Back to Journals » International Journal of Nanomedicine » Volume 15
Acute Damage to the Sperm Quality and Spermatogenesis in Male Mice Exposed to Curcumin-Loaded Nanoparticles
Authors Xia X, Wang L, Yang X , Hu Y, Liu Q
Received 7 November 2019
Accepted for publication 14 February 2020
Published 17 March 2020 Volume 2020:15 Pages 1853—1862
DOI https://doi.org/10.2147/IJN.S237254
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Linlin Sun
Xiaoyu Xia,1,* Li Wang,1,* Xiao Yang,2 Yanqin Hu,1 Qiang Liu1
1Shanghai Key Laboratory of Reproductive Medicine, Department of Histoembryology, Genetics and Developmental Biology, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, People’s Republic of China; 2Shanghai Key Laboratory of Orthopedic Implants, Department of Orthopedics, Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiang Liu
Department of Histoembryology, Genetics and Developmental Biology, Shanghai Jiao Tong University School of Medicine, 280 South Chongqing Road, Shanghai 200025, People’s Republic of China
Tel +86 21 63846590 Ext. 776761
Email [email protected]
Background: Curcumin has shown many pharmacological activities in both preclinical and clinical studies. Many technologies have been developed and applied to improve the solubility and bioavailability of curcumin, especially the nanotechnology-based delivery systems. However, there has been evidence that certain nanoparticles have potential reproductive toxicity in practice.
Methods: Curcumin-poly (lactic-co-glycolic acid) (PLGA)-PEG nanoparticles (Cur-PLGA-NPs for short) were prepared. The Cur-PLGA-NPs were evaluated with its effect on the proliferation of mouse testicular cell lines in vitro and spermatogenesis in vivo, while PLGA-NPs were used as control. For animal experiments, male BALB/c mice were treated with 20 mg/kg of Cur-PLGA-NPs for continuous 10 days via tail vein injection.
Results: We found the curcumin nanoparticles suppressed the proliferation of testicular cell lines in vitro. Furthermore, a short-term intravenous delivery of curcumin-loaded nanoparticles could be harmful to the differentiation of spermatogonia, the elongation of spermatids, as well as the motility of mature sperms.
Conclusion: In the present study, we disclosed the acute damage on mouse spermatogenesis and sperm parameters by curcumin-loaded nanoparticles. Our results suggested that the reproductive toxicity of nanoformulated curcumin needs to be prudently evaluated before its application.
Keywords: nano-curcumin, reproductive toxicity, Sertoli cell, sperm motility, spermatogenesis
Introduction
Curcumin is extracted from the plant Curcuma longa and has been used as food additives and traditional medications for thousands of years in Asia.1 In recent decades, curcumin has been proved to have a wide range of pharmacological activities, including antioxidant, anti–inflammatory, anti-mutagen, and anti-carcinogenic effects.2,3 However, the clinical application of curcumin had long been hampered by its poor solubility in aqueous solvents, the low oral bioavailability and the rapid clearance in practice. Therefore, many technologies have been developed to overcome this limitation, including liposomes, phospholipid complexes, microemulsions, as well as polymeric micelles and nanoparticles.4,5 Specifically, the nanoformulated curcumin is promising for therapeutical applications. For example, the combination of nano-curcumin and chemotherapy is likely to increase the effectiveness of cancer treatment as well as reduce the adverse outcomes.6 Another case is, curcumin-loaded nanoparticles could successfully pass the blood–brain barrier, providing the neuroprotective effects in various central nervous system (CNS)-related diseases.7
However, there have been contradictory scientific reports about the impacts of curcumin on reproductive systems, which partially dependent on the doses and cell/animal models used in different studies. In mouse and rat models, curcumin displayed a protective function on ovary and testis under exogenous stress or pathological conditions.8 In contrast, growing evidence supported that curcumin would adversely affect female and male reproduction. For instance, in vitro studies revealed that incubating murine sperms with curcumin would significantly suppress the sperm motility, capacitation, acrosomal reaction, as well as the in vitro fertilization outcomes.9,10 Similarly, when incubated with curcumin, the motility of human spermatozoa was decreased.10–12 In male mice and rats, curcumin exhibited the antifertility functions in vivo.13–15 Particularly, curcumin possessed the potential androgen-antagonizing property, as it could down-regulate the expression of human androgen receptors,16 HSD17B3,17 HSD3B2 and CYP11A1 genes,18 as well as the mouse Cyp11a1 and StAR genes.19
To summarize, the multifaceted bioactivities of curcumin contribute to its diverse effects on the reproductive systems. The potential reproductive toxicity of curcumin is also dependent on the dosage, preparations and delivery approaches. Along with the rise of the researches on nanoformulated curcumin, it is essential to analyze the reproductive safety of nano-curcumin carefully. In the present study, we prepared curcumin-loaded poly (lactic-co-glycolic acid) nanoparticles,20 tried to evaluate its influence on testicular cell viability in vitro, as well as the short-term impact on spermatogenesis and sperm parameters in mouse model.
Materials and Methods
Curcumin-Loaded PLGA Nanoparticles Preparation
Curcumin was purchased from Sigma-Aldrich (St Louis, USA). Curcumin-Poly (lactic-co-glycolic acid) (PLGA)-PEG nanoparticles (Cur-PLGA-NPs for short) were prepared by double-emulsion method.20 The identical concentration of free curcumin or PLGA nanoparticles (PLGA-NPs for short) was used as controls for in vitro and in vivo experiments.
Cell Culture and Proliferation Assay
Mouse Sertoli cell line TM4 and Leydig cell line TM3 were obtained from the Cell Bank of Chinese Academy of Sciences, Shanghai, China. TM4 and TM3 cells were cultured in DMEM supplemented with 10% FBS, 100 U/mL penicillin/streptomycin at 37°C in a humidified 5% CO2 incubator. Spermatogonial stem cell line C18-4 were cultured in DMEM/F12, supplemented with 10% FBS, 2 mM L-glutamine and 50 U/mL penicillin/streptomycin in a humidified incubator at 34°C.21
Uncapsuled curcumin was diluted to 0.5 M in DMSO, stored at −20°C until use. Then, curcumin or PLGA-NPs or Cur-PLGA-NPs were diluted into the culture media to the final concentration indicated. After the treatment, the cell proliferation MTS assay was performed according to the manufacture’s protocol (GENMED, GMS10043, China). The absorbance of each well was measured at 540 nm using Multiskan™ GO (Thermo Fisher Scientific, USA). The treatment was performed in triplicate and repeated three times.
Electron Microscopy Analysis
TM4 cells were cultured with 50 μM PLGA-NPs or Cur-PLGA-NPs for 48 hrs. For electron microscopy analysis, the cells were collected and fixed with glutaraldehyde, postfixed in reduced osmium, dehydrated, and embedded as previously described.22 The sections were examined under CM-120 transmission electron microscope (PHILIPS, Eindhoven, Netherlands) at 80 kV.
Design of Animal Experiment
All experiments were conducted following the Guide for the Care and Use of Laboratory Animals of National Institutes of Health. This study was approved by the animal ethics committee of Shanghai Jiao Tong University School of Medicine (DAS-A-2016-038). Male BALB/c mice were purchased from the Shanghai Laboratory Animal Center and hosted in the Animal Center of Shanghai Jiao Tong University School of Medicine under the standardized SPF conditions.
Six-week mice were randomly divided into two groups (n=6 per group) as follows: Group 1, PLGA-NPs; Group 2, Cur-PLGA-NPs. For Group 2, mice were injected 20 mg/kg body weight (~55 mM/kg b.w.)8,13 of Cur-PLGA-NPs in saline via tail vein. For Group 1, mice were injected 20 mg/kg body weight of PLGA-NPs. The injections were executed for continuous 10 days.
After the treatment, blood was obtained from the retro-orbital venous plexus and used for testosterone measurement assay (n≥4 per group). Then, mice were sacrificed, the weight of testes, epididymis and seminal vesicles were recorded, as well as the body weight (n≥4 per group). Sperms were collected immediately and subjected to the computer-assisted sperm analysis (CASA) (n=5 per group). At the same time, testes were fixed in Bouin’s solution for subsequent haematoxylin-eosin (H-E) staining, TdT-mediated dUTP Nick-End Labeling (TUNEL) and immunohistochemistry assays.
Testosterone Measurements
After in vivo treatment to the mice, the testosterone levels in serum were measured using the immunoassay kit (R&D Systems, Minneapolis, USA) according to the manufacturer’s protocols.
CASA Analysis
The cauda epididymis was dissected from an individual mouse and then incubated in Tyrode’s Solution (Sigma-Aldrich, St Louis, USA) at 37°C for 15 min to release the sperms. The supernatant was collected, sperm counts and motility were evaluated using the computer-assisted sperm analysis (CASA) system (Hamilton Thorne, Beverly, USA).
Histopathological Examination
Five-micrometer-thick serial paraffin sections were prepared and H-E staining was executed by standard protocols. The images were captured under ECLIPSE E600 microscope (Nikon, Tokyo, Japan) and analyzed by Image-Pro Plus 6.0. The relative volume (%) of lumen was evaluated according to the previous publications.23,24 The staging of seminiferous tubules was determined,25 200 tubules from each group were counted and the percentage of every spermatogenic stage was quantified.
TUNEL Assay
The paraffin sections were incubated in buffered citrate (pH 6.0) for 15 mins at 105°C. TUNEL analysis was performed using in situ Cell Death Detection Kit (Roche, Indianapolis, USA) according to the manufacturer’s instructions. Tubules from every group were counted and the percentage of the TUNEL positive tubules was quantified, as well as the average number of apoptotic cells per tubule.
Immunohistochemistry
For antigen retrieval, the paraffin sections were incubated in buffered citrate (pH 6.0) for 15 mins at 105°C and then soaked in methanol containing 0.3% H2O2 to neutralize endogenous peroxidase activity. For primary antibody incubation, the sections were incubated with 1:50 rabbit anti-Pan-Acetylated Histone4 antibody (Abcam, ab10807, Cambridge, USA) or normal rabbit IgG (negative control) at 4°C overnight. On the following day, the signals were visualized using a Histostain-Plus Kit, DAB (Life Technologies, Carlsbad, USA) according to the manufacturer’s instructions.
Statistical Analysis
Statistical analysis data are expressed as mean ± SEM or as proportions. For continuous variables, the differences between groups were analyzed by one-way ANOVA, followed by Student’s t-test. For categorical variables, Pearson’s χ2 test was used to analyze the correlation between variables. P<0.05 was considered to be statistically significant.
Results
The Cytotoxicity of Cur-PLGA-NPs on Testicular Cell Lines
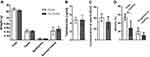
In the previous study, we have proved that curcumin affected the growth of spermatogonial stem cell line C18-4 in a dose-dependent manner. C18-4 cells treated with 50 μM curcumin entered into the apoptotic process after 48 h.26 In the present study, we prepared curcumin-PLGA nanoparticles (Supplemental Figure 1), considering the biosafety of PLGA nanoparticles has been verified convincingly.27,28 The average size and surface charge (zeta-potential) of the Cur-PLGA-NPs were 208.0 nm and 0.161± 3.45 mV, respectively. Firstly, we evaluated the cytotoxicity of Curcumin-PLGA nanoparticles to testicular cell lines, including C18-4, Leydig cell line TM3 and Sertoli cell line TM4. As shown in Figure 1, the given treatment of PLGA-NPs had no significant effect on the growth of cells. However, comparing to blank control, PLGA-NPs and curcumin, 50 μM Cur-PLGA-NPs treatment exhibited the obvious negative impact by 48 h incubation (P<0.05) (Figure 1A–C). These results confirmed that nano-curcumin exhibited stronger biological activity than naked curcumin.
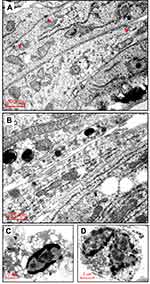
Among the cell lines we tested, TM4 seemed more sensitive to the curcumin/Cur-PLGA-NPs treatment. Interestingly, the proliferation of TM4 cells treated with Cur-PLGA-NPs was promoted by 24 h, but declined rapidly by 48 h (Figure 1C). The electron microscope observation revealed that, in the 50 μM PLGA-NPs group, there were continuous cell junctions formed between the TM4 cells, highlighted by the anchoring junctions (Figure 2A, arrowhead). In contrast, after the 50 μM Cur-PLGA-NPs treatment for 48 h, the anchoring junctions were disappeared and the TM4 cells were detached (Figure 2B). Furthermore, a considerable amount of the TM4 cells had gone into the program of the apoptosis, showing the characteristics of nuclei fragmentation and chromatin condensation, as well as the vacuolus and degenerated organelles in the cytoplasm (Figure 2C and D).
These results implied that Sertoli cells would probably be damaged by Cur-NPs delivery in vivo, which could be a risk factor to the blood-testis barrier (BTB) and the spermatogenesis. In order to elucidate this question, we applied a moderate treatment of 20 mg/kg body weight8,13 Cur-PLGA-NPs in the following tests, by tail intravenous injection for continuous 10 days, with the PLGA-NPs used as control.
A Short-Term Treatment of Cur-PLGA-NPs Reduced the Sperm Motility in vivo
As shown in Figure 3, after 10-day in vivo treatment, the weight of sex organs, the level of testosterone and the quality of semen were measured in Cur-PLGA-NPs delivered male mice. Comparing to the PLGA-NPs controls, the gross weight of sex organs was unchanged (Figure 3A), as well as the serum concentration of testosterone (Figure 3B), which infer the normal function of Leydig cells. However, there was a slight inclination of the decreased sperm concentration in the Cur-PLGA-NPs treated group (Figure 3C). Meanwhile, the CASA analysis disclosed the depressed total motility of sperms after the short-term Cur-PLGA-NPs treatment (P < 0.05), and the relatively lower progressive motility (Figure 3D).
Cur-PLGA-NPs Disturbed the Progress of Spermatogenesis
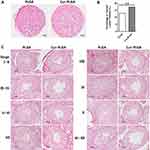
Consistently, the pathological analysis of testes provided interesting evidence. Although the average weight of the testis was not different between the two groups, the lumen of the seminiferous tubules was visibly enlarged in the Cur-PLGA-NPs treated ones (Figure 4A–C, Figure 6B). The relative volume of the tubular lumen rose from 12.78% in the control group to 17.11% in the Cur-PLGA-NPs treated group (P<0.01) (Figure 4B). This phenotype was caused by the general loss of cell layers in the spermatogenic epithelia, which could be found throughout all the existing stages of the spermatogenesis (Figure 4C).
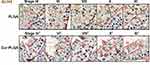
Another phenomenon was the abnormal proportion of given stages during the spermatogenesis process. The staging of seminiferous tubules was determined according to the previous publications,25 especially based on the characteristics of the developing spermatids. To quantify the percentage of each spermatogenic stage, at least 200 seminiferous tubules from 3 different mice were counted in each group. Surprisingly, the proportion of Stage IV–V and Stage VIII–IX were severely affected by the Cur-PLGA-NPs treatment, since the tubules at Stage IV–V were reduced, but the ones at Stage VIII–IX were increased (Figure 5A).
In fact, some of the tubule sections from the Cur-PLGA-NPs group could not be categorized into any spermatogenic stages, on account of the misarranged cell type combination (Figure 5B, I–II). Additionally, the vacuole in the basal compartment (arrowhead) implied the faulted BTB structure, and the multinucleated giant cells (arrow) were indications of the cellular apoptosis (Figure 5B. III–IV).29
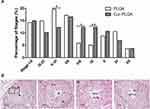
In that way, we then performed the TUNEL analysis on the testicular tissues. As shown in Figure 6, compared to the testicular tissue from the PLGA-NPs group (Figure 6A), apoptotic spermatogenic cells were remarkably accumulated in the Cur-PLGA-NPs group (P<0.01) (Figure 6B and E), especially the apoptotic spermatogonia (arrow) and early spermatocytes (Figure 6C and D). Taken together, a short-term in vivo treatment of Cur-PLGA-NPs would cause the acute injury to the testis.
Histone Acetylation Was Downregulated in Spermatogenic Cells After Cur-PLGA-NPs Treatment
One of the well-known properties of curcumin is the inhibition activity on kinds of histone acetylase (HATs).30,31 Herein, we investigated the histone acetylation expression after in vivo treatment of Cur-PLGA-NPs. In PLGA-NPs group, the signal of acetylated histone 4 (AcH4) was detected in the intermediate (arrowhead) and Type B (arrow) spermatogonia, as well as in the daughter leptotene spermatocytes (asterisk) (Figure 7, upper panel). In contrast, the AcH4 signal was diminished in the spermatogonia from the Cur-PLGA-NPs group (Figure 7, lower panel). Particularly, the progress of spermatogonia differentiation seemed blocked in some tubules from the Cur-PLGA-NPs group (Stage VI’, VIII’). Furthermore, the typical hyperacetylated signal in elongating spermatids was dramatically weakened (Stage X’, XI’), which would impede the maturation steps and contribute to the lower concentration/quality of sperms.
Discussion
Blood–testis barrier is mainly constituted by the tight junctions between the adjacent Sertoli cells, which is crucial to the homeostasis in the testis and the process of spermatogenesis.32 Noteworthy, there have been increasing evidence on nanoparticles penetrating the blood-testis barrier successfully and demonstrating the potential adverse effects on the reproductive system.33–36 It put forward the emergent question that whether the nanoformulated agents might induce the unintended reproductive dysfunction.
Curcumin is a prominent biomolecule in the field of drug development. The nano-based drug delivery system has effectively optimized the aqueous solubility and bioavailability of curcumin. Ahmed-Farid OAH reported that, 5 mg/kg b.w. curcumin nano-emulsion showed significant amelioration effects against the disrupted reproductive performance in rats induced by protein-deficient diet.37 In a randomized clinical trial, Alizadeh F reported that daily uptake of 80 mg curcumin nanomicelle for 10 weeks would improve the semen parameters in infertile men.38 On another hand, there have been studies focusing on the possible reproductive toxicity of nanoformulated curcumin. Using a rat model, Moshari S discovered that, a 48-day oral administration of 30 mg/kg b.w. of nanomicelle curcumin could induce DNA fragmentation in testis, accompanied by the suppression of the p53 and PCNA-related apoptotic program.14 Later, this group further testified that a 48-day nanomicelle curcumin treatment would negatively affect the spermatogenesis, sperm parameters and in vitro fertilization potential in rats.15 In our results, the curcumin-loaded nanoparticles demonstrated a more profound biological effect than the uncapsuled curcumin in vitro (Figures 1 and 2). We then delivered the dosage of 20 mg/kg b.w. curcumin-PLGA nanoparticles to male mice via intravenous injection for continuous 10 days that the administration via blood circulation could evade the issue of oral bioavailability and enhance the drug utilization. Strikingly, our results suggested that (Figures 3–6), the Cur-PLGA-NPs treatment, even for a short time, might endanger the blood-testis barrier function, be harmful to the spermatogenesis and sperm motility, ultimately the male fertility.
Generally, the spermatogenic cycle cost 35 days in mice, which spreading asynchronously along the seminiferous tubules.39 In our study, we observed the halted development of existing spermatogonia and elongating spermatids in Cur-PLGA-NPs group (Figure 5A, Figure 7). One appropriate interpretation is, the Cur-PLGA-NPs treatment probably caused the retarded transition of Stage II to VI and Stage VIII to X in the ongoing spermatogenic cycles. It leads to the reasonable assumption that, an accumulated, long-term treatment of nano-curcumin could eventually deplete the spermatogenic cells in the seminiferous tubules.
Mechanically, we detected the suppression of the histone 4 acetylation after Cur-PLGA-NPs treatment (Figure 7, lower panel), which is accordant to the reported activity of curcumin as HAT inhibitor.30,31 During spermatogenesis, the acetylation of histones is mainly happening in the spermatogonia under differentiating, as well as in the elongating spermatids. Importantly, the global hyperacetylation of histone 3 and 4 was found in mouse, rat and human elongating spermatids, critical to the later histone replacement and chromatin condensation.40,41 In Cur-PLGA-NPs group, the AcH4 signal was decreased in elongating spermatids, which implies the failure of spermatid maturation. However, considering the complexity of curcumin bioactivities, the mechanism of damage induced by curcumin-loaded nanoparticles in vivo should be cautiously analyzed.
In conclusion, our results revealed that curcumin-loaded nanoparticles might cause an acute injury to the testicular functions in male mice. There are extended questions as, whether the reproductive toxic effect of nano-curcumin is transient and reversible, and how to reduce the damage caused. It is also interesting and meaningful to evaluate the influences of nano-curcumin on female fertility in vivo. To sum up, the reproductive toxicity of nanoformulated curcumin needs to be evaluated thoroughly before its therapeutic applications.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers 81501308, 81571488, 81601334, 81771637, 81971436), Shanghai Municipal Commission of Health and Family Planning (grant number 20134Y169), Shanghai Key Laboratory of Embryo Original Diseases (grant number Shelab201901).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75.
2. Kotha RR, Luthria DL. Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules. 2019;24(16):E2930. doi:10.3390/molecules24162930
3. Di Martino RM, Luppi B, Bisi A, et al. Recent progress on curcumin-based therapeutics: a patent review (2012–2016). Part I: curcumin. Expert Opin Ther Pat. 2017;27(5):579–590. doi:10.1080/13543776.2017.1276566
4. Bisht S, Maitra A. Systemic delivery of curcumin: 21st century solutions for an ancient conundrum. Curr Drug Discov Technol. 2009;6(3):192–199. doi:10.2174/157016309789054933
5. Liu W, Zhai Y, Heng X, et al. Oral bioavailability of curcumin: problems and advancements. J Drug Target. 2016;24(8):694–702. doi:10.3109/1061186X.2016.1157883
6. Tan BL, Norhaizan ME. Curcumin combination chemotherapy: the implication and efficacy in cancer. Molecules. 2019;24(14):E2527. doi:10.3390/molecules24142527
7. Yavarpour-bali H, Ghasemi-kasman M, Pirzadeh M. Curcumin-loaded nanoparticles: a novel therapeutic strategy in treatment of central nervous system disorders. Int J Nanomedicine. 2019;14:4449–4460. doi:10.2147/IJN.S208332
8. Mohebbati R, Anaeigoudari A, Khazdair MR. The effects of Curcuma longa and curcumin on reproductive systems. Endocr Regul. 2017;51(4):220–228. doi:10.1515/enr-2017-0024
9. Naz RK. Can curcumin provide an ideal contraceptive? Mol Reprod Dev. 2011;78(2):116–123. doi:10.1002/mrd.v78.2
10. Naz RK. The effect of curcumin on intracellular pH (pHi), membrane hyperpolarization and sperm motility. J Reprod Infertil. 2014;15(2):62–70.
11. Naz RK, Lough ML. Curcumin as a potential non-steroidal contraceptive with spermicidal and microbicidal properties. Eur J Obstet Gynecol Reprod Biol. 2014;176:142–148. doi:10.1016/j.ejogrb.2014.01.024
12. Rithaporn T, Monga M, Rajasekaran M. Curcumin: a potential vaginal contraceptive. Contraception. 2003;68(3):219–223. doi:10.1016/S0010-7824(03)00163-X
13. Murphy CJ, Tang H, Van Kirk EA, Shen Y, Murdoch WJ. Reproductive effects of a pegylated curcumin. Reprod Toxicol. 2012;34(1):120–124. doi:10.1016/j.reprotox.2012.04.005
14. Moshari S, Nejati V, Najafi G, Razi M. Nanomicelle curcumin-induced DNA fragmentation in testicular tissue; Correlation between mitochondria dependent apoptosis and failed PCNA-related hemostasis. Acta Histochem. 2017;119(4):372–381. doi:10.1016/j.acthis.2017.03.007
15. Moshari S, Nejati V, Najafi G, Razi M. Insight into curcumin nanomicelle-induced derangements in male reproduction potential: an experimental study. Andrologia. 2018;50(2):e12842. doi:10.1111/and.12842
16. Nakamura K, Yasunaga Y, Segawa T, et al. Curcumin down-regulates AR gene expression and activation in prostate cancer cell lines. Int J Oncol. 2002;21(4):825–830.
17. Hu GX, Liang G, Chu Y, et al. Curcumin derivatives inhibit testicular 17beta-hydroxysteroid dehydrogenase 3. Bioorg Med Chem Lett. 2010;20(8):2549–2551. doi:10.1016/j.bmcl.2010.02.089
18. Ide H, Lu Y, Noguchi T, et al. Modulation of AKR1C2 by curcumin decreases testosterone production in prostate cancer. Cancer Sci. 2018;109(4):1230–1238. doi:10.1111/cas.2018.109.issue-4
19. Lin YC, Chiu CH, Liu HC, Wang JY. Curcumin downregulates 8-br-cAMP-induced steroidogenesis in mouse Leydig cells by suppressing the expression of Cyp11a1 and StAR independently of the PKA-CREB pathway. Endocr J. 2018;65(8):833–840. doi:10.1507/endocrj.EJ18-0010
20. Xie X, Tao Q, Zou Y, et al. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: characterizations and mechanisms. J Agric Food Chem. 2011;59(17):9280–9289. doi:10.1021/jf202135j
21. Hofmann MC, Braydich-stolle L, Dettin L, Johnson E, Dym M. Immortalization of mouse germ line stem cells. Stem Cells. 2005;23(2):200–210. doi:10.1634/stemcells.2003-0036
22. Fan Y, Liu Y, Xue K, et al. Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood-testis barrier. PLoS One. 2015;10(4):e0120775. doi:10.1371/journal.pone.0120775
23. Noorafshan A. Stereology as a valuable tool in the toolbox of testicular research. Ann Anat. 2014;196(1):57–66. doi:10.1016/j.aanat.2012.07.008
24. Ma L, Guo Y, Yuan Y, Li YG, Deng XZ, Yang ZW. Morphometric study of the testis and reproductive tract (including sperm granuloma) after vasectomy in mature rats. Asian J Androl. 2016;18(1):66–73. doi:10.4103/1008-682X.150038
25. Ahmed EA, de Rooij DG. Staging of mouse seminiferous tubule cross-sections. Methods Mol Biol. 2009;558:263–277.
26. Xia X, Cai H, Qin S, Xu C. Histone acetylase inhibitor curcumin impairs mouse spermiogenesis-an in vitro study. PLoS One. 2012;7(11):e48673. doi:10.1371/journal.pone.0048673
27. Kim YS, Park JS, Park M, et al. PLGA nanoparticles with multiple modes are a biologically safe nanocarrier for mammalian development and their offspring. Biomaterials. 2018;183:43–53. doi:10.1016/j.biomaterials.2018.08.042
28. Tiwari SK, Agarwal S, Seth B, et al. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/beta-catenin pathway. ACS Nano. 2014;8(1):76–103. doi:10.1021/nn405077y
29. Hansen DA, Esakky P, Drury A, Lamb L, Moley KH. The aryl hydrocarbon receptor is important for proper seminiferous tubule architecture and sperm development in mice. Biol Reprod. 2014;90(1):8. doi:10.1095/biolreprod.113.108845
30. Balasubramanyam K, Varier RA, Altaf M, et al. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem. 2004;279(49):51163–51171. doi:10.1074/jbc.M409024200
31. Kang J, Chen J, Shi Y, Jia J, Zhang Y. Curcumin-induced histone hypoacetylation: the role of reactive oxygen species. Biochem Pharmacol. 2005;69(8):1205–1213. doi:10.1016/j.bcp.2005.01.014
32. Johnson L, Thompson DL, Varner DD. Role of Sertoli cell number and function on regulation of spermatogenesis. Anim Reprod Sci. 2008;105(1–2):23–51. doi:10.1016/j.anireprosci.2007.11.029
33. Larson JK, Carvan MJ, Hutz RJ. Engineered nanomaterials: an emerging class of novel endocrine disruptors. Biol Reprod. 2014;91(1):20. doi:10.1095/biolreprod.113.116244
34. Brohi RD, Wang L, Talpur HS, et al. Toxicity of nanoparticles on the reproductive system in animal models: a review. Front Pharmacol. 2017;8:606. doi:10.3389/fphar.2017.00606
35. Ema M, Okuda H, Gamo M, Honda K. A review of reproductive and developmental toxicity of silver nanoparticles in laboratory animals. Reprod Toxicol. 2017;67:149–164. doi:10.1016/j.reprotox.2017.01.005
36. Wang R, Song B, Wu J, Zhang Y, Chen A, Shao L. Potential adverse effects of nanoparticles on the reproductive system. Int J Nanomedicine. 2018;13:8487–8506. doi:10.2147/IJN
37. Ahmed-farid OAH, Nasr M, Ahmed RF, Bakeer RM. Beneficial effects of curcumin nano-emulsion on spermatogenesis and reproductive performance in male rats under protein deficient diet model: enhancement of sperm motility, conservancy of testicular tissue integrity, cell energy and seminal plasma amino acids content. J Biomed Sci. 2017;24(1):66.
38. Alizadeh F, Javadi M, Karami AA, Gholaminejad F, Kavianpour M, Haghighian HK. Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: a randomized clinical trial. Phytother Res. 2018;32(3):514–521. doi:10.1002/ptr.v32.3
39. Zindy F, den Besten W, Chen B, et al. Control of spermatogenesis in mice by the cyclin D-dependent kinase inhibitors p18(Ink4c) and p19(Ink4d). Mol Cell Biol. 2001;21(9):3244–3255. doi:10.1128/MCB.21.9.3244-3255.2001
40. Qian MX, Pang Y, Liu CH, et al. Acetylation-mediated proteasomal degradation of core histones during DNA repair and spermatogenesis. Cell. 2013;153(5):1012–1024.
41. Goudarzi A, Shiota H, Rousseaux S, Khochbin S. Genome-scale acetylation-dependent histone eviction during spermatogenesis. J Mol Biol. 2014;426(20):3342–3349. doi:10.1016/j.jmb.2014.02.023
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.