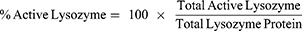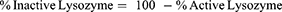Back to Journals » Clinical Ophthalmology » Volume 15
Activity of Deposited Lysozyme on Contemporary Soft Contact Lenses Exposed to Differing Lens Care Systems
Authors Heynen M , Ng A, Martell E, Subbaraman LN , Jones L
Received 20 January 2021
Accepted for publication 17 March 2021
Published 23 April 2021 Volume 2021:15 Pages 1727—1733
DOI https://doi.org/10.2147/OPTH.S296116
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Miriam Heynen,1 Alan Ng,1 Elizabeth Martell,1 Lakshman N Subbaraman,1 Lyndon Jones1,2
1Centre for Ocular Research and Education (CORE), School of Optometry and Vision Science, University of Waterloo, Waterloo, Ontario, Canada; 2Centre for Eye and Vision Research (CEVR), Hong Kong Science Park, Hong Kong
Correspondence: Miriam Heynen
Centre for Ocular Research and Education (CORE), School of Optometry and Vision Science, University of Waterloo, 200 University Avenue West, Waterloo, Ontario, N2L 3G1, Canada
Tel +1 519 888 4567
Fax +1 519 888 4303
Email [email protected]
Purpose: The amount of protein deposition on soft contact lenses and to what extent the proteins are denatured may have an impact on comfortable wearing times of contact lenses. The purpose of this study was to evaluate the effects of two lens care systems on total protein and the quantity and activity of lysozyme deposited on worn senofilcon A, silicone hydrogel contact lenses.
Participants and Methods: Thirty symptomatic soft contact lens wearers were enrolled into a 4-week prospective, randomized, bilateral eye, daily-wear, crossover, double-masked study. Participants were fitted with biweekly senofilcon A lenses and were assigned either a polyquaternium-1 and myristamidopropyl dimethylamine-containing system (OPTI-FREE RepleniSH) or a peroxide-based system (CLEAR CARE). After each wear period, proteins were extracted from the lenses and analyzed for total protein, total lysozyme quantity and activity.
Results: The use of either the peroxide-based system or the polyquaternium-1 and myristamidopropyl dimethylamine-containing system resulted in no difference (P> 0.05) to the amount of total protein deposited on the lenses (6.7 ± 2.8 micrograms/lens versus 7.3 ± 2.8 micrograms/lens, respectively) or to the amount of denatured lysozyme deposits (0.8 ± 0.7 versus 0.9 ± 0.7 micrograms/lens), respectively. The total amount of lysozyme deposited on the lenses was significantly lower when using the peroxide-based system (1.3 ± 0.9 micrograms/lens) compared to the polyquaternium-1 and myristamidopropyl dimethylamine-containing system (1.7 ± 1.0 micrograms/lens) (P=0.02).
Conclusion: The inactivation of lysozyme deposited on senofilcon A lenses when disinfected with the peroxide-based or the polyquaternium-1 and myristamidopropyl dimethylamine-containing systems were neither statistically nor clinically significant and the overall amounts of denatured lysozyme recovered from the lenses were low (< 1 microgram/lens).
Keywords: contact lens, contact lens care system, lysozyme, protein activity, silicone hydrogel
Introduction
Contact lenses are coated with tear film constituents almost immediately following their placement on the ocular surface and exposure to the tear film.1–4 Contact lens deposits can potentially cause discomfort, dryness, and a reduction in visual acuity,5,6 all of which are known factors leading to discontinuation from contact lens wear.7
A recent international survey8 reported that soft contact lenses account for approximately 90% of contact lenses fitted by practitioners, and that almost 70% of these soft lens wearers use planned replacement lenses. The use of lens care regimens for cleaning and disinfecting contact lenses is essential for reusable lens wearers, since failure to maintain proper hygiene of contact lenses may result in severe contact lens-related ocular complications such as microbial keratitis.9,10 Contemporary multi-purpose cleaning and disinfecting (MPS) lens care products, often including complex mixtures of wetting agents and surfactants, are designed to enhance on-eye wettability and reduce deposition and buildup of proteins and lipids on contact lenses.11 Hydrogen peroxide systems tend to have fewer formulation constituents than MPS, but they may contain additional components such as surfactants and wetting agents.11 Differences between these two types of lens care regimens may potentially result in differences in overall comfort, dryness, and visual performance for some contact lens patients.12,13
During wear, soft contact lenses deposit various tear film components, such as proteins and lipids,14–17 as well as other contaminants, such as bacteria and cosmetics.18,19 An abundant tear film protein typically accumulated on hydrogel lens materials is lysozyme.16,20 This bacteriolytic enzyme serves as an anti-microbial agent in the tear film and is most effective against Gram-positive bacteria.21 Previous studies have shown that high levels of lysozyme accumulate on conventional hydrogel lens materials, particularly United States Food and Drug Administration (FDA) group IV lenses, in comparison to silicone hydrogel lenses.2,16,20 One report has demonstrated an association between active lysozyme recovered from etafilcon A lens material and subjective comfort after several hours of lens wear.22 However, despite the low levels of deposition, the percentage of inactive or denatured lysozyme compared to total lysozyme is relatively greater on silicone hydrogel lens materials compared to conventional hydrogels.20,23,24
Although most contact lens care products are designed to remove deposits from the lens material, previous studies have reported the efficiency of lens care products on protein removal from both conventional and silicone contact lenses to be less than 50%.25–27 In addition to removing deposits and disinfecting contact lenses, it may be important for lens care regimens to maintain the active state of the deposited protein, for optimal lens performance and comfort.22 Proteins, specifically enzymes such as lysozyme, lose activity through processes that alter their conformational state or alter the substrate-binding site. This could arise from unfolding of the protein due to non-normative environments for pH, temperature or hydrophobic environments such as those found at air/water interfaces.28
The purpose of this investigation was to evaluate the effects of two different lens care systems on the activity of lysozyme deposited on a contemporary soft contact lens material.
Participants and Methods
A total of 30 symptomatic subjects (7 males, 23 females) were enrolled in this study (Clinical Registration Number: NCT00520351) and all were adapted soft lens wearers, wearing their habitual lenses on a daily wear basis (with a bi-weekly or monthly replacement schedule). This sample size was based on standard practice for a study of this type examining clinically sourced lenses for protein deposition. The range in age was from 18 to 43 years with an average age of 24.3 years.
Study participants did not use either the peroxide-based system (CLEAR CARE Cleaning & Disinfecting Solution, Alcon, Fort Worth, TX, USA) or the polyquaternium-1 and myristamidopropyl dimethylamine-containing system (OPTI-FREE RepleniSH, Multi-Purpose Disinfecting Solution, Alcon, Fort Worth, TX, USA) prior to commencement of the study, had a visual acuity of greater than 6/9 and were 18 years and older. Participants filled out a 3-part questionnaire and enrolled if the following criteria were fulfilled: 1) participants were not comfortable all day long in their contact lenses and either 2) took their contact lenses out sooner than they would like because they became uncomfortable or 3) despite late-in-the-day contact lens discomfort, continued wearing their contact lenses. Participants agreed to wear the study lens for 6 to 7 days per week and at least 12 hours per day.
Informed consent was obtained from all participants, ethics clearance (R/265/07/L) was acquired through the Office of Research Ethics at the University of Waterloo prior to commencement of the study and all aspects of the study conformed to the tenets of the Declaration of Helsinki.
This study was conducted as a 4-week prospective, randomized, bilateral eye, daily-wear, dispensing study in which investigators and participants were masked to the lens care product. During the first 2-week phase, all participants were fitted with senofilcon A silicone hydrogel contact lenses (Acuvue Oasys; Johnson & Johnson, Jacksonville, FL, USA) and randomly assigned to use one of the two lens care products: a 3% peroxide-based system or the polyquaternium-1 and myristamidopropyl dimethylamine-containing (Polyquad/Aldox)-containing system (Table 1). Participants were instructed to use both lens care products according to the masked package insert instructions for use. Following the first phase, test lenses were collected and participants were re-fitted with new senofilcon A lenses and assigned the other lens care product.
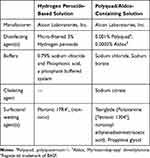 |
Table 1 Components of the Formulation of the Lens Care Systems Evaluated in the Study |
After each 2-week phase, worn lenses were collected from each participant. Proteins were extracted from lenses for 24 hours in 1.5 millilitres of 50:50 solution of 0.02% trifluoroacetic acid: acetonitrile23,24 in high-density polyethylene vials. Following extraction, aliquots of lens extracts for analysis of total protein, lysozyme activity and total lysozyme (0.65 millilitres, 0.375 millilitres and 0.275 millilitres, respectively) were transferred to Axygen microcentrifuge tubes (Corning, Corning, NY, USA) and evaporated to dryness using a vacuum concentrator, Savant SpeedVac (Thermo Fisher Scientific, Waltham MA, USA). Dried samples were stored at −80°C prior to quantification procedures.
Total Protein Measurement
A modified Bradford protein assay29 was used to quantify total protein in this study. Aliquots of contact lens extracts dedicated for protein assays were re-suspended in 20 microlitres phosphate buffer solution (0.137 M sodium chloride, 0.0027 M potassium chloride, 0.0119 M phosphate buffer, pH 7.4), and neutralized with 170 microlitres 0.0735 M phosphate pH 7.2. Neutral pH was confirmed with pH paper. Calcium chloride (10 microlitres, 500 mM) was added and all tubes were mixed well using a vortex, followed by 5-minute precipitation incubation on ice. Ethanol (1 millilitre, 100%) was added to each tube, mixed, and precipitates were pelleted by centrifugation (15,000x relative centrifugal force x g, 1 minute). The supernatants containing interfering substances, such as lipids and contact lens polymers, were removed by aspiration and the protein pellets were washed with 1 millilitre of 90% (volume/volume) ethanol. The centrifugation and aspiration steps were repeated once more with 90% ethanol. The tubes containing the protein pellet were dried in a vacuum concentrator. Bio-Rad Protein Assay (Biorad, Mississauga, ON, CAN) reagent (50 microlitres) was added to each tube and the protein pellet was completely dissolved by vortexing and short incubations in a boiling water bath (two minutes maximum per tube). All tubes were cooled to room temperature and 200 microlitres of 0.15 M sodium chloride was added. After mixing, the volume was transferred to a 96-well microtiter plate and the optical density at 595 nm and 450 nm was read within 10 minutes. The ratio of the two wavelengths were compared to a standard curve of known amounts of bovine serum albumin to determine the total amount of proteins in each sample.
Total Lysozyme Measurement
Electrophoresis and immunoblotting procedures were adapted from previous studies23,30 and were used for quantifying total lysozyme amounts on aliquots of contact lens extracts. Previous in-house experiments demonstrated greater than 90% recovery of lysozyme from senofilcon A contact lenses. Four-point lysozyme standard curves were separately generated on each Western blot with samples. The standards and samples were incubated with primary antibody at room temperature with shaking for 2½ hours (1:1000 polyclonal rabbit anti-human lysozyme in 5% bovine serum albumin blocking solution). Blots were washed and then incubated with secondary antibody for 1 hour (1:20000 goat anti-rabbit Immunoglobulin G-Horseradish peroxidase at room temperature with shaking). Bound antibody was visualized by enhanced chemiluminescence (ECL Plus, Amersham, Mississauga, ON, CAN) detection and results were captured with a Storm840 Imaging System (Molecular Dynamics, Inc., Sunnyvale, CA, USA).
Lysozyme Activity Measurement
Lysozyme activity was quantified from aliquots of contact lens extracts and neutrophil lysozyme standard at constant pH and temperature, using a fresh suspension of Micrococcus lysodeikticus bacteria for each sample. Previous work has demonstrated that lysozyme standards processed in the same procedure as that used in contact lenses extraction retained 94.0% ± 0.6% activity compared to an unprocessed control.31 Desiccated Micrococcus lysodeikticus cells (Sigma, Oakville, ON, CAN) (10 milligrams) were left to swell overnight at 4°C in 10 millilitres of 50 mM sodium phosphate buffer (pH 6.3). The following day, the cells were diluted to an initial optical density of 1.2 at 450 nanometres in a spectrophotometer fitted with a cuvette holder (Multiskan Spectrum Plate Reader, Thermo Fisher Scientific, Waltham, MA, USA). Once at the appropriate concentration, 1 millilitre of the micrococcal solution was placed in each 4 millilitre cuvette and assayed at 30°C. The initial optical density at 450 nanometres was measured for all samples (time=0) and then at 30-second intervals for 4 minutes after the addition of the appropriate volume of sample (maximum 10 microlitres). The rate of change in optical density at 450 nanometres over time was calculated using linear regression and the slope used to define activity. Activity of samples was compared to activity of known amounts of lysozyme standard and values transformed to determine the amount of active lysozyme extracted from the lens.
Percentage Lysozyme Inactivation
Once the lysozyme activity and total lysozyme protein amounts were calculated for each sample, the percentages of native and inactive lysozyme were determined. The percentage of lysozyme extracted from the lens in the native form is given in Equation 1.
where total active lysozyme was measured using the activity assay and total lysozyme protein was measured by Western blotting. The percentage of inactive lysozyme was calculated as given in Equation 2.
Statistical Analysis
Data analysis was conducted using SigmaStat 3.1 (Systat Software, Inc., San Jose, CA, USA). Data are presented as mean ± standard deviation (unless otherwise stated) and a significance level of P=0.05 was used for all analyses.
Thirty participants were originally enrolled in both phases of the study, totaling a maximum of 60 possible lenses for analysis. However, some lenses were not analyzed for various reasons (such as lens damage during lens wear; 3 during a cycle with peroxide, 1 during a cycle with Polyquad/Aldox) and patient discontinuation (2 consent withdrawal) from the study. Thus, 25 hydrogen peroxide-based and 29 Polyquad/Aldox-containing samples were analyzed.
Results
Participants reported that their total wear time was similar while using the two care regimes (peroxide: 12.4 ± 2.4 vs MPS: 12.5 ± 2.1 hrs; P>0.05). The reported comfortable wear times were also not different between solutions (peroxide: 8.1 ± 2.6 vs MPS: 8.1 ± 2.6 hrs; P>0.05).
Table 2 summarizes the effects of the two lens care products on total protein and lysozyme deposition on worn senofilcon A lenses. In addition, the amount of inactive lysozyme, expressed in both micrograms/lens and percentage of total lysozyme, is reported. Table 2 shows that despite the wide ranges of protein deposits among participants following a 2-week lens wear period, there were no significant differences between using a hydrogen peroxide system or Polyquad/Aldox-containing system when comparing the total amount of protein deposited on the lens material (6.7 ± 2.8 vs 7.3 ± 2.8 micrograms/lens respectively; P=0.24). Total lysozyme deposition was significantly greater when using the Polyquad/Aldox-containing solution compared to the peroxide-based solution (1.7 ± 1.0 vs 1.3 ± 0.9 micrograms/lens respectively; P=0.02) (Table 2). However, when comparing absolute amounts of inactive lysozyme on senofilcon A lenses, there were no significant differences found between the hydrogen peroxide or Polyquad/Aldox-containing systems (P>0.05) (Table 2).
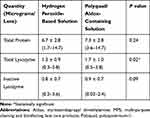 |
Table 2 Summary of ex vivo Lens Protein Deposition (Mean ± Standard Deviation; Minimum–Maximum in Brackets) |
Discussion
This study investigated the impact of two different contact lens cleaning and disinfecting solutions on the removal and inactivation of lysozyme from worn senofilcon A silicone hydrogel contact lenses. This study examined the differences in using a Polyquad/Aldox-containing MPS (OPTI-FREE RepleniSH) and a hydrogen peroxide-based system (CLEAR CARE) on lysozyme deposition on daily worn senofilcon A lenses. The MPS contains a mixture of various wetting agents, surfactants and biocides for cleaning and disinfecting lenses. Two other components in OPTI-FREE RepleniSH are nonanoyl ethylenediaminetriacetic acid and sodium citrate that have a variety of functions, including protein removal, chelation and wetting the contact lens.11 In contrast, the hydrogen peroxide-based system is primarily a strong disinfectant, which destroys pathogens by oxidation.6,11,13 The results from the current study revealed no significant differences between the two lens care products in total protein removal from the senofilcon A silicone hydrogel lens material following a 2-week wearing period. These results are in agreement with previous studies25,27 which investigated the effectiveness of protein removal from poly-hydroxyethyl methacrylate (pHEMA)-based lens materials. In these studies, the preserved care products removed similar ranges of protein deposits from pHEMA lenses compared to hydrogen peroxide-based solutions. To date, there is no clear consensus on the advantages or disadvantages of deposits on silicone hydrogel and pHEMA contact lenses which are replaced biweekly.22,32
Although total protein removal was similar, the efficiency of removing deposited lysozyme from senofilcon A lenses was significantly higher when using the hydrogen peroxide system compared to the Polyquad/Aldox-containing system. These findings are similar to a previous study investigating lysozyme removal from a different silicone hydrogel material (lotrafilcon B).26 In the current study, there were statistically significant differences between the two lens care products on lysozyme removal efficiency from senofilcon A. These differences were not considered clinically relevant since there was no significant difference in clinical measures or subjective comfort. Earlier work has demonstrated that silicone hydrogel lenses tend to deposit significantly lower amounts of lysozyme compared to FDA group IV hydrogel lens materials.20
It has been previously suggested that relatively hydrophobic surfaces (such as those typically exhibited by silicone hydrogel lenses) inactivate deposited proteins at a higher rate than do pHEMA-based lens materials.33,34 Proteins that change their conformational state following sorption to contact lenses can potentially initiate inflammatory responses possibly leading to local or general papillary responses.35 However, the absolute quantities of native and inactive lysozyme remaining on the senofilcon A lens material with the use of either lens care product is two orders of magnitude less than that found on an FDA group IV lens, etafilcon A, following use of an MPS care product.3,30 There was no significant difference in the percentage of inactive lysozyme determined when using either solution. This suggests that despite their differences in composition, both solutions share similar abilities to maintain lysozyme activity on worn senofilcon A lenses.
While there were no differences in physical characteristics of selected lens deposits between the two care systems reported in this study, other studies comparing peroxide-based systems and MPS on silicone hydrogels have reported differences in ocular surface and clinical outcomes. For example, the use of peroxide-based systems on silicone hydrogel lenses had a reduced number of corneal infiltrative events,4,13,36–38 less solution-induced corneal staining39 and less epithelial cell shedding40 compared to MPS care products. That there are differences in clinical outcomes based on what type of care product is used suggests an interaction between the contact lens and the ocular surface. Further work is warranted to understand the factors that influence contact lens deposits on human worn lenses and subsequent interaction with the patients’ ocular surface and their tear film.
Conclusion
The results demonstrate that patients using senofilcon A silicone hydrogel lenses disinfected with the Polyquad/Aldox-containing solution and hydrogen peroxide solution show similar amounts of total protein deposits. The efficiency of removing deposited lysozyme from senofilcon A lenses was statistically higher when using the hydrogen peroxide system compared to the Polyquad/Aldox-containing system. However, both lens care solutions had similar degrees of lysozyme inactivation. The degree of inactivation of deposited proteins on contact lenses is influenced by the lens material, though variability between participants should also be considered. Consequently, these variations may overwhelm any differences in lysozyme inactivation seen between lens care products.
Data Sharing Statement
The individual participant clinical data that support the findings of this study are not available for review.
Consent for Publication
Participants have consented for the submission of results of the study for publication.
Acknowledgments
Over the past 3 years CORE has received research support or lectureship honoraria from Alcon, Allergan, Allied Innovations, Aurinia Pharma, BHVI, CooperVision, GL Chemtec, iMedPharma, J&J Vision, Lubris, Menicon, Nature’s Way, Novartis, Ophtecs, Ote Pharma, PS Therapy, Santen, Shire, SightGlass, SightSage and Visioneering.
Funding
This study was funded by Alcon Research, LLC, USA.
Disclosure
L Jones is a consultant and/or serves on advisory boards for Alcon, CooperVision, Johnson & Johnson Vision, Novartis and Ophtecs. L Jones also reports grants from Alcon during the conduct of the study. His research group has contracts and/or receives personal fees from Alcon, Allergan, Allied Innovations, Aurinia Pharma, BHVI, CooperVision, GL Chemtech, i-Med Pharma, Johnson & Johnson Vision, Lubris, Menicon, Natures Way, Novartis, Ophtecs, Ote Pharma, PS Therapy, Santen, Shire, SightGlass, SightSage, and Visioneering, outside the submitted work. L Subbaraman and A Ng were employed at CORE while the study was conducted. A Ng is currently employed at Market Lane Optical, Woodbridge, Ontario, Canada and L Subbaraman is currently employed at Alcon Research, LLC, Vision Care Franchise, Johns Creek, Georgia, USA. The authors report no other conflicts of interest in this work.
References
1. Lin ST, Mandell RB, Leahy CD, et al. Protein accumulation on disposable extended wear lenses. CLAO J. 1991;17:44–50.
2. Keith DJ, Christensen MT, Barry JR, et al. Determination of the lysozyme deposit curve in soft contact lenses. Eye Contact Lens. 2003;29:79–82. doi:10.1097/01.ICL.0000061687.11408.B7
3. Jones L, Senchyna M, Glasier MA, et al. Lysozyme and lipid deposition on silicone hydrogel contact lens materials. Eye Contact Lens. 2003;29:S75–79. doi:10.1097/00140068-200301001-00021
4. Nichols JJ. Deposition on silicone hydrogel lenses. Eye Contact Lens. 2013;39:20–23. doi:10.1097/ICL.0b013e318275305b
5. Gellatly KW, Brennan NA, Efron N. Visual decrement with deposit accumulation of HEMA contact lenses. Am J Optom Physiol Opt. 1988;65:937–941. doi:10.1097/00006324-198812000-00003
6. Jones L, Brennan NA, Gonzalez-Meijome J, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens materials, design, and care subcommittee. Invest Ophthalmol Vis Sci. 2013;54:TFOS37–TFOS70. doi:10.1167/iovs.13-13215
7. Dumbleton K, Woods CA, Jones LW, et al. The impact of contemporary contact lenses on contact lens discontinuation. Eye Contact Lens. 2013;39:93–99. doi:10.1097/ICL.0b013e318271caf4
8. Morgan PB, Woods C, Tranoudis I, et al. International contact lens prescribing in 2019. Contact Lens Spectrum. 2020;35:26–32.
9. Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008;115:1655–1662. doi:10.1016/j.ophtha.2008.04.002
10. Lim CHL, Stapleton F, Mehta JS. Review of contact lens–related complications. Eye Contact Lens. 2018;44:S1–S10. doi:10.1097/ICL.0000000000000481
11. Kuc CJ, Lebow KA. Contact lens solutions and contact lens discomfort: examining the correlations between solution components, keratitis, and contact lens discomfort. Eye Contact Lens. 2018;44:355–366. doi:10.1097/ICL.0000000000000458
12. Keir N, Woods CA, Dumbleton K, et al. Clinical performance of different care systems with silicone hydrogel contact lenses. Cont Lens Anterior Eye. 2010;33:189–195. doi:10.1016/j.clae.2010.01.006
13. Nichols JJ, Chalmers RL, Dumbleton K, et al. The case for using hydrogen peroxide contact lens care solutions: a review. Eye Contact Lens. 2019;45:69–82. doi:10.1097/ICL.0000000000000542
14. Minarik L, Rapp J. Protein deposits on individual hydrophilic contact lenses: effects of water and ionicity. CLAO J. 1989;15:185–188.
15. Hart DE, Tidsale RR, Sack RA. Origin and composition of lipid deposits on soft contact lenses. Ophthalmology. 1986;93:495–503. doi:10.1016/S0161-6420(86)33709-6
16. Subbaraman LN, Glasier MA, Senchyna M, et al. Kinetics of in vitro lysozyme deposition on silicone hydrogel, PMMA, and FDA groups I, II, and IV contact lens materials. Curr Eye Res. 2006;31:787–796. doi:10.1080/02713680600888799
17. Heynen M, Lorentz H, Srinivasan S, et al. Quantification of non-polar lipid deposits on senofilcon a contact lenses. Optometry Vision Sci. 2011;88:1172–1179. doi:10.1097/OPX.0b013e31822a5295
18. Srinivasan S, Otchere H, Yu M, et al. Impact of cosmetics on the surface properties of silicone hydrogel contact lenses. Eye Contact Lens. 2015;41:228–235. doi:10.1097/ICL.0000000000000101
19. Willcox MDP. Microbial adhesion to silicone hydrogel lenses: a review. Eye Contact Lens. 2013;39:61–66. doi:10.1097/ICL.0b013e318275e284
20. Omali NB, Subbaraman LN, Coles-Brennan C, et al. Biological and clinical implications of lysozyme deposition on soft contact lenses. Optom Vis Sci. 2015;92:750–757. doi:10.1097/OPX.0000000000000615
21. Nakimbugwe D, Masschalck B, Atanassova M, et al. Comparison of bactericidal activity of six lysozymes at atmospheric pressure and under high hydrostatic pressure. Int J Food Microbiol. 2006;108:355–363. doi:10.1016/j.ijfoodmicro.2005.11.021
22. Subbaraman LN, Glasier MA, Varikooty J, et al. Protein deposition and clinical symptoms in daily wear of etafilcon lenses. Optom Vis Sci. 2012;89:1450–1459. doi:10.1097/OPX.0b013e318269e583
23. Subbaraman LN, Jones L. Kinetics of lysozyme activity recovered from conventional and silicone hydrogel contact lens materials. J Biomater Sci Polym Ed. 2010;21:343–358. doi:10.1163/156856209X415873
24. Ng A, Heynen M, Luensmann D, et al. Impact of tear film components on the conformational state of lysozyme deposited on contact lenses. J Biomed Mater Res B Appl Biomater. 2013;101:1172–1181. doi:10.1002/jbm.b.32927
25. Franklin VJ. Cleaning efficacy of single-purpose surfactant cleaners and multi-purpose solutions. Cont Lens Anterior Eye. 1997;20:63–68. doi:10.1016/S1367-0484(97)80042-2
26. Luensmann D, Heynen M, Liu L, et al. The efficiency of contact lens care regimens on protein removal from hydrogel and silicone hydrogel lenses. Mol Vis. 2010;16:79–92.
27. Jung J, Rapp J. The efficacy of hydrophilic contact lens cleaning systems in removing protein deposits. CLAO J. 1993;19:47–49. doi:10.1097/00140068-199301000-00008
28. Postel C, Abillon O, Desbat B. Structure and denaturation of adsorbed lysozyme at the air-water interface. J Colloid Interface Sci. 2003;266:74–81. doi:10.1016/S0021-9797(03)00571-X
29. Zuo SS, Lundahl P. A micro-Bradford membrane protein assay. Anal Biochem. 2000;284:162–164. doi:10.1006/abio.2000.4676
30. Senchyna M, Jones L, Louie D, et al. Quantitative and conformational characterization of lysozyme deposited on balafilcon and etafilcon contact lens materials. Curr Eye Res. 2004;28:25–36. doi:10.1076/ceyr.28.1.25.23496
31. Glasier MA, Keech A, Sheardown H, et al. Conformational and quantitative characterization of lysozyme extracted from galyfilcon and senofilcon silicone hydrogel contact lenses. Curr Eye Res. 2008;33:1–11. doi:10.1080/02713680701830278
32. Zhao Z, Naduvilath T, Flanagan JL, et al. Contact lens deposits, adverse responses, and clinical ocular surface parameters. Optom Vis Sci. 2010;87:669–674. doi:10.1097/OPX.0b013e3181ea1848
33. Castillo EJ, Koenig JL, Anderson JM. Characterization of protein adsorption on soft contact lenses. IV. Comparison of in vivo spoilage with the in vitro adsorption of tear proteins. Biomaterials. 1986;7:89–96. doi:10.1016/0142-9612(86)90062-1
34. Weeks A, Boone A, Luensmann D, et al. The effects of hyaluronic acid incorporated as a wetting agent on lysozyme denaturation in model contact lens materials. J Biomater Appl. 2013;28:323–333. doi:10.1177/0885328212446936
35. Kenny SE, Tye CB, Johnson DA, et al. Giant papillary conjunctivitis: a review. Ocul Surf. 2020;18:396–402. doi:10.1016/j.jtos.2020.03.007
36. Carnt NA, Evans VE, Naduvilath TJ, et al. Contact lens-related adverse events and the silicone hydrogel lenses and daily wear care system used. Arch Ophthalmol. 2009;127:1616–1623. doi:10.1001/archophthalmol.2009.313
37. Lazon de la Jara P, Papas E, Diec J, et al. Effect of lens care systems on the clinical performance of a contact lens. Optom Vis Sci. 2013;90:344–350. doi:10.1097/OPX.0b013e318288e10c
38. Szczotka-Flynn L, Jiang Y, Raghupathy S, et al. Corneal inflammatory events with daily silicone hydrogel lens wear. Optometry Vision Sci. 2014;91:3–12. doi:10.1097/OPX.0000000000000105
39. Zhang X, Marchetti C, Lee J, et al. The impact of lens care solutions on corneal epithelial changes during daily silicone hydrogel contact lens wear as measured by in vivo confocal microscopy. Cont Lens Anterior Eye. 2017;40:33–41. doi:10.1016/j.clae.2016.11.006
40. Gorbet M, Peterson R, McCanna D, et al. Human corneal epithelial cell shedding and fluorescein staining in response to silicone hydrogel lenses and contact lens disinfecting solutions. Curr Eye Res. 2014;39:245–256. doi:10.3109/02713683.2013.841255
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.