diagnosing criteria for AKI.
Abbreviations
ACE 2, angiotensin converting enzymes 2; AKI, acute kidney injury; ARF, acute renal failure; CKD, chronic kidney disease; ESRD, end stage renal disease; HDU, high dependency unit; ICU, intensive care unit; KDIGO, kidney disease improving global outcomes; NSAID, non-steroidal anti-inflammatory drugs; SPSH, Saint Peter Specialized Hospital; WBC, white blood cell.
Ethical Approval
Ethical approval was obtained from the Ethical Review Board of Gamby Medical and Business College with a reference number GCA.A/421/2014. Formal permission was obtained from the management bodies of Eka Kotebe General Hospital and St. Peter Specialized Hospital, and patient consent to review their medical records was not required by the approving ethics committee. Confidentiality was maintained through omission of personal identification.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Adhikari SP, Meng S, Wu YJ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9:1–2.
2. Chauhan S. Comprehensive review of coronavirus disease 2019 (COVID-19). Biomed J. 2020;43(4):334–340. doi:10.1016/j.bj.2020.05.023
3. Majersik JJ, Reddy VK. Acute neurology during the COVID-19 pandemic Supporting the front line Jennifer J. Neurology. 2020;94(24):10551057. doi:10.1212/WNL
4. Emery SL, Erdman DD, Bowen MD, et al. Polymerase chain reaction assay for SARS-associated coronavirus. Emerg Infect Dis. 2004;10(2):311–316. doi:10.3201/eid1002.030759
5. Lai CC, Ko W-C, Lee P-I, et al. Extra-respiratory manifestations of COVID-19. Int J Antimicrob Agents. 2020;56(2):106024. doi:10.1016/j.ijantimicag.2020.106024
6. Gabarre P, Dumas G, Dupont T, et al. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46(7):1339–1348. doi:10.1007/s00134-020-06153-9
7. Birnie K, Verheyden V, Pagano D, et al. Predictive models for kidney disease: improving global outcomes (KDIGO) defined acute kidney injury in UK cardiac surgery. Crit Care. 2014;18(6):606. doi:10.1186/s13054-014-0606-x
8. Yildirim C, Ozger HS, Yasar E, et al. Early predictors of acute kidney injury in COVID −19 patients. Nephrology. 2021;26(6):513–521. doi:10.1111/nep.13856
9. Han X, Ye Q. Kidney involvement in COVID-19 and its treatments. J Med Virol. 2021;93(3):1387–1395. doi:10.1002/jmv.26653
10. Silver SA, Beaubien-Souligny W, Shah PS, et al. The prevalence of acute kidney injury in patients hospitalized with COVID-19 Infection: a systematic review and meta-analysis. Kidney Med. 2021;3(1):83–98 e1. doi:10.1016/j.xkme.2020.11.008
11. Radulescu D, Tutta L-A, David C. AKI in moderate and severe COVID-19 patients: reports of two universty hospitals; 2021.
12. Alfano G, Ferrari A, Fontana F. Incidence, risk factors and outcomes of AKI in patients with COVID-19. Clin Exp Nephrol. 2021;25(11):1203–1214. doi:10.1007/s10157-021-02092-x
13. Saghafi A, Aghaali M, Saghafi H. AKI in hospitalized COVID-19 patients in Iran; a systematic review and meta analysis. J Ren Inj Prev. 2021;10(2):e09. doi:10.34172/jrip.2021.09
14. Lumretugul N, Dini LP, Cooney E. AKI prevalence, progression and longterm outcomes in critically ill patients with COVID-19 a cohort study. Ann Intensive Care. 2021;11(1):1. doi:10.1186/s13613-020-00796-z
15. Lessa R, Fernando L, Salles EF. AKI in patients with COVID-19 in a Brazilian ICU: incidence, predictors and in hospital mortality. Bras J Nefrol. 2021;43:349–358. doi:10.1590/2175-8239-jbn-2020-0144
16. Chan L, Dhary KC, Saha A. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32(1):151–160. doi:10.1681/ASN.2020050615
17. Bashir AM, Sadik M, Mohammed YG. Prevalence of AKI in COVID-19 patients retrospective single center study; 2022.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.