Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Acute Generalized Pustular Psoriasis Developed Resistance to Adalimumab Was Successfully Treated with Narrowband Ultraviolet B and Acitretin: A Case Report
Authors Yang X, Wang J, Wang H, Kong M, Chen Q
Received 27 September 2022
Accepted for publication 8 November 2022
Published 25 November 2022 Volume 2022:15 Pages 2541—2546
DOI https://doi.org/10.2147/CCID.S391463
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Jeffrey Weinberg
Xianjie Yang,* Juan Wang,* Huan Wang, Minmin Kong, Qiquann Chen
Department of Dermatology, Southwest Hospital, Army Medical University, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiquann Chen, Email [email protected]
Abstract: Acute generalized pustular psoriasis (GPP) is a severe but rare variant of psoriasis, characterized by an acute eruption of extensive erythema with numerous non-follicular pustules. In rare cases, local pustular psoriasis like acrodermatitis continua of Hallopeau (ACH) may progress into acute GPP if improperly treated. ACH and GPP are rare in the clinic and their treatment is more complex and often treatment-resistant compared to psoriasis vulgaris (PV). A variety of anti-psoriasis biologics emerging in recent years have been reported for the treatment of ACH and acute GPP. Biologics is considered to be an upgraded treatment option for traditional anti-psoriasis agents. But there are few reports of GPP patients developing resistance to biologics, or what if biologics fails. Herein, we report a case of acute GPP that developed from ACH, initially responded extremely well to adalimumab, but the treatment failed when the patient treated with the drug again, which is thought to have developed resistance to adalimumab, finally successfully treated with narrowband ultraviolet B (NB-UVB) and acitretin.
Keywords: acute generalized pustular psoriasis, acrodermatitis continua of hallopeau, adalimumab, narrowband ultraviolet B, acitretin
Introduction
Generalized pustular psoriasis (GPP), which manifests as an acute or subacute, widely distributed eruption of pustules arising on inflamed, erythematous skin, is a severe but rare variant of psoriasis. This rare condition affects male and female of all ages, including infants, children, and adolescents, but mostly affects adults.1 The aetiology of GPP is not fully delineated. Many patients with GPP may have a preceding history of another form of psoriasis, and some GPP may develop from local pustular psoriasis like acrodermatitis continua of hallopeau (ACH) or palmoplantar pustulosis (PPP). The severe GPP flare cause a dramatic reduction in quality of life and even has potentially life-threatening. Very few drugs have been approved specifically for the treatment of GPP. Biologics approved for PV, such as tumor necrosis factor (TNF)-alpha inhibitors (including infliximab, adalimumab and etanercept), anti-interleukin (IL) 17 (ixekizumab and secukinumab) and anti-IL-23 (ustekinumab) biologic agents, have been adopted for the treatment of GPP.2 Although there are more and more reports on the treatment of GPP with these biologics, there are few reports on their treatment failure, and no attention has been paid to what to do if the biologics fail. Besides, treatment options for pediatric GPP are more limited and few drugs are approved for use, but adalimumab is one of the therapy approved by FDA. Herein, we report a rare case of acute GPP that developed from ACH, initially responded extremely well to adalimumab, but the treatment failed because the patient did not administrate the biologics on time and developed resistance to it, finally successfully treated with narrowband ultraviolet B (NB-UVB) and acitretin.
Case Report
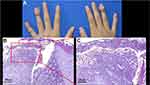
A 14-year-old adolescent female was referred to our department in July 2021 with generalized pustular and erythematous on the trunk and extremities for a week. She did not complain of chills, fever or arthralgia, but complained of swelling and pain and mild itching in the lesions. During the history collection, the patient reported a history of recurrent pustules on the distal portion of the middle finger of her right hand since 2015, which had been diagnosed as onychomycosis and treated with oral and topical antifungal in local hospitals for half a year but failed in 2017. In the summer of 2019, pustules gradually developed to multiple fingers of her both hands (Figure 1A), she visited our clinic and had a biopsy, bacterial culture was sterile and the pathologic results confirmed the diagnosis of ACH (Figure 1B and C). Since then she received intermittently treatment with oral thiamphenicol, compound glycyrrhizin, minocycline, and traditional Chinese medicine (TCM), and so on, combined with topical halometasone and tazarotene cream. The treatment response was still poor and more fingers were involved. She returned to our out-patient three months ago. According to the patient’s previous treatment response, in order to improve the treatment effect, taking into account the patient’s age and economic status, oral cyclosporine (150mg/day, 4mg/kg/day) was given, but there was no improvement after one month of adherence, so she stopped the medication on her own. However, about half a month after she stopped cyclosporine, the symptoms of her fingers became worse and pustules also appeared on some of the toes of both feet, and erythema and pustules immediately appeared on the trunk and extremities. She was admitted and diagnosed with acute GPP at a local hospital where she had received detailed examination ruled out latent infections such as tuberculosis and hepatitis B. Then, she was treated with adalimumab (80mg at first dose). The erythema and pustules all over her body quickly subsided within a week after first injection. However, she did not receive subsequent injections, mistakenly believing that the disease had healed, and GPP relapsed a month after she stopped taking adalimumab. Then she was given adalimumab with an 80mg dose again at the local hospital, but her symptoms were still active a week later. After a second injection of 40mg adalimumab, instead of improving, her symptoms got worse. In desperation, she stopped further adalimumab injections and came to our out-patient again.
Physical examination revealed widespread erythema and dense pustules on the trunk and limbs (Figure 2A–E). The erythema, swelling, crusting, focal erosion, and pustules were present on distal portions of all fingers (except the index and middle fingers of the right hand), as well as the fourth toe of the right foot and the third toe of the left foot. Nails of these fingers and toes were dystrophic and the nail plate were also studded with pustules (Figure 3A and B). Routine laboratory testing revealed white blood cell count of 15,860/μL (normal range, 4000–11,000/μL), neutrophil count of 12,670/μL (normal range, 2500–7000/μL) and neutrophil percentage of 79.9% (normal range, 50–70%), the count and percentage of eosinophils were both normal, serum levels of C-reactive protein (CRP) of 78.32 mg/L (normal range, 0–2 mg/L) and TNF-α of 162.00ng/mL (normal range, 7.4–15.4 ng/mL). The immunological laboratory tests including antinuclear antibodies, thyroid autoantibodies, rheumatoid factors, complement and so on were all normal. The thorax scanned by computed tomography (CT) and abdominal ultrasound showed no obvious abnormalities. Tuberculin purified protein derivative (PPD) skin test was normal. Hepatitis-virus-related tests were also normal. The family history of the patient was unremarkable for psoriasis or other inflammatory pathologies.
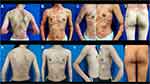 |
Figure 2 Manifestations on the trunk and limbs of the patient before treatment with narrowband ultraviolet B and acitretin (A–E) and after treatment with narrowband ultraviolet B and acitretin (a–e). |
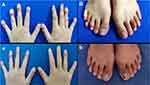 |
Figure 3 Manifestations of the fingers and toes before treatment with narrowband ultraviolet B and acitretin (A and B) and after treatment with narrowband ultraviolet B and acitretin (a and b). |
Based on the patient’s history of ACH, skin lesion characteristics and laboratory examination, the diagnosis of acute GPP was clear, with a Generalized Pustular Psoriasis Physician Global Assessment (GPPGA) total score of 4. Based on her previous experience with adalimumab, the patient and her guardians strongly refused to be treated with any biologics again. Therefore, we formulated a regular regimen of NB-UVB phototherapy combined with oral acitretin (20mg/day), which was accepted by the patient and her guardians. Due to lack of suitable skin areas for UVB minimal erythema dose (MED) testing, the patient was treated with NB-UVB (SS-10, Shanghai Sigma High Technology Co., LTD.) with an empirical starting dose of 300 mJ/cm2 (on the basis of Fitzpatrick skin type III), 3 times a week, and the dose is increased by 0–100 mJ/cm2 each time, depending on the skin reaction after each irradiation, and the maximum single dose did not exceed 3000 mJ/cm2. Surprisingly, the erythema and pustules on her trunk and extremities almost disappeared after two weeks of treatment. The patient was satisfied with the treatment response and was willing to continue the treatment. After another two weeks of treatment, the lesions all over her body had totally subsided, leaving only pigmentation (Figure 2a–e). Symptoms of fingers and toes also improved (Figure 3a and b). The GPPGA total score had been reduced to 1. NB-UVB was then reduced to once a week and the dose of acitretin was reduced to 10mg/day. There has been no recurrence of eruptions after more than 1 year of follow up.
Discussion
Our patient started the disease with ACH, which usually presents as sterile pustular eruption on the tip of one or more digits, is an uncommon variant of local pustular psoriasis. The natural progression of ACH is chronic and progressive, and it is not easy to treat. So far, there is no standard treatment for the disease with a high level of evidence.3 ACH generally does not respond well to many local and systemic therapies, and it has been reported that sudden withdrawal of some systemic therapies may induce the evolution of GPP, such as acitretin, methotrexate, and cyclosporine,4,5 which may be the cause of the outbreak of GPP in our patient.
The introduction of biologics has provided a new class of therapy that has revolutionized the therapy of ACH and GPP. Adalimumab, which have been approved by the FDA for use in pediatric patients, is one of the earliest and most well-documented biologics for the treatment of GPP, a summary of literatures of GPP treated with adalimumab is shown in Supplementary Table 1.6–13 However, there had been case reports suggesting that adalimumab might induce generalized pustulosis or GPP-like eruption.14,15 Our patient responded well to the first dose of adalimumab, but her GPP relapsed after discontinuation of the drug, and then the patient developed resistance to the drug. It is not clear why our patient developed drug resistance. But we all know that misuse is an important trigger for drug resistance, which has been well documented in antibiotics.16 Therefore, we speculate that the trigger for the acquired resistance to adalimumab in this patient was due to improper discontinuation, but this needs to be confirmed by more studies. Due to the rarity of this disease, most of the current evidences on biological agents for the treatment of GPP are case reports or small-scale, uncontrollable clinical trials, and there is no clear recommendation of biological agents for the treatment of GPP. Failure of treatment with biologics is not rare in clinical practice, and drug resistance to biological agents also exists. Some patients may not initially respond to biologics or, as in our case, they may initially respond well but later develop resistance.12 When patients do not respond to or develop resistance to previous biologics, switching to other types of biologics may be the preferred treatment option, but if patients refuse or develop resistance to the new biologics, the treatment will be extremely challenging.17
Acitretin plays a role in regulation of keratinocyte growth and differentiation and inhibition of neutrophil function, which has immunomodulatory effect.18 Acitretin is an effective traditional drugs for non-pregnant adult GPP, and can also be used in adolescents and children, even in infants.19 Although acitretin is usually effective when used alone, it is still not enough, and a growing number of studies report that acitretin can achieve better results when combined with other drugs or therapies.20 While ultraviolet phototherapy is widely used in treating PV, it is rarely mentioned in GPP treatment. After a comprehensive literature search, there are limited reports on ultraviolet phototherapy for GPP so far, shown in Supplementary Table 2.21–23 These limited case reports suggest that ultraviolet phototherapy is safe and effective for the treatment of GPP. In terms of mechanism, ultraviolet phototherapy can induce depletion of Langerhans cells and intra-epidermal T cells, decrease leucocyte adhesion to the microvasculature and induction of the immunosuppressive cytokine interleukin 10, playing good immunomodulatory and anti-inflammatory effect, and it has played a good therapeutic effect in the treatment of various inflammatory skin diseases including PV, atopic dermatitis, pityriasis rosea, and so on.24 It has been identified that combination therapy of psoriasis with acitretin and ultraviolet phototherapy offers multiple advantages, both clinically and mechanistically, over use of either modality alone.25,26 Since GPP is a complex systemic inflammatory disease, improper use of immunosuppressants, or biologic agents that target inflammatory pathways, may further induce inflammatory disorders in GPP, which may contribute to exacerbation of GPP symptoms or drug resistance. The immunomodulatory effects of ultraviolet phototherapy combined with acitretin may be a solution to this condition. To our knowledge, there have been no reports of switching treatment regimens to phototherapy or acitretin when patients develop resistance to biologics.
Overall, GPP is a rare variant of psoriasis currently lacks a standard treatment regimen. Although previous reports have shown that biologics are effective for GPP treatment, the phenomenon of GPP patients developing resistance to biologics cannot be ignored. Our case brings some implications for coping with this situation. When GPP patients develop resistance to biologics, phototherapy combined with acitretin may be one of the treatment options.
Ethics Statement
Written informed consents were obtained from the participant and her mother for the publication of any potentially identifiable images in this case report.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82003359).
Disclosure
The authors declare no conflict of interest in this work.
References
1. Zheng M, Jullien D, Eyerich K. The prevalence and disease characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23(Suppl S1):5–12. doi:10.1007/s40257-021-00664-x
2. Falto‐Aizpurua LA, Martin‐Garcia RF, Carrasquillo OY, Nevares‐Pomales OW, Sánchez‐Flores X, Lorenzo‐Rios D. Biological therapy for pustular psoriasis: a systematic review. Int J Dermatol. 2020;59(3):284–296. doi:10.1111/ijd.14671
3. Smith MP, Ly K, Thibodeaux Q, Bhutani T, Liao W, Beck KM. Acrodermatitis continua of Hallopeau: clinical perspectives. Psoriasis. 2019;9:65–72. doi:10.2147/ptt.S180608
4. Ranugha PS, Kumari R, Thappa DM. Acrodermatitis continua of hallopeau evolving into generalised pustular psoriasis. Indian J Dermatol. 2013;58(2):161. doi:10.4103/0019-5154.108096
5. Hong SB, Kim NI. Generalized pustular psoriasis following withdrawal of short-term cyclosporin therapy for psoriatic arthritis. J Eur Acad Dermatol Venereol. 2005;19(4):522–523. doi:10.1111/j.1468-3083.2005.01195.x
6. Callen JP, Jackson JH. Adalimumab effectively controlled recalcitrant generalized pustular psoriasis in an adolescent. J Dermatolog Treat. 2005;16(5–6):350–352. doi:10.1080/09546630500430604
7. Zangrilli A, Papoutsaki M, Talamonti M, Chimenti S. Long-term efficacy of Adalimumab in generalized pustular psoriasis. J Dermatolog Treat. 2008;19(3):185–187. doi:10.1080/09546630701759587
8. Kimura U, Kinoshita A, Sekigawa I, Takamori K, Suga Y. Successful treatment with Adalimumab in a patient with psoriatic arthritis and generalized pustular psoriasis. J Dermatol. 2012;39(12):1071–1072. doi:10.1111/j.1346-8138.2012.01563.x
9. Gallo E, Llamas-Velasco M, Daudén E, García-Diez A. Refractory generalized pustular psoriasis responsive to a combination of Adalimumab and Acitretin. Int J Dermatol. 2013;52(12):1610–1611. doi:10.1111/j.1365-4632.2012.05472.x
10. Kawakami H, Maeda T, Abe N, et al. Efficacy of Adalimumab and methotrexate combination therapy on generalized pustular psoriasis patients unresponsive to infliximab monotherapy due to anti-infliximab antibody development. J Dermatol. 2015;42(1):94–95. doi:10.1111/1346-8138.12704
11. Gkalpakiotis S, Arenberger P, Gkalpakioti P, et al. A case of acute generalized pustular psoriasis of von Zumbusch treated with Adalimumab. J Eur Acad Dermatol Venereol. 2015;29(10):2063–2064. doi:10.1111/jdv.12597
12. Matsumoto A, Komine M, Karakawa M, Kishimoto M, Ohtsuki M. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44(2):202–204. doi:10.1111/1346-8138.13632
13. Morita A, Yamazaki F, Matsuyama T, et al. Adalimumab treatment in Japanese patients with generalized pustular psoriasis: results of an open-label Phase 3 study. J Dermatol. 2018;45(12):1371–1380. doi:10.1111/1346-8138.14664
14. Kucharekova M, Winnepenninckx V, Frank J, Poblete-Gutiérrez P. Generalized pustulosis induced by Adalimumab in a patient with rheumatoid arthritis - a therapeutic challenge. Int J Dermatol. 2008;47(Suppl 1):25–28. doi:10.1111/j.1365-4632.2008.03954.x
15. Kimura U, Kinoshita A, Haruna K, et al. Generalized pustular psoriasis-like eruptions induced after the first use of Adalimumab in the treatment of psoriatic arthritis. J Dermatol. 2012;39(3):286–287. doi:10.1111/j.1346-8138.2011.01344.x
16. Abushaheen MA, Muzaheed Fatani M, Fatani AJ, et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis Mon. 2020;66(6):100971. doi:10.1016/j.disamonth.2020.100971
17. Takeichi T, Akiyama M. Generalized Pustular Psoriasis: clinical Management and Update on Autoinflammatory Aspects. Am J Clin Dermatol. 2020;21(2):227–236. doi:10.1007/s40257-019-00492-0
18. Gottlieb S, Hayes E, Gilleaudeau P, Cardinale I, Gottlieb AB, Krueger JG. Cellular actions of etretinate in psoriasis: enhanced epidermal differentiation and reduced cell-mediated inflammation are unexpected outcomes. J Cutan Pathol. 1996;23(5):404–418. doi:10.1111/j.1600-0560.1996.tb01430.x
19. Mahé E, Amy De La Bretêque M, Phan C. Perspectives on the pharmacological management of psoriasis in pediatric and adolescent patients. Expert Rev Clin Pharmacol. 2021;14(7):807–819. doi:10.1080/17512433.2021.1911641
20. Kearns DG, Chat VS, Zang PD, Han G, Wu JJ. Review of treatments for generalized pustular psoriasis. J Dermatolog Treat. 2021;32(5):492–494. doi:10.1080/09546634.2019.1682502
21. Hunt MJ, Lee SH, Salisbury EL, Wills EJ, Armati R. Generalized pustular psoriasis responsive to PUVA and oral cyclosporin therapy. Australas J Dermatol. 1997;38(4):199–201. doi:10.1111/j.1440-0960.1997.tb01697.x
22. Ozawa A, Ohkido M, Haruki Y, et al. Treatments of generalized pustular psoriasis: a multicenter study in Japan. J Dermatol. 1999;26(3):141–149. doi:10.1111/j.1346-8138.1999.tb03444.x
23. Hönigsmann H, Gschnait F, Konrad K, Wolff K. Photochemotherapy for pustular psoriasis (von Zumbusch). Br J Dermatol. 1977;97(2):119–126. doi:10.1111/j.1365-2133.1977.tb15055.x
24. Torres AE, Lyons AB, Hamzavi IH, Lim HW. Role of phototherapy in the era of biologics. J Am Acad Dermatol. 2021;84(2):479–485. doi:10.1016/j.jaad.2020.04.095
25. Lebwohl M. Acitretin in combination with UVB or PUVA. J Am Acad Dermatol. 1999;41(3):S22–4. doi:10.1016/s0190-9622(99)70362-2
26. Silva FS, Oliveira H, Moreiras A, et al. Cytotoxic and genotoxic effects of Acitretin, alone or in combination with psoralen–ultraviolet A or narrow-band ultraviolet B-therapy in psoriatic patients. Mutat Res. 2013;753(1):42–47. doi:10.1016/j.mrgentox.2012.12.017
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.