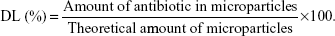Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Activity of daptomycin- and vancomycin-loaded poly-epsilon-caprolactone microparticles against mature staphylococcal biofilms
Authors Ferreira I, Bettencourt A, Gonçalves L, Kasper S, Bétrisey B, Kikhney J, Moter A , Trampuz A, Almeida AJ
Received 6 March 2015
Accepted for publication 28 April 2015
Published 7 July 2015 Volume 2015:10(1) Pages 4351—4366
DOI https://doi.org/10.2147/IJN.S84108
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Thomas Webster
Inês Santos Ferreira,1 Ana F Bettencourt,1 Lídia MD Gonçalves,1 Stefanie Kasper,2 Bertrand Bétrisey,3 Judith Kikhney,2 Annette Moter,2 Andrej Trampuz,4 António J Almeida1
1Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, University of Lisbon, Lisbon, Portugal; 2Biofilmcenter, German Heart Institute Berlin, Berlin, Germany; 3Infectious Diseases Service, Department of Medicine, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland; 4Center for Musculoskeletal Surgery, Charité – University Medicine Berlin, Berlin, Germany
Abstract: The aim of the present study was to develop novel daptomycin-loaded poly-epsilon-caprolactone (PCL) microparticles with enhanced antibiofilm activity against mature biofilms of clinically relevant bacteria, methicillin-resistant Staphylococcus aureus (MRSA) and polysaccharide intercellular adhesin-positive Staphylococcus epidermidis. Daptomycin was encapsulated into PCL microparticles by a double emulsion-solvent evaporation method. For comparison purposes, formulations containing vancomycin were also prepared. Particle morphology, size distribution, encapsulation efficiency, surface charge, thermal behavior, and in vitro release were assessed. All formulations exhibited a spherical morphology, micrometer size, and negative surface charge. From a very early time stage, the released concentrations of daptomycin and vancomycin were higher than the minimal inhibitory concentration and continued so up to 72 hours. Daptomycin presented a sustained release profile with increasing concentrations of the drug being released up to 72 hours, whereas the release of vancomycin stabilized at 24 hours. The antibacterial activity of the microparticles was assessed by isothermal microcalorimetry against planktonic and sessile MRSA and S. epidermidis. Regarding planktonic bacteria, daptomycin-loaded PCL microparticles presented the highest antibacterial activity against both strains. Isothermal microcalorimetry also revealed that lower concentrations of daptomycin-loaded microparticles were required to completely inhibit the recovery of mature MRSA and S. epidermidis biofilms. Further characterization of the effect of daptomycin-loaded PCL microparticles on mature biofilms was performed by fluorescence in situ hybridization. Fluorescence in situ hybridization showed an important reduction in MRSA biofilm, whereas S. epidermidis biofilms, although inhibited, were not eradicated. In addition, an important attachment of the microparticles to MRSA and S. epidermidis biofilms was observed. Finally, all formulations proved to be biocompatible with both ISO compliant L929 fibroblasts and human MG63 osteoblast-like cells.
Keywords: antibiotic release, Staphylococcus aureus, Staphylococcus epidermidis, fluorescence in situ hybridization, isothermal microcalorimetry
Introduction
Staphylococci are known to be one of the main concerns in orthopedic implant-associated infections due to their capability of readily forming biofilms that show increasing tolerance toward antibiotics and are able to effectively evade the immune system.1 In biofilms, bacteria are surrounded by an extracellular polysaccharide matrix, which prevents antibiotic penetration. Hence lower concentrations of the drug are found inside these communities.2 In addition, sessile bacteria have a low replication rate, which is associated with a stationary metabolic state. Since most antibiotics target active metabolic pathways, such as RNA transduction, enzymatic activity, and DNA translation, their activity is limited within biofilms.3 These two key features make sessile staphylococci up to 1,000 times more tolerant to antibiotics than the planktonic form; hence higher concentrations of antibiotics and longer treatment times are required for eradication.2,3 The persistence of the infection endangers the functionality of the orthopedic implant since it reduces bone and tissue regeneration, prevents osteoblast adhesion and proliferation, and induces chronic inflammation.1,2,4
For the last decade, research has been focused on improving the effectiveness of clinically available antibiotics against biofilms by encapsulating them into micro- and nanoparticles.1,5 This strategy has several advantages such as: 1) possibility of targeted and triggered drug release, 2) incorporation of lipophilic as well as hydrophilic drugs, 3) protection of the encapsulated antibiotic, and 4) reduction of unwanted side effects. Liposomes, have been widely studied and different antimicrobials have been successfully encapsulated with improved antibiofilm activity.5 In contrast, a lesser number of polymeric particles have been successfully developed. The main advantages of polymeric particles versus liposomes lie on their higher stability during storage and the possibility of tailoring the rate and duration of drug release, thus enhancing the antibiofilm effect by increasing the long-term antibiotic concentration in situ.5
The aim of the present work was to develop novel daptomycin-loaded poly-epsilon-caprolactone (PCL) microparticles in order to achieve a sustained drug release and thus higher antibiofilm activity against pre-grown staphylococci biofilms, which typically occur in implant-associated bone infections. Daptomycin, a glycopeptide selective against Gram-positive bacteria, has shown superior activity against staphylococcal biofilms compared to other antibiotics commonly used in bone infection treatment, such as ciprofloxacin and vancomycin, due to the fact that it targets the cell membrane by opening ion channels that ultimately lead to cell lysis.6 Consequently, it does not target metabolic active pathways, making it more active against metabolically stationary bacteria, such as sessile bacteria. The PCL is a slow biodegradable polymer that has been often used in medical devices and in micro- and nanoencapsulation of antibiotics, such as vancomycin and gentamicin.7 Particles made of PCL have proven to be a useful strategy to encapsulate both lipophilic and hydrophilic antibiotics with high encapsulation efficiencies and controlled drug release profiles without losing antibacterial activity. To date, no published studies have addressed the encapsulation of daptomycin in PCL microparticles.
The antibiofilm activity of antibiotic-loaded PCL microparticles was assessed in real time by isothermal microcalorimetry and the surviving biofilms were characterized by fluorescence in situ hybridization (FISH). For comparison purposes, similar formulations were prepared with vancomycin, often recommended for Staphylococcus infection control.2 In addition, the biocompatibility of the formulations was also characterized using an ISO-compliant cell line and osteoblasts.
Materials and methods
Chemicals and test strains
Daptomycin (Cubicin, 350 mg) was kindly provided by Novartis (Basel, Switzerland) and vancomycin hydrochloride (Vancomicina, 1,000 mg) was purchased from Farma APS Produtos Farmacêuticos, Lda. (Lisboa, Portugal). PCL (average MW =45,000 g/mol) and poly(vinyl alcohol) (MW =13,000–23,000, 87%–89% hydrolyzed) were purchased from Sigma-Aldrich (St Louis, MO, USA). All other reagents were analytical grade. Mueller–Hinton broth (MHB; CM 0405, Oxoid, UK) and tryptic soy broth (236950, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) was freshly prepared and sterilized in autoclave (121°C, 15 minutes) before use. The study microorganisms were methicillin-resistant Staphylococcus aureus (MRSA; ATCC 43300) and polysaccharide intercellular adhesin (PIA)-positive Staphylococcus epidermidis 8400 (kindly provided by Mack et al).8 Bacteria were stored at −70°C using the cryovial bead preservation system (Microbank; 79 Pro-Lab Diagnostics, Richmond Hill, ON, Canada).
Preparation of antibiotic-loaded PCL microparticles
Antibiotic-loaded microparticles were prepared using a modification of a previously described double-emulsion w/o/w-solvent evaporation method.9,10 Briefly, PCL was dissolved in 5 mL dichloromethane and emulsified by homogenization using an Ultra-Turrax T10 basic (IKA, Staufen, Germany) for 3 minutes with a 10% (w/w) poly(vinyl alcohol) solution, where the antibiotics were previously solubilized. The resulting (w/o) emulsion was added to 30 mL of 1.25% (w/w) poly(vinyl alcohol) solution and emulsified by homogenization using a Silverson Laboratory Mixer Emulsifier L5M (Silverson Machines Inc., Buckinghamshire, UK) for 7 minutes at maximum rotation speed. The resulting w/o/w double emulsion was magnetically stirred at room temperature for 4 hours to evaporate the organic solvent. PCL microparticles were harvested by centrifugation (5,723× g, 10 minutes, 4°C; Allegra 64R High Speed Centrifuge, Beckman Coulter Inc., Brea, CA, USA), washed three times and resuspended in a 0.5% (w/V) sucrose solution. All microparticles were subsequently freeze-dried (Christ Alpha 1–4, B. Braun Biotech International, Melsungen, Germany) to obtain a fine, free-flowing dry powder. For preparation of fluorescence labeled microparticles, nile red was added to the polymer solution in dichloromethane. Mean yield of production was calculated according to the equation: y= (practical yield/theoretical yield) ×100. All batches were prepared in triplicate and plain microparticles were used as controls.
Particle characterization
Particle morphology was analyzed a by transmission electron microscopy. The suspension sample was applied to the copper grid, dried at room temperature, and analyzed on a Hitachi 8100 with ThermoNoran light elements, EDS detector, and digital image acquisition.
Size distribution of lyophilized microparticles was determined by light scattering, using the Malvern Mastersizer 2000 – Hydro SM (Malvern Instruments, Worcestershire, UK). Diluted samples were loaded to the sample dispersion unit under constant agitation. The size distribution measurements were performed using at least three replicate samples. Size distribution of microparticles was characterized using the volume mean diameter (μm) and the width of particle size distribution is given by the span. Surface charge of lyophilized microparticles was determined by electrophoretic light scattering using the Malvern Nanosizer Z (Malvern Instruments) and water as a dispersant.
Encapsulation efficiency (EE, %) was determined spectrophotometrically (Spectrophotometer U-2001, Hitachi Instruments Inc., Tokyo, Japan) by quantification of the antibiotics in the supernatants (ie, non-encapsulated antibiotic) obtained during particle preparation. Antibiotic detection was performed at 220.5 nm for daptomycin and 280 nm for vancomycin.11,12 The EE is expressed as the percentage of encapsulated antibiotic reported to the initial amount used for particle preparation. Drug loading (DL, %) was calculated according to the equation:
|
All results are presented as mean ± standard deviation (SD).
Differential scanning calorimetry analysis of plain, daptomycin-, and vancomycin-loaded PCL microparticles was performed in a Q200 (TA Instruments, New Castle, DE, USA). Samples (1–3 mg) were placed in sealed aluminum pans and heated at 10°C/min under a nitrogen atmosphere from 25°C–240°C. An empty aluminum pan was used as reference.
In vitro release
Daptomycin and vancomycin release from PCL microparticles was assessed using dialysis membranes with a pore size of 100 kD (Float-A-Lyzer G2®, Spectrum Laboratories Inc., Rancho Dominguez, CA, USA). Briefly, microparticles (12 mg) were suspended in phosphate buffered saline (PBS; pH 7.4, supplemented with 0.01% [w/V] sodium azide) and added to the dialysis membranes. Samples, in triplicate, were incubated at 37°C under constant agitation (350 rpm). At predetermined intervals, a 1 mL aliquot was collected and an equal volume of PBS was added to keep the total volume constant. Released antibiotics were quantified by previously optimized high-performance liquid chromatography methods (Beckman Coulter System Gold with 126 solvent module and 166 UV-Vis detector coupled with a Stark Holland Midas autosampler). Linearity and reproducibility were analyzed and considered adequate for sample analysis. Briefly, quantification of daptomycin was performed using a Merck LiChospher 125-4, RP18 5 μm, LiChroCART 100 column and the following chromatographic conditions: 0.7 mL/min flow; injection volume of 20 μL; mobile phase of 35% acetonitrile and 65% PBS (pH 7.4), and detection at 230 nm.11 As for vancomycin, the same type of column was used and the chromatographic conditions were as follows: 1.2 mL/min flow; injection volume of 40 μL; mobile phase of 10% acetonitrile and 90% H2KPO4 (pH 2.75), and detection at 280 nm.12 All samples were analyzed in triplicate. Results are presented as mean ± SD.
Susceptibility testing of daptomycin and vancomycin
In vitro determination of minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) of non-encapsulated daptomycin and vancomycin against Gram-positive biofilm-forming staphylococci, namely methicillin-resistant S. aureus ATCC 43300 (MRSA) and PIA-positive S. epidermidis 8400, was performed by the macro-broth dilution method.13 In addition, the minimal heat inhibitory concentration (MHIC) was determined by isothermal microcalorimetry (TAM III, TA Instruments). In both methods, serial twofold dilutions of daptomycin and vancomycin were prepared in MHB. For inoculum preparation, bacteria were resuspended in 2 mL sterile saline and adjusted to turbidity of McFarland 0.5 (corresponding to approximately 108 colony forming unit (CFU)/mL; Densimat, BioMérieux, SA, France). A 1:100 dilution of the bacterial suspension was prepared in sterile saline and added to the samples in order to achieve a 1–5×105 CFU/mL inoculum. Samples were incubated aerobically for 24 hours at 35°C±2°C. The samples for isothermal microcalorimetry were sealed and vortexed and measurements of heat flow (W) were performed for 24 hours at 10 seconds intervals. The isothermal microcalorimetry results are presented as curves of heat flow (μW) versus time (hours). All samples were tested in triplicate. The MHIC was defined as the lowest antibiotic concentration that completely inhibited visible growth at 24 hours or did not exhibit heat flow production in the isothermal microcalorimeter.14 The MBC was defined as the lowest antimicrobial concentration, which killed ≥99.9% of the initial bacterial count (ie, ≥3 log10 CFU/mL) in 24 hours using MHB.13 For MBC determination, all samples that did not exhibit turbidity or heat flow production (ie, bacterial growth) after 24 hours were diluted with sterile saline, spread onto Mueller–Hinton agar plates and incubated for 24 hours at 35°C±2°C.
In vitro growth of staphylococcal biofilms
Biofilms of MRSA and PIA-positive S. epidermidis 8400 were grown onto polyurethane (PU) pieces of fixed dimensions. An overnight culture of MRSA or S. epidermidis was appropriately diluted in tryptic soy broth in order to achieve a final inoculum of 1–5×108 CFU/mL. Each PU piece was then incubated with 0.5 mL of the final bacterial suspension at 37°C for 48 hours. Fresh medium (tryptic soy broth supplemented with 50 mg/L Ca2+) was added at 24 hours. After 48 hours, biofilms were washed with PBS to remove remaining planktonic bacteria.
Antibacterial activity of antibiotic-loaded PCL microparticles by isothermal microcalorimetry
Planktonic bacteria
The in vitro determination of MIC and MBC of encapsulated daptomycin and vancomycin against MRSA and PIA-positive S. epidermidis was performed by isothermal microcalorimetry (TAM III, TA Instruments). Daptomycin- and vancomycin-loaded microparticles suspensions were prepared by serial twofold dilutions in MHB. The highest microparticle concentration tested was 10 mg/mL and the lowest was 0.04 mg/mL. Growth media for daptomycin studies were supplemented with 50 mg/L Ca2+. Negative controls (ie, without bacteria) were used: MHB alone and a suspension of microparticles in MHB. Also, a bacteria growth control (GC) was included. Inoculum preparation was performed as stated previously in the susceptibility testing of daptomycin and vancomycin section in order to achieve a 1–5×105 CFU/mL inoculum. Samples were sealed and vortexed and measurements of heat flow (W) were performed for 24 hours at 10 seconds intervals. Results are presented as curves of heat flow (μW) versus time (hours). All samples were tested in triplicate. The MHIC and MBC values were determined as described earlier in the susceptibility testing of daptomycin and vancomycin section.
Sessile bacteria
For determination of antibiofilm activity of antibiotic-loaded PCL, MRSA, and PIA-positive S. epidermidis, biofilms were grown onto PU pieces as previously described in the in vitro growth of staphylococcal biofilms section. After 48 hours of biofilm growth, each PU piece was added to a microcalorimetry glass ampoule and incubated for 24 hours at 37°C (TAM III, TA Instruments) with different concentrations of microparticles (20, 10, 5, 2.5, 1.25 mg/mL). The ampoules were hermetically sealed and measurements of heat flow (W) were performed for 24 hours at 10 seconds intervals. The minimal biofilm inhibitory concentration (MBIC) was defined as the lowest microparticle concentration leading to absence of recovering biofilm, indicated by absence of growth related heat flow.14 Results are presented as curves of heat flow (μW) versus time (hours). All samples were tested in triplicate.
Interaction between PCL microparticles and biofilms by fluorescence in situ hybridization
Biofilms of MRSA and PIA-positive S. epidermidis were grown for 48 hours on PU pieces as described earlier in the in vitro growth of staphylococcal biofilms section. Each sample was then incubated with different concentrations of nile red-labeled microparticles (20, 10, 5, 2.5, and 1.25 mg/mL) at 37°C for 24 hours. The samples were subsequently washed with PBS, fixated and embedded in a cold polymerizing resin (Technovit 8100; Kulzer, Hanau, Germany) according to the manufacturer’s instructions. After polymerization of the resin, the blocks were sectioned in 2 μm sections on a rotary microtome (Medim America, Type DDM 0036, Wilmington, DE, USA.) using steel knives with hard metal blades. The slides were mounted and each sample was permeabilized prior to probe binding with an enzymatic step including lysozyme and lysostaphin. FISH was used to characterize the biofilms.15 Biofilms were hybridized with the pan-bacterial probe EUB 338FITC and the staphylococci-specific probe STAPHYFITC as well as stained with the unspecific nucleic acid stain 4′,6-diamidino-2-phenylindole.16,17 All samples were prepared in triplicate.
Cell viability assays
The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) and resazurin (Alamar Blue, Invitrogen Life Technologies, Carlsbad, CA, USA) reduction assays were used to investigate in vitro cytotoxicity of plain and antibiotic-loaded microparticles in two different cell lines: L929 mouse fibroblast (ATCC CCL-1™), and MG63 human osteoblast-like cells (ATCC CRL-1427).18,19
Cell viability was assessed after 24-hour incubation with different concentrations of PCL microparticles (10–2,000 μg/mL) in RPMI 1640 medium. After incubation, cells were exposed to a MTT dye solution (5 mg/mL in PBS at pH 7.4) for 3 hours at 37°C, after which the complete media was removed and the intracellular formazan crystals were solubilized and extracted with dimethylsulfoxide. After 15 minutes at room temperature, the absorbance was measured at 570 nm in a microplate reader (FLUOstar Omega, BMG Labtech, Offenburg, Germany).18 For the Alamar Blue assay, the resazurin solution was added to each well and incubated for 3 hours at 37°C. A 3% sodium dodecyl sulfate (SDS) solution was used to stop the reaction and fluorescence was measured at 530/590 nm (FLUOstar Omega, BMG Labtech). For both assays, culture medium and SDS served as negative and positive controls, respectively. The relative cell viability (% of control) was calculated and compared with the untreated control.18
Statistical evaluation of data was performed using one-way analysis of variance (ANOVA). A Tukey–Kramer multiple comparison test (GraphPad Prism 6, GraphPad Software, San Diego, CA, USA), was used to compare the significance of the difference between the groups, a P-value <0.05 was accepted as significant.
Results
Microparticle characterization
Transmission electron microscopy analysis revealed a spherical shape within the micrometer size range (Figure 1). Different concentrations of antibiotics were added to the formulation to study the effect on EE and DL (Figure 2). Increasing the antibiotic percentage added to the formulation steadily increased DL values, with maximum values of 18.9%±2.5% and 12.6%±2.1% for 30% (w/w) of daptomycin and vancomycin, respectively. The EE values did not follow the same trend. Daptomycin EE decreased as the antibiotic percentage in formulation increased from 2.5% to 15%, with a minimum value of 42.9%±0.5%, but it increased to 83.0%±3.6% as 30% of antibiotic was added to the formulation. Vancomycin presented EE values between 54.3%±4.0% and 73.0%±7.1% for 7.5% and 5% of antibiotic in the formulation, respectively. Nevertheless, the most promising formulations for the antibacterial effect assessment would be those with higher DL (ie, mg of antibiotic/mg of microparticles), which in this case are the microparticles loaded with 30% daptomycin or vancomycin. These formulations, as well as the plain microparticles, were further characterized taking into consideration particle size distribution and surface charge. A summary of the characteristics of the final formulations is presented in Table 1. Plain and antibiotic-loaded PCL microparticles presented a monomodal particle size distribution within the micrometer range. All formulations presented a negative surface charge. The encapsulation of daptomycin or vancomycin did not alter particle size distribution or surface charge (Table 1).
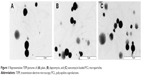  | Figure 1 Representative TEM pictures of (A) plain, (B) daptomycin, and (C) vancomycin-loaded PCL microparticles. |
Additional physical characterization of freeze-dried microparticles was performed by differential scanning calorimetry (Figure 3). As shown in Figure 3, PCL (raw material) presents an endothermic peak at 59.7°C, which corresponds to the melting temperature of the polymer. The same peak is observed in plain and antibiotic-loaded PCL microparticles. The antibiotic-loaded PCL microparticles did not show the daptomycin and vancomycin melting peaks (226.6°C and 212.0°C, respectively).
In vitro release
Daptomycin and vancomycin cumulative release from PCL microparticles is presented in Figure 4. Overall, daptomycin release is higher and steadily increases up to 72 hours (10.4%±1.38%), whereas vancomycin release reaches its maximum at 24 hours (4.03%±1.41%). In terms of concentration of released antibiotic, these values equal 12.1±1.6 and 4.4±1.5 μg/mL, respectively.
Susceptibility testing of daptomycin and vancomycin
For both staphylococci strains, the MIC and MBC obtained with the macro-broth dilution method was 0.25 μg/mL and 2 μg/mL for daptomycin and vancomycin, respectively. In order to confirm the correlation between the macro-broth dilution method and isothermal microcalorimetry, the MHIC of the antibiotics against both strains was determined (Figure 5). The MHIC values obtained by isothermal microcalorimetry were consistent with the MIC and MBC obtained by the macro-broth dilution method.
Antibacterial activity of antibiotic-loaded PCL microparticles by isothermal microcalorimetry
The in vitro determination of MHIC of encapsulated daptomycin and vancomycin against planktonic and sessile bacteria was performed by isothermal microcalorimetry.
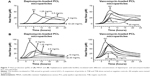
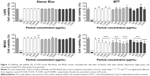
Regarding planktonic bacteria, 24 hours thermograms for MRSA and S. epidermidis incubated with different concentrations of antibiotic-loaded PCL microparticles were obtained (Figure 6). It was possible to identify the MHIC (ie, concentration of microparticles that completely inhibits heat flow production caused by bacterial growth) as well as to characterize the concentration-dependent effect of the microparticles on bacterial growth.
Table 2 summarizes the MHIC values of daptomycin- and vancomycin-loaded PCL microparticles against planktonic MRSA and S. epidermidis.
It was possible to confirm that the released antibiotics retained their antibacterial activity. As presented in Table 2, daptomycin-loaded PCL microparticles showed lower MHIC values for both strains, meaning that lower amounts of microparticles were required to inhibit bacterial growth in vitro. A comparison of the MHIC values for the encapsulated antibiotics shows that encapsulated vancomycin had the same value for both strains (10 mg/mL), whereas daptomycin-loaded microparticles presented a twofold higher MHIC value for S. epidermidis (0.625 mg/mL) than for MRSA although the daptomycin MIC/MBC values for both strains are equal (ie, 0.25 μg/mL; Table 2).
The activity of the antibiotic-loaded PCL microparticles against MRSA and S. epidermidis pre-grown biofilms was also assessed by isothermal microcalorimetry (Figure 7). In this case, the method quantified the heat flow associated with the recovery of the biofilm once fresh medium, with or without microparticles, was added. Daptomycin-loaded microparticles were able to inhibit MRSA biofilm recovery at 10 mg/mL, whereas vancomycin-loaded microparticles at the highest concentration (20 mg/mL) did not completely inhibit biofilm recovery. Nevertheless, vancomycin microparticles were able to delay biofilm recovery (growth peak at 21 hours) when compared to the GC. Regarding S. epidermidis biofilms, 20 mg/mL of daptomycin-loaded microparticles were required to inhibit biofilm recovery and no inhibition was achieved with vancomycin microparticles. It was possible to observe that subinhibitory concentrations of microparticles (ie, 1.25–10 mg/mL for encapsulated daptomycin and 10 and 20 mg/mL for encapsulated vancomycin) decreased the maximum heat flow production, as can be seen by the decrease in the peak height as compared to the GC.
Table 3 summarizes the MBIC values of daptomycin- and vancomycin-loaded PCL microparticles.
Daptomycin-loaded microparticles presented a higher antibiofilm effect against MRSA and S. epidermidis biofilms than vancomycin microparticles. In addition, encapsulated daptomycin MBIC values against MRSA and S. epidermidis were 40- and 80-fold increased, respectively, when compared to the MHIC values (Figure 5).
Interaction between PCL microparticles and biofilms by fluorescence in situ hybridization
Further characterization of the biofilms before and after incubation with PCL microparticles was performed by FISH. This molecular biological imaging technique enables the characterization of the microparticles’ effect on biofilm size and structure in situ as well as the visualization of particle-biofilm interaction (Figures 8–10).
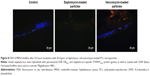
In the untreated samples (ie, controls), the biofilms of MRSA or S. epidermidis were 20–30 μm thick and had a confluent appearance. The signal of the EUB 338FITC and STAPHYFITC probes was low, which correlates to few FISH-positive (ie, labeled) cells.
The microparticles used in this assay were labeled with nile red, which enabled their visualization with the Cy3 filter set (orange). Except for the MRSA biofilms treated with daptomycin microparticles, it was possible to observe orange-labeled masses inside the biofilms, indicating that there is a strong interaction between the microparticles and sessile bacteria (Figures 8 and 9).
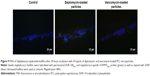
The MRSA biofilms treated with daptomycin-loaded microparticles revealed in the FISH analysis a reduction in biofilm thickness from 20 μm (as seen in the control) to a one-layered irregular biofilm (Figure 8). In contrast, the treatment of S. epidermidis biofilms with daptomycin-loaded microparticles showed that it was not possible to eradicate the biofilm, displaying only a disruption of the regularity of the biofilm (Figure 9). In addition, it was possible to observe an increase in the number of EUB338- and STAPHY-labeled cells in the outer layers of the S. epidermidis biofilm as compared to the controls (Figure 10).
The vancomycin-loaded microparticles showed no observable antibiofilm effect, since the FISH images of MRSA and S. epidermidis biofilms revealed no difference between the treated and control biofilms (Figures 8 and 9).
Cell viability assay
The in vitro cytotoxicity of the microparticles was evaluated with the MTT and Alamar Blue assays using L929 mouse fibroblasts and MG63 human osteoblast-like cells (Figure 11).
Regarding the ISO-compliant cells L929, no significant reduction of cellular viability was observed after incubation with plain and antibiotic-loaded PCL microparticles in the Alamar Blue or in the MTT assay. Since these formulations are intended to be in contact with osteoblasts, the cellular viability of MG63 human osteoblast-like cell was also assessed. The Alamar Blue assay did not reveal any cytotoxicity, whereas the MTT assay revealed a reduction in cellular viability for nearly all concentrations and types of microparticles used. No significant differences between plain and antibiotic-loaded microparticles were found, except between 10 μg/mL of plain and daptomycin-loaded microparticles.
Discussion
Characterization of polymeric microparticles
Plain and antibiotic-loaded PCL microparticles presented a spherical form with a diameter of 1–2 μm (Figure 1). Regarding DL (Figure 2), it was possible to observe a steady increase for both daptomycin and vancomycin as the concentrations of the antibiotics in the formulation increased from 2.5% to 30% (w/w). The EE values obtained for daptomycin decreased as the antibiotic percentage in the formulation increased from 2.5% to 15%. From 15% to 30% of daptomycin in the formulation, the EE strongly increased from 42.9%±0.5% to 83.0%±3.6%. This may be explained by the formation of micelles of daptomycin above 1 mg/mL, which is observed when 30% of daptomycin is used for particle preparation.20 In the case of the w/o/w double emulsion-solvent evaporation method, daptomycin micelles will have the lipophilic tail of the molecule facing the organic phase of the emulsion, thus enabling the increase in EE. Unlike daptomycin, vancomycin presents lower EE values, which are consistent with the values found in the literature. For PCL microparticles prepared by the same method with 7.5% and 25% of vancomycin, the reported EE values were 57.3%±4.2% and 49.6%±3.6%, respectively.21,22 The formulations chosen for further physicochemical characterization and assessment of antibacterial activity were the ones loaded with 30% of antibiotic due to the higher DL values (ie, mg of antibiotic/mg of microparticles) (Table 1). The particle size distribution of plain and antibiotic-loaded PCL microparticles presented a monomodal population within the micrometer range, which is consistent with reports of polymeric microparticles prepared with the same method.9,10 The negative charge from all formulations is attributed to the fact that PCL is negatively charged.10 In addition, the encapsulation of daptomycin or vancomycin did not influence particle size distribution or surface charge (Table 1). Furthermore, the differential scanning calorimetry (DSC) thermograms revealed that PCL in the microparticles maintained its crystalline state, whereas both antibiotics were amorphous (Figure 3).23 The same findings for the thermal behavior of drug-loaded PCL microparticles prepared by the solvent evaporation method have been reported elsewhere.23,24 The amorphization of the encapsulated antibiotics may be due to the rapid precipitation of both drugs during particle preparation.23
In vitro release
The in vitro release profile is an important predictor of the antibacterial activity of antibiotic-loaded microparticles. In this case, after 2 hours of incubation, it was possible to achieve released concentrations of daptomycin and vancomycin above the reported MIC/MBC values for the strains used in this study, 8.2%±1.3% and 4.4%±1.4% corresponding to 9.1±1.4 μg/mL and 4.4±1.5 μg/mL respectively, remaining so up to 72 hours (Figure 4). Overall, daptomycin release is expressively higher than vancomycin (12.1±1.6 μg/mL vs 4.4±1.5 μg/mL). The lower release of vancomycin can be explained by the lowest encapsulation values observed for these microparticles in comparison with daptomycin. In addition, vancomycin is a more hydrophobic molecule than daptomycin and presents a larger molecular size.25,26 These factors may hinder vancomycin diffusion through the polymeric matrix, thus reducing the concentration of released antibiotic.
Susceptibility testing of daptomycin and vancomycin
The values for the MIC and MBC of non-encapsulated daptomycin and vancomycin were 0.25 μg/mL and 2.0 μg/mL respectively, for both strains. These results are consistent with the values in the literature.14,27
Regarding both strains, the MHIC obtained by isothermal microcalorimetry were consistent with the MIC and MBC obtained by the macro-broth dilution method (Figure 5). In addition, it was possible to observe that MRSA and S. epidermidis growth was delayed at 0.125 μg/mL of daptomycin and at 1 μg/mL of vancomycin, when compared to the GC. In conclusion, isothermal microcalorimetry proved to be a suitable method to evaluate the antibacterial activity of soluble antibiotics against different strains, yielding the same results as the gold standard method (ie, macro-dilution broth).
Antibacterial activity of PCL microparticles by isothermal microcalorimetry
The MHIC of encapsulated daptomycin and vancomycin against planktonic and sessile bacteria was assessed by isothermal microcalorimetry (Figure 6 and Table 2). Daptomycin- and vancomycin-loaded PCL microparticles are insoluble and turbid when in suspension; hence it was not possible to assess their antibacterial activity through the macro-broth dilution method. In addition, due to their micrometric size these microparticles readily sediment causing changes in the sample’s turbidity, which in turn hinders the use of OD 600 nm measurement to assess bacterial growth in the sample (ie, micro-broth dilution method). In the last years, the use of isothermal microcalorimetry in microbiology has been increasing among others for characterization of antibacterial and antibiofilm activity of several antibiotics against staphylococci strains.14,28,29 It is a highly sensitive method able to assess in real-time changes in bacterial growth based on the measurement of heat flow produced by replicating bacteria.29 Thus, it is not affected by the turbidity of the sample like the broth dilution methods, making it very useful for the study of the antibacterial effect of insoluble compounds, such as microparticles.30
Regarding planktonic bacteria, daptomycin-loaded PCL microparticles presented lower MHIC values (0.313 mg/mL for MRSA and 0.625 mg/mL for S. epidermidis) than vancomycin-loaded microparticles (10 mg/mL for both strains) (Table 2). This is consistent with the MIC/MBC values of daptomycin and vancomycin for both strains as well as with the release profiles obtained (Figure 4). Not only did daptomycin present higher antibacterial activity (ie, lower MIC/MBC values) but it was also released in higher concentrations than vancomycin. Although the MIC/MBC values of solubilized daptomycin for both strains were equal (ie, 0.25 μg/mL), daptomycin-loaded microparticles presented a twofold higher MHIC value for S. epidermidis (0.625 mg/mL) when compared to the MRSA values. As shown in Figure 6B, S. epidermidis growth with 0.313 mg/mL of daptomycin-loaded microparticles started at approximately 20 hours, which is close to the end of the incubation time (24 hours). In some publications, a heat flow threshold for the MHIC is set and any heat flow production below does not correspond to a significant bacterial growth. For example, Mihailescu et al and Entenza et al set the threshold at 10 μW and 20 μW, respectively.14,28 In this case, a more conservative approach was chosen and the MHIC corresponded to the concentration of microparticles that completely inhibited bacterial growth, hence the difference between the MHIC values of daptomycin-loaded microparticles against MRSA and S. epidermidis.
The antibiofilm activity of antibiotic-loaded PCL microparticles was assessed by the quantification of heat flow associated with the recovery of the biofilm once fresh medium, with or without microparticles, was added (Figure 7 and Table 3). Overall, daptomycin-loaded microparticles presented a higher antibiofilm effect against MRSA and S. epidermidis biofilms than vancomycin microparticles. This may be explained by the lower release rate of vancomycin (Figure 4) as well as the higher intrinsic antibiofilm activity of daptomycin compared to vancomycin. Biofilms are characterized by their increased tolerance toward antibiotics, which has been demonstrated in vitro by reports of ten- to 1,000-fold increase in the MBIC values when compared to the MIC.3 The structure of biofilms, as well as the low metabolic activity of the cells, is thought to be accountable for the increased tolerance to antibiotics.3 The penetration of some antibiotics into biofilms may be hindered by their multilayered structure and extracellular polysaccharide matrix. In addition, some bacteria within the biofilm present a decreased metabolic activity, which prevents antibiotic action, since most antibiotics target important metabolic pathways, such as protein production, enzymatic activity, and DNA transcription. Previous reports indicate that daptomycin shows higher antibiofilm activity than vancomycin against MRSA biofilms with MBIC values of 40 and >1,024 μg/mL for daptomycin and vancomycin, respectively.14 The reason for this difference lies in the different mechanism of action of each antibiotic, since vancomycin inhibits the peptidoglycan synthesis, which only occurs in metabolically active bacteria, whereas daptomycin targets the cell membrane by opening pores and causing cell lysis.31,32 For this reason, it is not surprising that vancomycin-loaded microparticles did not exhibit a considerable antibiofilm effect. Regarding daptomycin-loaded microparticles, our results showed the MBIC of daptomycin-loaded microparticles for MRSA biofilms was 40-fold higher than the MHIC for the planktonic form of the same strain, whereas for S. epidermidis biofilms there was a 80-fold higher MBIC compared to the MHIC. This increase was expected due to the differences in terms of antibiotic tolerance between planktonic and sessile bacteria. Nevertheless, this increase of the MBIC values of daptomycin microparticles was considerably lower than the 320-fold increase of the MBIC for solubilized daptomycin against MRSA reported by Mihailescu et al.14 In fact, additional reports of higher antibiofilm activity of encapsulated antibiotics include the reduction of S. aureus biofilms by vancomycin-loaded chitosan microparticles and the prolonged antibiofilm activity of gentamicin-loaded PLGA microparticles against Pseudomonas aeruginosa.33,34 There are three main mechanisms that may be accountable for the higher antibiofilm effect of these systems; prevention of drug degradation, controlled drug release, and interaction between microparticles and biofilm. In this case both antibiotics are stable; thus, no degradation is expected. In fact, controlled drug release was observed with increasing amounts of daptomycin being released up to 72 hours, which may prolong the antibiofilm effect. Finally, increasing the interaction between microparticles and biofilms will increase the residence time of the microparticles near the biofilm, thus increasing the local antibiotic concentration.
Interaction between PCL microparticles and biofilms by fluorescence in situ hybridization
FISH enabled to gain further insights on the interaction between MRSA or S. epidermidis biofilms and PCL microparticles (Figures 8–10). It allowed the assessment of the microparticles’ effect on biofilm size and structure as well as the visualization of particle–biofilm interaction.
FISH has been used to characterize medical biofilms by using strain-specific probes labeled with a fluorescent dye to target the ribosomal 16S RNA. The high copy number of 16S rRNA in each replicating and metabolically active cell offers sufficient target to visualize single bacterial cells within biofilms.35 The concomitant use of two or more specific probes labeled with the same fluorescent dye (ie, EUB 338FITC and STAPHYFITC here) intensifies the FISH signal by increasing the number of fluorescent molecules per cell, thus improving sensitivity and detection of active cells.35 In the untreated biofilms (ie, control) a low number of FISH-positive cells were observed. This is due to the fact that most bacteria within mature biofilms present a low metabolic activity, thus the 16S rRNA content is low.
The untreated biofilms and the biofilms treated with vancomycin-loaded microparticles presented no observable differences for both strains by FISH analysis, meaning that these microparticles were microscopically not active against staphylococci biofilms (Figures 8 and 9). These findings correlate to the isothermal microcalorimetry results, in which vancomycin microparticles did not prevent the recovery of MRSA and S. epidermidis biofilms (Figure 7).
Regarding daptomycin-loaded microparticles, it was possible to observe a strong difference in the activity against MRSA and S. epidermidis biofilms. MRSA biofilm was successfully reduced to single bacterial cells by these microparticles. Also in this case, FISH complemented the isothermal microcalorimetry results; both methods in combination showed that these microparticles not only inhibited biofilm recovery but also reduced biofilm mass. In contrast, S. epidermidis biofilms were more tolerant toward daptomycin-loaded microparticles than MRSA biofilms, a result, which was also corroborated by the microcalorimetry analysis (Figure 7). The FISH images revealed that daptomycin-loaded microparticles were not able to eradicate the biofilm, although some disruption of the biofilm structure was observed. In addition, clusters of FISH-positive cells were present in the outer biofilm layers, which is of the upmost importance since these are most probably viable cells that survived the treatment with daptomycin-loaded microparticles and would have been able to regrow the biofilm, thus compromising treatment efficacy and causing reinfection in the clinical setting (Figure 10).
FISH also provided valuable insights on the interaction between microparticles and biofilm as can be seen in the orange-labeled remnants found enclosed by the surviving biofilms. The attachment of positively charged nano- and microparticles to biofilms has previously been reported and attributed to the overall negative charge of bacteria and extracellular matrix.5 In this case, PCL microparticles are negatively charged, meaning that the interaction may be due to other factors, such as hydrogen binding or similar hydrophobicity. Isothermal microcalorimetry alone was not able to provide such information, thus these two techniques complement each other in terms of characterizing the antibiofilm effect of polymeric microparticles as well as their interaction with biofilms.
Cell viability studies
The determination of in vitro cytotoxicity of antibiotic-loaded PCL microparticles is a fundamental aspect in the assessment of their biocompatibility. In this case, the cytotoxicity assessment of plain and antibiotic-loaded PCL microparticles was performed with two cell lines (mouse fibroblasts L929 and MG63 human osteoblast-like cells). The MTT and Alamar Blue assays were used for assessment of cell viability.
Regarding the ISO-compliant cells L929, no significant reduction of cellular viability was observed after incubation with plain and antibiotic-loaded PCL microparticles. Since these formulations are intended to be in contact with osteoblasts, the cellular viability of MG63 human osteoblast-like cell was also assessed. Although the Alamar Blue assay did not reveal a significant cytotoxicity, it was possible to verify that the MTT assay revealed a slight reduction in cell viability. Nevertheless, results show that neither the plain nor the different antibiotic-loaded microparticles led to a reduction in cell viability below 50%, even for concentrations as high as 2,000 mg/mL, meaning that the cytotoxicity of the microparticles was very limited.36 The fact that the cytotoxicity assays presented different results can be explained by the different evaluated endpoints. Both assays rely on the assessment of cytotoxicity by quantification of the products of enzymatic reactions.37 The MTT assay is based on the quantification of formazan crystals produced by the reduction of the tetrazolium salt by the mitochondrial succinic dehydrogenases. In contrast, the Alamar Blue assay is based on the reduction of resazurin to resofurin by mitochondrial, cytosolic, and microsomal enzymes; hence it is not necessarily specific for mitochondrial dysfunction. In this case, it is possible to observe that PCL microparticles may hinder mitochondrial activity, thus decreasing cell viability but such is compensated by the less specific enzymes present in the cytoplasm and microsomes of the cells, hence no cytotoxicity is observed in the Alamar Blue assay. Overall, the cellular viability of both cell lines presented acceptable values, which leads to the conclusion that both daptomycin- and vancomycin-loaded PCL microparticles are biocompatible.
Conclusion
Daptomycin-loaded PCL microparticles presented the highest antibacterial effect against clinically relevant planktonic MRSA and S. epidermidis, proving to be more effective than vancomycin-loaded microparticles. This formulation also showed superior activity against MRSA biofilms by inhibiting biofilm recovery as well as significantly decreasing biofilm mass. Regarding S. epidermidis biofilms, daptomycin-loaded PCL microparticles were also superior, since they were able to inhibit biofilm recovery, but no significant biofilm mass decrease was observed. All PCL microparticles presented a high interaction with MRSA and S. epidermidis biofilms. Furthermore, all formulations proved to be biocompatible with both mouse fibroblasts L929 and MG63 human osteoblast-like cells.
Isothermal microcalorimetry showed to be a highly sensitive and accurate tool to evaluate the antibiofilm effect of antibiotic-loaded PCL microparticles without the previously mentioned drawbacks of the routine microbiology methods. Moreover, FISH provided crucial information regarding biofilm structure and viability as well as particle–biofilm interactions. Combining these techniques proved to be essential in order to fully characterize the antibiofilm activity of PCL microparticles against MRSA and S. epidermidis.
Daptomycin-loaded PCL microparticles showed potential to be a useful strategy to successfully manage Gram-positive biofilm-associated infections, due to their enhanced antibiofilm activity and to their considerable interaction with biofilms, especially when compared to the free drug. The interaction between polymeric nano- and microcarriers and Gram-positive biofilms is still unclear. The presented study showed further insights on this, clarifying the antibiofilm activity of daptomycin-loaded PCL microparticles against mature staphylococcal biofilms.
Acknowledgments
This work was supported by the Portuguese government (Fundação para a Ciência e a Tecnologia) and FEDER Grants SFRH/BD/69260/2010 and SFRH/BSAB/1210/2011, research project EXCL/CTM-NAN/0166/2012, and strategic project PEst-OE/SAU/UI4013/2011. The authors acknowledge Novartis Pharma (Basel, Switzerland) for the gift of daptomycin.
Disclosure
The authors report no conflicts of interest in this work.
References
Desai TA, Uskokovic V. Nanoparticulate drug delivery platforms for advancing bone infection therapies. Expert Opin Drug Deliv. 2014; 11(12):1899–1912. | ||
Høiby N, Bjarnsholt T, Moser C, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections. Clin Microbiol Infect. 2015;21(Suppl 1):S1–S25. | ||
Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322–332. | ||
Valour F, Trouillet-Assant S, Rasigade JP, et al. Staphylococcus epidermidis in orthopedic device infections: the role of bacterial internalization in human osteoblasts and biofilm formation. PLoS One. 2013;8(6): e67240. | ||
Forier K, Raemdonck K, De Smedt SC, Demeester J, Coenye T, Braeckmans K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J Control Release. 2014;190:607–623. | ||
Steenbergen JN, Alder J, Thorne GM, Tally FP. Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections. J Antimicrob Chemother. 2005;55(3):283–288. | ||
Sinha VR, Bansal K, Kaushik R, Kumria R, Trehan A. Poly-epsilon-caprolactone based microspheres and nanospheres: an overview. Int J Pharm. 2004;278(1):1–23. | ||
Mack D, Siemssen N, Laufs R. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect Immun. 1992;60(5):2048–2057. | ||
Bettencourt A, Florindo HF, Ferreira IFS, et al. Incorporation of tocopherol acetate-containing particles in acrylic bone cement. J Microencapsul. 2010;27(6):533–541. | ||
Florindo HF, Pandit S, Gonçalves LMD, Alpar HO, Almeida AJ. Streptococcus equi antigens adsorbed onto surface modified poly-ε-caprolactone microspheres induce humoral and cellular specific immune responses. Vaccine. 2008;26:4168–4177. | ||
Martens-Lobenhoffer J, Kielstein JT, Oye C, Bode-Böger SM. Validated high performance liquid chromatography-UV detection method for the determination of daptomycin in human plasma. J Chromatogr B, Anal Technol Biomed Life Sci. 2008;875(2):546–550. | ||
Council of Europe. Vancomycin hydrochloride. In: European Pharmacopoeia. 8th ed. Strasbourg: Council of Europe; 2013:3192–3194. | ||
Clinical and Laboratory Standards Institute (CLSI). Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline M26-A. Wayne (PA): CLSI; 1999. | ||
Mihailescu R, Tafin UF, Corvec S, et al. High activity of fosfomycin and rifampin against methicillin-resistant Staphylococcus aureus biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2014;58(5):2547–2553. | ||
Schillinger C, Petrich A, Lux R, et al. Co-localized or randomly distributed? Pair cross correlation of in vivo grown subgingival biofilm bacteria quantified by digital image analysis. PLoS One. 2012;7(5):e37583. | ||
Amann RI, Blinder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56(6):1919–1925. | ||
Trebesius K, Leitritz L, Adler K, Schubert S, Autenrieth IB, Heesemann J. Culture independent and rapid identification of bacterial pathogens in necrotising fasciitis and streptococcal toxic shock syndrome by fluorescence in situ hybridisation. Med Microbiol Immunol. 2000;188:169–175. | ||
Cadete A, Figueiredo L, Lopes R, Calado CCR, Almeida A J, Gonalves LMD. Development and characterization of a new plasmid delivery system based on chitosan-sodium deoxycholate nanoparticles. Eur J Pharm Sci. 2012;45:451–458. | ||
International Organization for Standardization (ISO). ISO Specification 10993–10995: Biological Evaluation of Medical Devices – Part 5: Tests for In Vitro Cytotoxicity. 3rd ed. Geneva, Switzerland: ISO; 2009. | ||
Kelleher TJ, Lai JJ, Decourcey JP, Lynch P, Zenoni M, Tagliani A, inventors; Cubist Pharmaceuticals Inc., assignee. High purity lipopeptides, lipopeptide micelles and processes for preparing same. United States patent 6696412 B1. 2004. | ||
Le Ray AM, Chiffoleau S, Iooss P, et al. Vancomycin encapsulation in biodegradable poly(epsilon-caprolactone) microparticles for bone implantation. Influence of the formulation process on size, drug loading, in vitro release and cytocompatibility. Biomaterials. 2003; 24(3):443–449. | ||
Le Ray AM, Gautier H, Laty MK, et al. In vitro and in vivo bactericidal activities of vancomycin dispersed in porous biodegradable poly(ε-caprolactone) microparticles. Antimicrob Agents Chemother. 2005; 49(7):3025–3027. | ||
Hombreiro Pérez M, Zinutti C, Lamprecht A, et al. The preparation and evaluation of poly(epsilon-caprolactone) microparticles containing both a lipophilic and a hydrophilic drug. J Control Release. 2000; 65(3):429–438. | ||
Lamprecht A, Rodero Torres H, Schäfer U, Lehr CM. Biodegradable microparticles as a two-drug controlled release formulation: a potential treatment of inflammatory bowel disease. J Control Release. 2000;69: 445–454. | ||
Domenech O, Francius G, Tulkens PM, Van Bambeke F, Dufrêne Y, Mingeot-Leclercq MP. Interactions of oritavancin, a new lipoglycopeptide derived from vancomycin, with phospholipid bilayers: effect on membrane permeability and nanoscale lipid membrane organization. Biochim Biophys Acta – Biomembr. 2009;1788(9):1832–1840. | ||
Lemaire S, Van Bambeke F, Mingeot-Leclercq MP, Tulkens PM. Modulation of the cellular accumulation and intracellular activity of daptomycin towards phagocytized Staphylococcus aureus by the P-glycoprotein (MDR1) efflux transporter in human THP-1 macrophages and madin-darby canine kidney cells. Antimicrob Agents Chemother. 2007;51(8):2748–2757. | ||
John A-K, Baldoni D, Haschke M, et al. Efficacy of daptomycin in implant-associated infection due to methicillin-resistant Staphylococcus aureus: importance of combination with rifampin. Antimicrob Agents Chemother. 2009;53(7):2719–2724. | ||
Entenza JM, Bétrisey B, Manuel O, et al. Rapid detection of Staphylococcus aureus strains with reduced susceptibility to vancomycin by isothermal microcalorimetry. J Clin Microbiol. 2014;52(1): 180–186. | ||
Von Ah U, Wirz D, Daniels AU. Isothermal micro calorimetry – a new method for MIC determinations: results for 12 antibiotics and reference strains of E. coli and S. aureus. BMC Microbiol. 2009;9:106. | ||
Lewis G, Daniels AU. Use of isothermal heat-conduction microcalorimetry (IHCMC) for the evaluation of synthetic biomaterials. J Biomed Mater Res B Appl Biomater. 2003;66:487–501. | ||
Straus SK, Hancock REW. Mode of action of the new antibiotic for Gram-positive pathogens daptomycin: comparison with cationic antimicrobial peptides and lipopeptides. Biochim Biophys Acta – Biomembr. 2006;1758:1215–1223. | ||
Mascio CTM, Alder JD, Silverman JA. Bactericidal action of daptomycin against stationary-phase and nondividing Staphylococcus aureus cells. Antimicrob Agents Chemother. 2007;51(12):4255–4260. | ||
Chakraborty SP, Sahu SK, Pramanik P, Roy S. In vitro antimicrobial activity of nanoconjugated vancomycin against drug resistant Staphylococcus aureus. Int J Pharm. 2012;436(1–2):659–676. | ||
Abdelghany SM, Quinn DJ, Ingram RJ, et al. Gentamicin-loaded nanoparticles show improved antimicrobial effects towards Pseudomonas aeruginosa infection. Int J Nanomedicine. 2012;7:4053–4063. | ||
Moter A, Göbel U. Fluorescence in situ hybridization (FISH) for direct visualization of microorganisms. J Microbiol Methods. 2000;41: 85–112. | ||
Mura S, Hillaireau H, Nicolas J, et al. Influence of surface charge on the potential toxicity of PLGA nanoparticles towards Calu-3 cells. Int J Nanomedicine. 2011;6:2591–2605. | ||
Rampersad SN. Multiple applications of alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors (Basel). 2012;12(9):12347–12360. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.