Back to Journals » Journal of Pain Research » Volume 13
Acute Cytokine Response During Breast Cancer Surgery: Potential Role of Dexamethasone and Lidocaine and Relationship with Postoperative Pain and Complications – Analysis of Three Pooled Pilot Randomized Controlled Trials
Authors van den Heuvel SAS , van der Wal SEI, Bronkhorst EM , Warlé MC, Ronday M, Plat J , van Alfen N, Joosten LAB , Lerou JGC , Vissers KCP , Steegers MAH
Received 4 March 2020
Accepted for publication 6 May 2020
Published 27 May 2020 Volume 2020:13 Pages 1243—1254
DOI https://doi.org/10.2147/JPR.S252377
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Robert B. Raffa
Sandra AS van den Heuvel,1 Selina EI van der Wal,1 Ewald M Bronkhorst,2 Michiel C Warlé,3 May Ronday,4 Judith Plat,4 Nens van Alfen,5 Leo AB Joosten,6 Jos GC Lerou,1 Kris CP Vissers,1 Monique AH Steegers7
1Department of Anesthesiology, Pain and Palliative Medicine, Radboud University Medical Centre, Nijmegen, the Netherlands; 2Department of Health Evidence, Radboud University Medical Centre, Nijmegen, the Netherlands; 3Division of Vascular and Transplant Surgery, Department of Surgery, Radboud University Medical Centre, Nijmegen, the Netherlands; 4Department of Anesthesiology, Alexander Monro Breast Cancer Hospital, Bilthoven, the Netherlands; 5Donders Institute for Brain Cognition and Behavior, Department of Neurology, Radboud University Medical Centre, Nijmegen, the Netherlands; 6Department of Internal Medicine and Radboud Centre for Infectious Diseases, Radboud University Medical Centre, Nijmegen, the Netherlands; 7Department of Anesthesiology, Pain and Palliative Medicine, Amsterdam University Medical Centre, Amsterdam, the Netherlands
Correspondence: Sandra AS van den Heuvel
Department of Anesthesiology, Pain and Palliative Medicine, Radboud University Nijmegen Medical Center, Internal mail 630, P.O. Box 9101, Nijmegen 6525 GA, the Netherlands
Tel +31 243615244
Email [email protected]
Purpose: An imbalance in perioperative cytokine response may cause acute pain and postoperative complications. Anesthetic drugs modulate this cytokine response, but their role in non-major breast cancer surgery is unclear. In an exploratory study, we investigated whether intravenous lidocaine and dexamethasone could modulate the cytokine response into an anti-inflammatory direction. We also evaluated interrelationships between cytokine levels, pain scores and postoperative complications. Our goal is to develop multimodal analgesia regimens optimizing outcome after breast cancer surgery.
Patients and Methods: Forty-eight patients undergoing a lumpectomy were randomly assigned to placebo or lidocaine (1.5 mg⋅kg− 1 followed by 2 mg⋅kg− 1⋅hour− 1) supplemented by dexamethasone zero, 4 or 8 mg, yielding six groups of eight patients. Interleukin (IL)-1β, IL-1Ra, IL-6, IL-10 levels and pain scores were measured at baseline and four hours postoperatively. We assessed postoperative complications occurring within 30 days. We noted persistent pain and infections as potential immune-related complications (PIRC). We used multiple regression to disentangle the effects of the individual study drugs (given by their partial regression coefficients (b)). Odds ratios (OR) estimated the link between pain scores and complications.
Results: Dexamethasone 8 mg increased IL-10 (b=12.70 (95% CI=8.06– 17.34), P< 0.001). Dexamethasone 4 mg and 8 mg decreased the ratio IL-6/IL-10 (b=− 2.60 (− 3.93 to − 1.26), P< 0.001 and b=− 3.59 (− 5.04 to − 2.13), P< 0.001, respectively). We could not show modulatory effects of lidocaine on cytokines. High pain scores were linked to the occurrence of PIRC’s (OR=2.028 (1.134– 3.628), P=0.017). Cytokine levels were not related either to acute pain or PIRC.
Conclusion: Dexamethasone modulated the perioperative cytokine response into an anti-inflammatory direction. An overall lidocaine effect was not found. Patients with higher pain scores suffered from more 30-day PIRCs. Cytokine levels were not associated with pain or more postoperative complications, even not with PIRC. Larger studies in breast cancer surgery are needed to confirm these explorative results.
Keywords: acute pain, anesthetic agents, immune response, lumpectomy, perioperative outcomes
Plain Language Summary
Cytokines are small proteins that are crucial in controlling the activity of the body’s immune system. The body’s response to an injury or other trigger like surgery is called “inflammation”.
Surgery is a cornerstone of breast cancer treatment, but induces the release of pro-inflammatory and anti-inflammatory cytokines. An imbalance in the pro-inflammatory direction results in more pain and more morbidity after an operation. This may contribute to the potential negative impact of breast cancer surgery on the quality of life in up to half of the patients. Anesthetic drugs used during surgery, such as lidocaine and dexamethasone, may however counteract the cytokine imbalance beneficially.
What Is Unknown?
- The impact of dexamethasone and lidocaine on cytokine responses is unclear for patients undergoing conservative breast cancer surgery, like lumpectomy without axillary lymph node dissection.
- The interrelationships between cytokine levels, pain scores and complications after lumpectomy are unknown.
We investigated 48 patients undergoing breast cancer surgery to answer two questions. Do lidocaine and/or dexamethasone steer the immune response into a beneficial anti-inflammatory direction? Does an interrelationship exist between cytokine levels, pain scores and postoperative complications?
What This Article Adds
- The immune response of patients receiving dexamethasone 4 mg or 8 mg goes into an anti-inflammatory direction.
- Patients experiencing less pain suffer less from postoperative complications.
Results for lidocaine were inconclusive and we could not demonstrate a link between patient’s cytokine levels and complications. Larger studies are thus needed to confirm our results and to help optimizing perioperative outcomes.
Introduction
Surgery is a cornerstone of breast cancer treatment, but induces a systemic inflammatory response characterized by the release of cytokines. Proinflammatory cytokines like interleukin (IL)-1β, IL-6 and tumor necrosis factor alpha are released after tissue injury. Anti-inflammatory cytokines, like IL-10 and IL-1Ra, are also released to restore homeostasis.1 An imbalance between pro- and anti-inflammatory cytokines activates an inflammatory cascade resulting in increased postsurgical morbidity. Increased levels of IL-6 and ratio of IL-6/IL-10 are proven predictors for developing infectious complications after surgery.2–4
Postoperative pain, chronic pain and postoperative infectious complications contribute to the overall negative impact of breast cancer surgery on the quality of life in up to half of the patients.5,6 Acute postoperative pain, which is a predictive factor for the development of chronic pain,7,8 may be related to the inflammatory responses after surgery.9 This may help to explain why higher pain scores after surgery contribute to postoperative complications, especially health care-associated infections.10
Various pharmacological regimens proved to counteract the surgery-induced proinflammatory response beneficially, thus reducing morbidity after operation.11 Dexamethasone, given in a high dose of up to 100 mg before cardiac surgery with cardiopulmonary bypass, shifted the cytokine profile into the anti-inflammatory direction.12 Intravenous (IV) lidocaine attenuates the hyperinflammatory response after tissue injury, thus reducing pain and improving outcomes.13,14
For patients undergoing breast cancer surgery, and specifically in conservative surgery like lumpectomy without axillary lymph node dissection, the impact of dexamethasone and IV lidocaine on cytokine responses is unclear. The question thereby arises if a single low dose of dexamethasone could be effective enough. Four mg is commonly administered for its anti-emetic effect and 8 mg for its additional analgesic effect.15 Furthermore, the interrelationships between cytokine levels, pain scores and postoperative complications after lumpectomy have not yet been investigated. Understanding immune-mediated mechanisms underlying acute pain and complications could help develop multimodal analgesia regimens and optimize outcome after breast cancer surgery.
Therefore, we performed an explorative study including patients undergoing a lumpectomy for breast cancer. First, we aimed to evaluate whether a low dose of dexamethasone or IV lidocaine modulates the early cytokine response into an anti-inflammatory direction. Second, we aimed to confirm the link between higher pain scores and 30-day surgical complications. Third, we aimed to investigate whether a proinflammatory-oriented cytokine balance is associated with higher pain scores and immune-mediated surgical complications.
Patients and Methods
We consecutively performed three double blinded randomized controlled trials at the Alexander Monro Hospital, a non-teaching hospital (10 hospital beds) specialized in breast cancer care (Bilthoven, The Netherlands). The Institutional Review Board approved all study protocols and documents. The study was registered prior to patient enrollment at the European Clinical Trial Register (EudraCT 2012–002222-70; Principal Investigator: Prof. K.C.P. Vissers; Date of registration: November 15, 2013). The protocol can be accessed after a reasonable request at protocols.io (https://www.protocols.io/edit/targeting-the-inflammatory-response-after-breast-c-bc56iy9e). Procedures followed were in accordance with the Declaration of Helsinki and adhere to the applicable Consolidated Standards of Reporting Trials (CONSORT) guidelines. Participating patients gave written informed consent.
Patients
We assessed potential participants for eligibility during the preoperative anesthesia consultation. Female patients aged older than 18 years undergoing primary breast cancer surgery without axillary lymph node dissection were eligible for inclusion. Exclusion criteria were: allergy to amide type local anesthetics, myocardial ischemia within the previous six months, hypokalemia (<3.5 mmol∙L−1), renal impairment (creatinine clearance < 60 mL∙min−1∙1.73m−2) or liver function impairment (international normalized ratio > 1.8), pregnancy or breastfeeding, use of anti-arrhythmic drugs, chronic opioid use, a history of chronic pain, corticosteroid use, and the inability to provide written informed consent.
Patient Groups
In the three consecutive studies, patients received no dexamethasone or dexamethasone 4 mg or dexamethasone 8 mg before induction of general anesthesia. In each of the studies, patients were randomly assigned in a 1:1 ratio to one of two groups (IV lidocaine or IV placebo) using a sealed envelope with blocks of eight.
An external pharmacy delivered the blinded study medication to the anesthetic department in identically appearing 50 mL syringes. Lidocaine 1.5 mg∙kg−1 or placebo (saline 0.9% in the same volume) was administered as a bolus in ten minutes before induction of anesthesia, followed by a continuous infusion of IV lidocaine at 2 mg∙kg−1∙hour−1 or placebo until one hour after end of surgery. The lidocaine dosing regimen is consistent with most references, but the optimal dosing is unknown.13 Potential adverse events caused by IV lidocaine were noted. They were reported by patients or observed by site staff monitoring patient’s vital signs.
Measurements
There were four measuring points: at baseline, when patients arrived on the operation theater complex before surgery (t0), admission on post-anesthesia care unit (PACU) (t1), 4 hours after end of surgery (t2), and 30 days after surgery.
Pain scores were assessed using the Numeric Rating Scale (NRS 0 = no pain, NRS 10 = worst pain imaginable).16 NRS was recorded at t0, t1, and t2.
Cytokine levels of IL-6, IL-10, IL-1β and IL-1Ra were measured at t0 and t2. Ethylenediaminetetraacetic anticoagulated blood was centrifugated immediately after withdrawal at 2000 x g for ten minutes at 4°C, after which plasma was stored at −80°C. Cytokines were analyzed batch-wise by Simplex AssaysTM according to the manufacturer’s instructions as previously described.17 Baseline levels of low abundance cytokines, including IL-6, IL-10 and IL-1β, proved to be below the lower limit of quantification by standard enzyme-linked immunosorbent assays. The Ella microfluidic analyzer (Protein Simple, San Jose, CA, USA) was therefore used to assess cytokine concentrations in the fg∙mL−1 to low pg∙mL−1 range.18
We classified any medical adverse outcome occurring between admission and 30 days after surgery according to the Clavien Dindo Classification (CDC) of surgical complications. The CDC systematically scores postoperative complications in five grades and is a preferred method for outcome assessment after surgery.19 A complication with a potential immune-mediated pathway, ie an infection or persistent postsurgical pain, was noted as a potential immune-related complication (PIRC).3,9,20 The study physician searched retrospectively for complications and then classified them according to CDC. A medical researcher, who was not involved in the study or patient’s care, checked the records to ensure completeness of data.
Perioperative Management
Acetaminophen 1 gr PO was given one hour before operation. We induced anesthesia with IV propofol and sufentanil and maintained it with continuous infusion of IV propofol supplemented with sufentanil according to clinical needs. The total dose of sufentanil at end of surgery was recorded. Intraoperative monitoring included pulse oximetry, capnography, electrocardiography and non-invasive blood pressure measurement. IV diclofenac 75 mg was administered at the end of surgery unless there was a contraindication for non-steroidal anti-inflammatory drugs.
Patients remained at the PACU for at least one hour until discontinuation of the study medication. Nurses assessed postoperative nausea and vomiting and the Aldrete score per standard protocols. Patients with NRS ≥ 4 at admission on the PACU received IV piritramide 2.5 mg at ten minutes intervals until NRS ≤ 3. Clonidine 75 μg was given at ten minutes intervals when, despite administration of piritramide, the NRS remained ≥4 on condition that the heart rate was ≥80 bpm and systolic blood pressure was ≥120 mmHg. Postoperative pain management on the ward consisted of acetaminophen 1 gram four times daily, diclofenac 50 mg three times daily and piritramide 0.1 mg∙kg−1 six times daily as needed.
After discharge patients received standard care. One week post-surgery a follow-up visit was scheduled with a nurse and the surgeon. Thereafter, patients received regular clinical care by their radiotherapist, surgeon or nurse, depending on their oncologic plan. Postoperative complications were documented in patients’ records.
Statistical Analysis
Our study is based upon the analysis of pooled data obtained in three consecutive randomized controlled trials. As this was an explorative study, no formal sample size calculation was made. Our results should, therefore, be regarded as explorative and hypothesis-generating.
Results for all variables are given as mean (SD) [minimum–maximum], unless stated otherwise. The differences between plasma levels of cytokines at t2 and those at t0 are given as ΔIL-6, ΔIL-10, ΔIL-1β and ΔIL-1Ra. The balance between pro- and anti-inflammatory cytokines is described by the ratio of IL-6/IL-10 and the ratio of IL-1β/IL-1Ra. The differences between these ratios at t2 and those at t0 are given as ΔIL-6/IL-10 and ΔIL-1β/IL-1Ra.
Multiple linear regression analysis was used to evaluate the null hypothesis that lidocaine or dexamethasone had no effect on ΔIL-6, ΔIL-10, ΔIL-1β, ΔIL-1Ra, ΔIL-6/IL-10 and ΔIL-1β/IL-1Ra. Descriptive analysis preceded formal statistical analysis. Based on a striking pattern in the dataset, we introduced “post hoc” the duration of surgery as an independent variable.
Univariate binary logistic regression models using pooled data were estimated to evaluate the potential association between NRS at t2 and the occurrence of any complication according to CDC or a PIRC. The association between the cytokine levels and their ratios at t0 and t2 on one hand, and the occurrence of a PIRC on the other hand, was evaluated similarly.
A Spearman’s Rho correlation was used to determine if NRS at t2 was related to each of the cytokine levels at t0 and t2.
Statistical analysis was performed using IBM SPSS Statistics V25.0 and GraphPad Prism V8.0 (GraphPad Software). P<0.05 was considered statistically significant.
Results
We analyzed data for 48 patients with eight patients in each of six groups (Figure 1).
 |
Figure 1 Consolidated Standards of Reporting Trials (CONSORT) flow chart of the three consecutive randomized controlled trials. |
The six groups were similar in respect of physical and clinical characteristics, except surgery duration (Table 1). All patients underwent breast-conserving surgery with a sentinel node biopsy. The intraoperative and direct postoperative course was uneventful. All patients could be discharged from the PACU one hour after admission (Aldrete score ≥ 13).
 |
Table 1 Patients’ Perioperative Characteristics in the Three Consecutive Randomized Controlled Trials |
We supply patients’ characteristics of pooled lidocaine groups and pooled dexamethasone groups in the Supplementary Table S1 and all raw cytokine levels in the Supplementary Table S2. IL-1β levels at baseline were lower in the study with dexamethasone 8 mg than those in the other two studies.
Pain and Its Treatment
Pain scores and pain treatment are summarized in Table 1. At t1, 94% of the patients had NRS ≤ 4 and 96% at t2. Opioid and diclofenac use was similar among patient groups during and after operation. Rescue drug clonidine was not administered in the third trial where dexamethasone 8 mg was given alone or combined with IV lidocaine.
Postoperative Complications
A total of 23 postoperative complications occurred in 22 (45.8%) patients (Table 1). Sixteen patients (33% of the total) had a CDC grade I complication. Four patients (8%) had a CDC grade II, and two patients had a CDC grade III complication. The groups were similar in respect of complications.
Eight patients suffered from a PIRC: persistent pain (n=3), mucositis (1), surgical site infection treated with antibiotics (2), and abscess treated with antibiotics and surgery (2). Fourteen patients suffered from the following complications: hematoma (6), seroma (3), nausea (3), constipation (1), light headedness (1), and a transient ischemic attack (1). One of these fourteen patients suffered from two CDC grade I complications.
Effects of Dexamethasone and Lidocaine on Postoperative Cytokine Levels
Figures 2 and 3 reveal patterns in the individual observations. Observations above the zero line in Figures 2 and 3 are increases in cytokine levels or their ratios between t0 and t2.
In most patient groups the levels of IL-6 and IL-10 (Figure 2A and B) and IL-1Ra (Figure 3B) seemed to increase after surgery. In contrast, IL-1β levels (Figure 3A) appeared not to change. Lidocaine had little or no effect on the cytokine levels and their ratios in comparison with placebo (Figures 2A and C, 3A–C), except that it seemed to increase IL-10 levels when combined with dexamethasone 8 mg (Figure 2B). Dexamethasone 4 and 8 mg appeared to reverse ΔIL-6/IL-10 (Figure 2C) but seemed to have no effect on IL-6 (Figure 2A), IL-1β (Figure 3A), and IL-1Ra (Figure 3B) levels. Dexamethasone 8 mg appeared to increase the IL-10 level (Figure 2B). Figures 2C and 3C suggest that a longer duration of surgery increased ΔIL-6/IL-10 but decreased ΔIL-1β/IL-1Ra.
Multiple regression confirms our descriptive analysis and untangles the separate effects of lidocaine, dexamethasone 4 mg, dexamethasone 8 mg and duration of surgery (Table 2). An overall effect of lidocaine on measured cytokines was not found. There is only suggestive evidence that dexamethasone 4 mg increased IL-10 level (P=0.045; positive “b” in Table 2 means increase). Dexamethasone 4 mg decreased IL-6/IL-10 (P<0.001; negative “b” means decrease). Dexamethasone 8 mg increased the IL-10 level (P<0.001) and decreased IL-6/IL-10 (P<0.001). As a “post hoc” independent variable the duration of surgery increased postoperative IL-6 levels (P=0.026) and IL-6/IL-10 (P=0.04), but decreased IL-1β/IL-1Ra (P=0.016).). Eg the result for surgery time in Table 2 means that the average increase in IL-6 level was 0.162 pg∙mL−1 per minute or 9.72 pg∙mL−1 per hour of surgery time, independently from the other independent variables.
 |
Table 2 The Effects (Column “b”) of Lidocaine, Dexamethasone 4 mg, Dexamethasone 8 mg and Surgery Duration on the Levels of Interleukins and Their Ratios After Surgery |
IL-1Ra increased from 268 pg·mL-1 to 8075 pg·mL-1 in one patient of the dexamethasone 8 mg-lidocaine group (Figure 3B). As this outlier was not an influential observation, it was not excluded from regression analysis.
Relationships Between Cytokine Levels, Pain Scores and Postoperative Complications
Patients who experienced higher pain scores at t2 seemed to be prone to suffer from any complication according to CDC within 30 days after surgery (OR=1.464, 95% CI=0.941–2.278; P=0.091) (Table 3). A statistically significant association exists between having postoperative pain and the occurrence of a PIRC: the odds ratio associated with a one-point increase in NRS is 2.028 (95% CI=1.134–3.628; P=0.017). This implies that patients who score an NRS of 5 have eight times the odds of having immune-related complications as those with an NRS of 2 (2.0283=8.341, where the exponent 3 is the difference between 5 and 2).
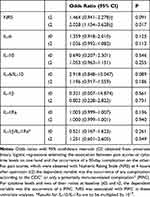 |
Table 3 Relationships Between Cytokine Levels, Pain Scores and Postoperative Complications |
Cytokine levels showed no relation with the occurrence of a PIRC (Table 3), although there is some suggestive evidence that the preoperative ratio of IL-6/IL-10 could relate to the occurrence of a PIRC (OR=2.918, 95% CI=0.848–10.047; P=0.089).
No correlation was found between NRS at t2 and cytokine levels at t0 or t2. The low P-value (P=0.078), for the correlation between postoperative IL-10 levels and pain scores at t2 might be regarded as “indicative”. However, no correction for the number of tests was done (Supplementary Table S3).
Discussion
This prospective explorative study in 48 patients undergoing a lumpectomy for breast cancer has several findings, but the small sample size in each group precludes drawing firm conclusions. Dexamethasone 4 mg or 8 mg, but not IV lidocaine, modulated the early cytokine response into an anti-inflammatory direction. Raw cytokines levels were not clearly related to postoperative pain scores or the occurrence of a PIRC. However, there was suggestive evidence that a more pro-inflammatory cytokine balance at baseline could result in more PIRC’s.
Despite the small number of patients, we may be more confident in the evidence supporting a link between higher pain scores noted 4 hours postoperatively and PIRC in our homogenous group of patients as our finding accords with previous research.10 In a cohort of 1014 patients undergoing a broad spectrum of surgeries, higher pain scores or unacceptable pain after surgery were associated with more 30-day complications, especially health care-associated infections.10
Anesthetic agents may aid to improve postoperative outcomes21,22 by counteracting the neuroendocrine stress response to surgery or through a direct effect on immune cells. The choice of anesthetic agents can even be crucially important for patients with pre-existing immune dysfunction or tumor burden.21,22 However, the impact of lidocaine or dexamethasone, which have different immune-modulating effects on cytokine responses, has not been investigated after lumpectomy.
IV lidocaine is known to reduce a hyperinflammatory response,13 and to decrease proinflammatory cytokines in abdominal surgery.23,24 Under the circumstances of our study no overall effect of lidocaine on postoperative cytokine levels was found. Most likely, lumpectomy is a surgical procedure with less inflammatory stimuli,23 where lidocaine may have little effect.
Dexamethasone’s anti-inflammatory effects depend on its dosage in relation to the extent of tissue trauma. After high dose corticosteroids for major abdominal or thoracic surgery, earlier reports demonstrated a shift towards an anti-inflammatory balance characterized by suppressed IL-6 and increased IL-10 levels, while effects on IL-1 and IL-1Ra levels were absent or showed a decrease.12,25,26 A lower dose, like dexamethasone 8 mg, during abdominal surgery had no effect on IL-10 levels, and reduced27 or showed no effect on IL-6 levels.28 As lumpectomy can be regarded as minimally invasive surgery, when compared with abdominal or cardiac surgery, a less pronounced increase of pro-inflammatory cytokines could be expected. Accordingly, we found a decrease in IL-10 levels and in the ratio IL-6/IL-10, but no significant effect on the levels of IL-6 and IL-1β. Surprisingly, patients in the third pilot study had pre-existent lower levels of IL-1β, which could have masked a potential effect of dexamethasone 8 mg on the postoperative IL-1β levels in this group.
Increased IL-6 levels proved to be related to infectious complications3,4 and higher pain levels.29,30 Also in pathological pain models, elevated expressions of IL-6 and IL-1β in the spinal cord and dorsal root ganglia have been found.31,32 In our study we found a link between higher pain scores and more PIRC’s, suggesting a similar underlying immune-mediated mechanism. However, we failed to find a statistically significant relation between cytokine levels and pain scores or PIRC’s. Besides a small sample size, potential explanations are: the postoperative cytokine response was influenced by dexamethasone, the postoperative inflammatory response was minimal, the time of measurement was not well chosen, or the range of pain scores was too small. Interestingly, patients with a pre-existent higher IL-6 to IL-10 ratio tended to develop more postoperative complications, which can indicate a vulnerability in a subset of patients.
Our study was not powered to find a statistically significant difference in postoperative pain scores between patient groups, but a clear pattern was present. All patients, who received dexamethasone 8 mg, did not need extra clonidine as rescue analgesic. Those patients who received also lidocaine, apart from the dexamethasone 8 mg, had no pain immediately after surgery. These findings accord with those of others.33–36
Our study has important limitations that are to be avoided in future research. We performed the three studies in a consecutive order so that dexamethasone was not blindly administered (Figure 1). This may have influenced expectations and subsequently postoperative pain scores. A further limitation was the small number of patients in each group. Thus, negative results are to be interpreted with caution. Furthermore, we only measured cytokine levels measured at a single time point postoperatively. Hence, we cannot draw any firm conclusions whether the early postoperative immune imbalance could explain the acute pain and complications. Lastly, we used current literature3,9,20 to designate not only infections but also pain as complications with a potential immune-mediated mechanism. Notwithstanding, we conceivably missed other specific immune-mediated complications which could have influenced our results.
A strength is that we performed a single-center study with a small team and a strictly protocolized environment. Consequently, inclusion rate was rather low. An extra delaying factor was that we had to switch from a standard to a more sensitive measuring method to assess very low cytokine concentrations.18 Thus, time bias cannot be ruled out.
Conclusions
Our study suggests research subjects for future larger scale studies in patients undergoing a lumpectomy for breast cancer: (1) Confirming that dexamethasone 4 mg or 8 mg, but not IV lidocaine, modulates the cytokine response into an anti-inflammatory direction. These effects should be evaluated by measuring cytokine levels on several time points postoperatively and surgery duration should be included as a variable. (2) Investigating the relation between acute pain and postoperative complications, especially PIRC’s. (3) Investigating the relation between 30-day complications and the preoperative ratio IL-6/IL-10 or other immune-mediated mechanisms.
Perioperatively a dynamic balance exists between pro- and anti-inflammatory cytokines. It is still an ongoing debate about the kind of immune balance we should aim at.22 Although larger studies are needed, we provided new pieces of information that may help developing a multimodal approach to the prevention of complications after breast cancer surgery.
Abbreviations
CDC, Clavien Dindo Classification; CI, confidence interval; IL, interleukin; IV, intravenous; NRS, Numeric Rating Scale; OR, odds ratio; PACU, post anesthesia care unit; PIRC, potential immune-related complications; SD, standard deviation.
Data Sharing Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85(1):109–117. doi:10.1093/bja/85.1.109
2. Miyaoka K, Iwase M, Suzuki R, et al. Clinical evaluation of circulating interleukin-6 and interleukin-10 levels after surgery-induced inflammation. J Surg Res. 2005;125(2):144–150. doi:10.1016/j.jss.2004.12.001
3. Mokart D, Merlin M, Sannini A, et al. Procalcitonin, interleukin 6 and systemic inflammatory response syndrome (SIRS): early markers of postoperative sepsis after major surgery. Br J Anaesth. 2005;94(6):767–773. doi:10.1093/bja/aei143
4. Oka Y, Murata A, Nishijima J, et al. Circulating interleukin 6 as a useful marker for predicting postoperative complications. Cytokine. 1992;4(4):298–304. doi:10.1016/1043-4666(92)90070-8
5. Tian Y, Schofield PE, Gough K, Mann GB. Profile and predictors of long-term morbidity in breast cancer survivors. Ann Surg Oncol. 2013;20(11):3453–3460. doi:10.1245/s10434-013-3004-8
6. Verbelen H, Tjalma W, Meirte J, Gebruers N. Long-term morbidity after a negative sentinel node in breast cancer patients. Eur J Cancer Care. 2019;28:e13077.
7. Schug SA, Bruce J. Risk stratification for the development of chronic postsurgical pain. Pain Rep. 2017;2(6):e627. doi:10.1097/PR9.0000000000000627
8. Lavand’homme P. Transition from acute to chronic pain after surgery. Pain. 2017;158(Suppl 1):S50–S54. doi:10.1097/j.pain.0000000000000809
9. Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229(1–2):26–50. doi:10.1016/j.jneuroim.2010.08.013
10. van Boekel RLM, Warle MC, Nielen RGC, et al. Relationship between postoperative pain and overall 30-day complications in a broad surgical population: an observational study. Ann Surg. 2019;269(5):856–865.
11. Amaya F, Hosokawa T, Okamoto A, et al. Can acute pain treatment reduce postsurgical comorbidity after breast cancer surgery? A literature review. Biomed Res Int. 2015;2015:641508. doi:10.1155/2015/641508
12. El Azab SR, Rosseel PM, de Lange JJ, et al. Dexamethasone decreases the pro- to anti-inflammatory cytokine ratio during cardiac surgery. Br J Anaesth. 2002;88(4):496–501. doi:10.1093/bja/88.4.496
13. van der Wal SE, van den Heuvel SA, Radema SA, et al. The in vitro mechanisms and in vivo efficacy of intravenous lidocaine on the neuroinflammatory response in acute and chronic pain. Eur J Pain. 2016;20(5):655–674. doi:10.1002/ejp.794
14. Kranke P, Jokinen J, Pace NL, et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery. Cochrane Database Syst Rev. 2015;7:CD009642.
15. Waldron NH, Jones CA, Gan TJ, Allen TK, Habib AS. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth. 2013;110(2):191–200. doi:10.1093/bja/aes431
16. Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41(6):1073–1093. doi:10.1016/j.jpainsymman.2010.08.016
17. Aldo P, Marusov G, Svancara D, David J, Mor G. Simple Plex™: a novel multi-analyte, automated microfluidic immunoassay platform for the detection of human and mouse cytokines and chemokines. Am J Reprod Immunol. 2016;75(6):678–693. doi:10.1111/aji.12512
18. Ter Horst R, Jaeger M, Smeekens SP, et al. Host and environmental factors influencing individual human cytokine responses. Cell. 2016;167(4):1111–1124 e1113. doi:10.1016/j.cell.2016.10.018
19. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi:10.1097/SLA.0b013e3181b13ca2
20. Arnaoutoglou E, Kouvelos G, Papa N, et al. Prospective evaluation of post-implantation inflammatory response after EVAR for AAA: influence on patients’ 30 day outcome. Eur J Vasc Endovasc Surg. 2015;49(2):175–183. doi:10.1016/j.ejvs.2014.12.006
21. Cruz FF, Rocco PR, Pelosi P. Anti-inflammatory properties of anesthetic agents. Crit Care. 2017;21(1):67. doi:10.1186/s13054-017-1645-x
22. Hsing CH, Wang JJ. Clinical implication of perioperative inflammatory cytokine alteration. Acta Anaesthesiol Taiwan. 2015;53(1):23–28. doi:10.1016/j.aat.2015.03.002
23. Ortiz MP, Godoy MC, Schlosser RS, et al. Effect of endovenous lidocaine on analgesia and serum cytokines: double-blinded and randomized trial. J Clin Anesth. 2016;35:70–77. doi:10.1016/j.jclinane.2016.07.021
24. Yardeni IZ, Beilin B, Mayburd E, Levinson Y, Bessler H. The effect of perioperative intravenous lidocaine on postoperative pain and immune function. Anest Analg. 2009;109(5):1464–1469. doi:10.1213/ANE.0b013e3181bab1bd
25. Sato N, Koeda K, Ikeda K, et al. Randomized study of the benefits of preoperative corticosteroid administration on the postoperative morbidity and cytokine response in patients undergoing surgery for esophageal cancer. Ann Surg. 2002;236(2):184–190. doi:10.1097/00000658-200208000-00006
26. Holte K, Kehlet H. Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg. 2002;195(5):694–712. doi:10.1016/S1072-7515(02)01491-6
27. Zargar-Shoshtari K, Sammour T, Kahokehr A, Connolly AB, Hill AG. Randomized clinical trial of the effect of glucocorticoids on peritoneal inflammation and postoperative recovery after colectomy. Br J Surg. 2009;96(11):1253–1261. doi:10.1002/bjs.6744
28. Kirdak T, Yilmazlar A, Cavun S, Ercan I, Yilmazlar T. Does single, low-dose preoperative dexamethasone improve outcomes after colorectal surgery based on an enhanced recovery protocol? Double-blind, randomized clinical trial. Am Surg. 2008;74(2):160–167.
29. Lisowska B, Maldyk P, Kontny E, Michalak C, Jung L, Cwiek R. Postoperative evaluation of plasma interleukin-6 concentration in patients after total hip arthroplasty. Ortop Traumatol Rehabil. 2006;8(5):547–554.
30. Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 2008;140(1):135–144. doi:10.1016/j.pain.2008.07.024
31. Zhou YQ, Liu Z, Liu ZH, et al. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation. 2016;13(1):141. doi:10.1186/s12974-016-0607-6
32. Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev. 2009;60(1):57–64. doi:10.1016/j.brainresrev.2008.12.020
33. Gomez-Hernandez J, Orozco-Alatorre AL, Dominguez-Contreras M, et al. Preoperative dexamethasone reduces postoperative pain, nausea and vomiting following mastectomy for breast cancer. BMC Cancer. 2010;10:692. doi:10.1186/1471-2407-10-692
34. Wattwil M, Thorn SE, Lovqvist A, Wattwil L, Gupta A, Liljegren G. Dexamethasone is as effective as ondansetron for the prevention of postoperative nausea and vomiting following breast surgery. Acta Anaesthesiol Scand. 2003;47(7):823–827. doi:10.1034/j.1399-6576.2003.00172.x
35. Grigoras A, Lee P, Sattar F, Shorten G. Perioperative intravenous lidocaine decreases the incidence of persistent pain after breast surgery. Clin J Pain. 2012;28(7):567–572. doi:10.1097/AJP.0b013e31823b9cc8
36. Kim MH, Lee KY, Park S, Kim SI, Park HS, Yoo YC. Effects of systemic lidocaine versus magnesium administration on postoperative functional recovery and chronic pain in patients undergoing breast cancer surgery: a prospective, randomized, double-blind, comparative clinical trial. PLoS One. 2017;12(3):e0173026. doi:10.1371/journal.pone.0173026
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


