Back to Journals » Drug Design, Development and Therapy » Volume 10
Activity of a novel sulfonamide compound 2-nitro-N-(pyridin-2-ylmethyl)benzenesulfonamide against Leishmania donovani
Authors Dikhit MR, Purkait B, Singh R, Sahoo B, Kumar A, Kar RK, Ansari Md Y , Saini S, Abhishek K, Sahoo GC, Das S, Das P
Received 18 September 2015
Accepted for publication 8 December 2015
Published 26 May 2016 Volume 2016:10 Pages 1753—1761
DOI https://doi.org/10.2147/DDDT.S96650
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Manas R Dikhit,1,* Bidyut Purkait,1,* Ruby Singh,1 Bikash Ranjan Sahoo,2 Ashish Kumar,1 Rajiv K Kar,3 Md Yousuf Ansari,1,4 Savita Saini,1,5 Kumar Abhishek,1 Ganesh C Sahoo,1 Sushmita Das,6 Pradeep Das1
1Department of Molecular Parasitology and Biomedical Informatics, Rajendra Memorial Research Institute of Medical Sciences, Indian Council of Medical Research, Agamkuan, Patna, Bihar, India; 2Laboratory of Molecular Biophysics, Institute for Protein Research, Osaka University, Japan; 3Biomolecular Nuclear Magnetic Resonance and Drug Design Laboratory, Department of Biophysics, Bose Institute, Kolkata, West Bengal, 4Department of Pharmacoinformatics, 5Department of Biotechnology, National Institute of Pharmaceutical Education and Research, Hajipur, 6Department of Microbiology, All India Institute of Medical Sciences, Patna, Bihar, India
*These authors contributed equally to this work
Abstract: New treatments for visceral leishmaniasis, caused by Leishmania donovani, are needed to overcome sustained toxicity, cost, and drug resistance. The aim of this study was to evaluate the therapeutic effects of 2-nitro-N-(pyridin-2-ylmethyl)benzenesulfonamide (2NB) against promastigote and amastigote forms of L. donovani and examine its effect in combination with amphotericin B (AmB) against AmB-resistant clinical isolates. Effects were assessed against extracellular promastigotes in vitro and intracellular amastigotes in L. donovani-infected macrophages. Levels of inducible nitric oxide and Th1 and Th2 cytokines were measured in infected 2NB-treated macrophages, and levels of reactive oxygen species and NO were measured in 2NB-treated macrophages. 2NB was active against promastigotes and intracellular amastigotes with 50% inhibitory concentration values of 38.5±1.5 µg/mL and 86.4±2.4 µg/mL, respectively. 2NB was not toxic to macrophages. Parasite titer was reduced by >85% in infected versus uninfected macrophages at a 2NB concentration of 120 µg/mL. The parasiticidal activity was associated with increased levels of Th1 cytokines, NO, and reactive oxygen species. Finally, 2NB increased the efficacy of AmB against AmB-resistant L. donovani. These results demonstrate 2NB to be an antileishmanial agent, opening up a new avenue for the development of alternative chemotherapies against visceral leishmaniasis.
Keywords: visceral leishmaniasis, AmB resistance, benzenesulfonamide, ROS, NO, Th1/Th2 cytokines
Introduction
Currently, >350 million people in 98 countries are at risk of leishmaniasis, with approximately half a million new cases of visceral leishmaniasis (VL) diagnosed annually and >50,000 associated deaths. More than 90% of VL cases occur in just six countries, namely, India, Nepal, Bangladesh, Sudan, Ethiopia, and Brazil.1,2 There are no effective vaccines available against VL; thus, treatment relies solely on chemotherapy. Current health practice depends on a limited number of drugs (such as miltefosine and aminoglycosides) that have issues of toxicity, long-dose regimens, high cost, and the need for parenteral administration.3 The toxicity of miltefosine includes its teratogenic potential and its long half-life (~150 hours), which may facilitate the emergence of resistance,4,5 and aminoglycosides-related adverse effects, including elevated hepatic transaminases, ototoxicity, nephrotoxicity, and pain at the injection site.6,7 Drug efficacy against different clinical isolates is variable, and the emergence of acquired resistance to the pentavalent antimonials is a major concern, particularly in the state of Bihar, India, where 64% of cases show resistance to antimonials. Similarly, emerging resistance to amphotericin B (AmB) in Bihar emphasizes the need for new and effective treatments.3,8,9
Sulfonamide drugs have a broad-spectrum application through their antibacterial,10 anticarbonic anhydrase,11,12 and antiproton activities.13,14 In this study, 2-nitro-N-(pyridin-2-ylmethyl)benzenesulfonamide (2NB) (CID 779413), a benzenesulfonamide derivative, which is a chemokine receptor-binding heterocyclic compound, was used.15 Benzenesulfonamides are effective in case of a proliferative disease, such as cancer,16 and are also effective against Leishmania tropica, Toxoplasma, Entamoeba histolytica,17–19 and Plasmodium falciparum.20 Similarly, anticancer drugs, such as miltefosine, and some protein kinase inhibitors21 are effective against VL. Therefore, 2NB was selected and tested against Leishmania donovani. Sulfonamide anilide is an inhibitor of histone deacetylase.22 We have previously shown that high level of silent information regulator 2 (Sir2) of L. donovani, a histone deacetylase, is associated with AmB resistance in parasites.23 This led us to predict that our compound of interest 2NB may reverse AmB resistance in combination with AmB through inhibition of Sir2 activity.
More than 100 sulfonamide-containing drugs are currently on the market.24 Therefore, the use of 2NB could provide a rapid and cost-effective approach to antileishmanial drug discovery. Here, we tested the therapeutic potential of 2NB against Leishmania promastigotes and also the intracellular amastigotes via infected peritoneal mouse macrophages. We also evaluated the toxicity level of 2NB on peritoneal macrophages. Therefore, the major objective of this investigation is to evaluate the antileishmanial effect of 2NB and its potential to be used in combination with AmB against AmB-resistant clinical isolates.
Materials and methods
Experimental animals
Female BALB/c mice 6–8 weeks old were obtained from breeding stocks maintained at the animal husbandry of Rajendra Memorial Research Institute of Medical Sciences (RMRIMS), Patna, India. Mice were injected with 4% starch and sacrificed after 48 hours. Peritoneal macrophages were isolated and seeded onto well plates according to the experimental protocol described in (Cell cytotoxicity assay) section. Macrophages were infected with L. donovani promastigotes, and the effect of 2NB was tested on intracellular amastigotes. A total of 12 mice were used to obtain peritoneal macrophages for all the experiments.
Ethical statement
For animal use, the procedures used were reviewed and approved by the Animal Ethical Committee, RMRIMS, Indian Council of Medical Research (ICMR). The RMRI (ICMR) follows “The Guide for the Care and Use of Laboratory Animals,” 8th edition, by the Institute for Laboratory Animal Research. This study was approved by the Institutional Ethical Committee of RMRIMS.
Compound
2NB (CID 779413), a derivative of sulfonamide drug, was purchased from Asinex (Moscow, Russia). The compound 2NB (Figure 1) was dissolved in distilled water (dH2O) at a stock concentration of 5 mg/mL.
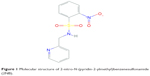  | Figure 1 Molecular structure of 2-nitro-N-(pyridin-2-ylmethyl)benzenesulfonamide (2NB). |
Parasite culture
L. donovani promastigotes, AG83 (MHOM/IN/1983/AG83), were maintained in M199 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) at 25°C in a BOD incubator. Parasites were subcultured every 7 days, and only stationary-phase cultures were used for experiments.
AmB-resistant and -sensitive clinical isolates of L. donovani (used in our previous study)9,23 of VL were obtained from the splenic aspirates of AmB responder and nonresponder patients of the indoor ward facility of the RMRIMS, Patna, India,9 and were grown in Roswell Park Memorial Institute (RPMI)-1640 (Thermo Fisher Scientific) medium (pH 7.4), supplemented with 10% FBS (Thermo Fisher Scientific) and 1% of penicillin (50 U/mL) and streptomycin (50 mg/mL) solution (Sigma-Aldrich Co., St Louis, MO, USA) at 250°C and maintained further under drug pressure.9
The resistant and sensitive nature of the parasites was confirmed by in vitro and ex vivo (macrophage infection) assay as described earlier by our group.9,23 Briefly, in in vitro drug sensitivity assay, 2×106 parasites were incubated with different concentrations of AmB, and the viability of the cells was evaluated either by counting the viable cells in a hemocytometer (Rohem, Nashik, India) by the trypan blue (Sigma-Aldrich Co.) (0.5 mg/mL) exclusion method or by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, and the 50% lethal doses (LD50) were determined for both the AmB-resistant and AmB-sensitive strains.9,23 Briefly, in ex vivo drug sensitivity assay,9,23 adhered macrophages (THP1 cells) were infected with parasites at a ratio of 1:10 (macrophages:parasite), and AmB at different concentrations was then added to the infected macrophages and incubated for 48 hours. The number of viable amastigotes per 100 macrophages was counted under the microscope after Giemsa staining, and the LD50 values for both resistant and sensitive parasites were calculated.9,23
Antileishmanial activity of 2NB (in vitro) and determination of IC50
In vitro drug sensitivity was performed by incubating 2×106 parasites in RPMI-1640 medium (supplemented with 10% FBS) with indicated different concentrations of 2NB at 1-day intervals for 3 consecutive days. Parasites were not treated with 2NB in the control experimental set. The viability of the parasites was evaluated using MTT assay,9 where the conversion of MTT to formazan by mitochondrial enzymes served as an indicator of cell viability. The amount of formazan produced was directly proportional to the number of metabolically active cells. The 50% inhibitory concentration (IC50) was determined after analyzing with MS Excel™ and Prism™.
Inhibitor assay of AmB-resistant parasites by 2NB
As used in our previous work, for this experiment, three AmB-resistant and three AmB-sensitive parasites were used.23 2NB was added at a concentration of 20 μg/mL to AmB-resistant and -sensitive parasites and incubated for 4 hours at 23°C in a BOD incubator. The parasites were subsequently washed with phosphate-buffered saline (pH 7.2) and treated with AmB. LD50 values of AmB were then determined. For positive control, known inhibitor of Sir2, that is, sirtinol, was also used to inhibit the parasites.
Determination of enzyme activity (deacetylase activity) of Sir2 in 2NB-inhibited AmB-resistant and -sensitive parasites
Total intracellular nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase activity of Sir2 was measured for wild-type, 2NB–treated, and sirtinol (known inhibitor of Sir2)-treated parasites according to our previous work23 using SIRT1/Sir2 deacetylase fluorometric assay kit (CycLex). Briefly, total cellular extracts were prepared and used as a cofactor for purified recombinant L. donovani SIR2 protein (LdSir2RP). In control reaction, NAD+ of the kit was used as a cofactor for the purified LdSir2RP. The results were recorded in an LS55 Spectrofluorimeter (PerkinElmer Inc., Waltham, MA, USA). The results were expressed as the rate of reaction for the first 20 minutes when there was a linear correlation between the fluorescence and the period of time.
Cell cytotoxicity assay
This assay was performed as described previously,9 with some modifications. Briefly, primary macrophages that were harvested from starch-induced peritoneal exudates in BALB/c mice were seeded (104 cells/well) in a 96-well plate with different concentrations of 2NB. After 48 hours of incubation, the medium was removed, 200 μL of fresh supplemented medium and 20 μL of Alamar blue (Sigma-Aldrich Co.) were added, and the absorbance was measured at 550 nm. There were three replicates in each test, and the data reported herein are the mean ± standard deviation of the three experiments.
Activity of 2NB against L. donovani-infected macrophages
Starch-induced peritoneal exudate-harvested macrophages from BALB/c mice were seeded in 16-well glass slides and infected with Leishmania promastigotes (at a ratio of 1:10= macrophages:parasite) as previously described.9,25 The infected macrophages were exposed to 2NB for 48 hours, after which the percentage of infected macrophages and the amastigotes per 100 macrophages was determined microscopically after Giemsa staining25,26 followed by IC50 calculation as described previously.9
Semi-quantitative reverse transcription–polymerase chain reaction
The messenger RNA level of inducible nitric oxide (iNOS) was performed by isolating the total RNA from 2NB-treated/untreated peritoneal macrophages using Trizol method. Reverse transcription was performed using an anchored oligo(dT) (H-dT11M, where M represents A, C, or G; GenHunter, Nashville, TN, USA).9 The synthesized complementary DNAs were amplified by polymerase chain reaction (PCR) for iNOS gene. The PCR product was run on 1.5% agarose gel, stained with ethidium bromide, and finally documented and quantified using the Bio-Rad gel documentation system and the associated Quantity One software. PCR product was normalized with respect to the glyceraldehyde-3-phosphate dehydrogenase reverse transcription PCR product.
Cytokine production
The ability of 2NB to induce the production of the cytokines was tested using peritoneal macrophage cells. These cells were cultured in 24-well plates, in two conditions, 1) infected with L. donovani at a ratio of ten parasites:one macrophage and 2) no infection and incubated for 6–8 hours at 37°C in 5% CO2. Free parasites were removed by washing with phosphate-buffered saline (pH 7.2), and the cultures were maintained in RPMI-1640 medium supplemented with 10% FBS for 24 hours at 37°C in 5% CO2. After incubation, the infected macrophages were treated with 100 μg/mL of 2NB. After 48 hours of treatment, the production of Th1 cytokines (interleukin [IL]-12 and tumor necrosis factor [TNF]-α) and Th2 cytokines (IL-10 and transforming growth factor [TGF]-β) was measured in the cell culture supernatants using BioVision enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions. All the assays were performed in triplicates.
Measurement of reactive oxygen species (ROS)
To measure the level of ROS, the cell permeable probe H2DCFDA (Sigma-Aldrich Co.) was used as described previously.27 Infected 2NB-treated/untreated peritoneal macrophages were incubated with H2DCFDA (2 mg/mL) at room temperature for 20 minutes in the dark. Relative fluorescence was measured in a PerkinElmer Inc., LS55 Spectrofluorometer at an excitation wavelength of 508 nm and emission wavelength of 529 nm. Fluorometric measurements were made in triplicate and expressed as mean fluorescence intensity units.
Quantification of NO
NO was quantified by the accumulation of nitrite in macrophage culture supernatants, and nitrite was detected by the Griess reaction as described previously.28
Statistical analysis
All the experiments were conducted at least in triplicate, and the results are expressed as mean ± standard deviation of the three experiments, and the data were statistically analyzed by a single analysis of variance test. A P-value of <0.01 was considered significant.
Results and discussion
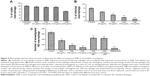
The commercially available compound 2NB was tested against L. donovani promastigotes (MHOM/IN/1983/AG83) in vitro and intracellular amastigotes cultured in mouse peritoneal macrophages. The in vitro assay revealed that 2NB showed significant activity against L. donovani promastigotes (Figure 2), with an IC50 value of 38.5±1.5 μg/mL. Treatment of promastigotes with 2NB demonstrated a dose-dependent inhibition of the parasite growth (Figure 2), indicating parasite-killing ability.
The bacteriostatic sulfonamide29 is active against Toxoplasma and Entamoeba.19,20 Typically, sulfonamides suppress bacterial growth by competitive blockade of para-aminobenzoic acid to prevent the synthesis of folic acid. Since, in humans, folate accumulation takes place through the diet,30 sulfonamide has no effect on human cellular machinery. Although antifolates such as pyrimethamine, sulfa drugs, and trimethoprim are effective against protozoan infectious diseases,31–33 antifolate chemotherapy has not been achieved against Leishmania infections.34 Here, we found potential antileishmanial activity by 2NB, consistent with sulfonamide activity against L. tropica.18 This suggests a different antileishmanial target in Leishmania promastigotes than the folate biosynthetic pathway, possibly the carbonic anhydrase as reported in L. chagasi.35
2NB in combination with AmB reverses the resistant property of the AmB-resistant parasites.23 In our previous study, we showed that histone deacetylase enzyme, Sir2, was highly overexpressed in AmB-resistant parasites compared to the sensitive parasites, and this upregulation of Sir2 was associated with AmB-resistant property of the parasites.23 Here, treatment of AmB-resistant parasites with 2NB decreased the LD50 of AmB ~2.5-fold compared to the untreated resistant parasites (Figure 3A; Table 1). There was no change in the LD50 of AmB for the sensitive parasites after inhibition with the compound 2NB (Table 1). The deacetylase activity of Sir2 of AmB-resistant parasites was found to be significantly higher compared to the sensitive parasites, which correlates our previous report of upregulation of Sir2 in AmB-resistant parasites.23 It was reported that sulfonamide anilides inhibit the histone deacetylase enzyme,22 and in this study, 2NB, being a sulfonamide compound, reduced the deacetylase activity of Sir2 of AmB-resistant L. donovani parasites ~2.6-fold compared to the untreated resistant parasites (Figure 3B). Therefore, these results demonstrate that our compound of interest 2NB can inhibit the deacetylase activity of Sir2 as evidenced by Figure 3 and can consequently reverse the AmB-resistant property of resistant parasites (Table 1), which correlates with our previous study.23 2NB at a concentration of 20 μg/mL had no significant toxic effect on the untreated resistant and sensitive parasites (data not shown). So, our compound of interest 2NB in combination with AmB may increase the efficacy of the AmB and may be used in combination with AmB for the treatment of resistant cases.
In order to test the efficacy of the drug on intracellular amastigotes, peritoneal macrophages were infected with L. donovani and treated with different concentrations of 2NB. The number of amastigotes was counted microscopically on 100 macrophages per sample, and the results were expressed as percent of reduction of the infection rate in comparison to that of the controls (Figure 4B).23,24 2NB was found to inhibit amastigote growth in a dose-dependent manner (Figure 4) with an IC50 value of 86.4±2.4 μg/mL and reduced the parasite burden in infected macrophages by >85% (P<0.001) as compared to untreated controls (Figure 4C). Furthermore, up to 2NB concentration of 150 μg/mL, no cytotoxicity was observed against the murine macrophages, which indicates the selectivity of 2NB against amastigotes compared with mammalian cells as evaluated by qualitative microscopic examination (Figure 4A). To the best of our knowledge, this is the first report of antileishmanial activity of 2NB that can also reduce parasite burdens in infected macrophages.
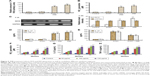
ROS and NO, important macrophage-derived microbicidal molecules, are essential to control Leishmania infection.27,36 Therefore, the generation of ROS and NO in the culture supernatants of 2NB-treated and untreated L. donovani-infected macrophages was estimated. In infected 2NB-treated macrophages, the level of ROS was increased up to ~5.4-fold (P<0.001) as compared to the infected control (Figure 5A). Similarly, 2NB increased NO generation in peritoneal macrophages in a dose- and time-dependent manner (Figure 5F) that was found to be maximal at 24 hours (27.87+2.1 mM/106 cells) at a dose of 120 μg/mL of 2NB (Figure 5B). We then checked whether 2NB treatment could enhance the generation of NO in infected macrophages. Nitrite generation was markedly increased after 2NB (120 μg/mL) treatment in infected peritoneal macrophages up to ~6-fold (P<0.001) as compared to untreated infected control (Figure 5B). Consequently, the mRNA level of iNOS was increased ~4-fold (P<0.001) with the treatment of 120 μg/mL 2NB in peritoneal macrophages infected with L. donovani (Figure 5C). Using an iNOS inhibitor, 2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine (AMT), the rate of infection was measured to confirm the involvement of NO in the inhibition of intracellular amastigote multiplication by 2NB. At 48 hours posttreatment, 15 mM AMT markedly reduced (82% reduction in parasite clearance) the inhibitory effect of 2NB in ex vivo culture condition (Figure 4C). For the cure of VL, iNOS upregulation and subsequent release of nitrogen metabolites are essential.36,37 However, both reactive nitrogen and oxygen intermediates are important factors for the cure of VL.38,39 Significantly enhanced generation of ROS and NO in 2NB-treated macrophages further suggested the overall activated state of cells for successful elimination of parasite ex vivo.
We then investigated the role of immune system in parasite killing by 2NB in infected macrophage model. Macrophage-produced cytokines can affect the intracellular growth of Leishmania, and its infection results in impaired microbicidal machinery of macrophages as evidenced by modification of Th1/Th2 paradigm, resulting in parasite survival.40–43 Along with NO production, the level of IL-12 and TNF-α was also increased in 2NB-treated macrophages in a dose- and time-dependent manner, and maximum induction of IL-12 and TNF-α was observed at 48 hours posttreatment (Figure 5G and H) with a dose of 120 μg/mL of 2NB. Therefore, in order to evaluate whether 2NB could modulate the infected macrophages for production of these pro- and anti-inflammatory cytokines, Th1 cytokines (IL-12, TNF-α) and Th2 cytokines (IL-10, TGF-β) levels were measured in supernatants from L. donovani-infected peritoneal macrophages, untreated or treated with 120 μg/mL 2NB. In untreated L. donovani-infected macrophages, the level of IL-12 (68±6.8 pg/mL) and TNF-α (84±8.1 pg/mL) did not appreciably change (Figure 5D). However, the levels of IL-10 (8.4-fold increase, P<0.001) and TGF-β (7.7-fold increase, P<0.001) were increased robustly after infection (Figure 5E). In contrast, 2NB (120 μg/mL) treatment significantly increased the production of pro-inflammatory cytokines, IL-12 (6.64-fold increase, P<0.001), and TNF-α (5.57-fold increase, P<0.001) in infected macrophages (Figure 5D). In contrast, 2NB (120 μg/mL) treatment decreased the level of anti-inflammatory cytokines, IL-10 (58% decrease, P<0.01), and TGF-β (56% decrease, P<0.01) in infected macrophages compared to the untreated infected macrophages (Figure 5E). In infected macrophages, 2NB treatment at a dose of 120 μg/mL resulted in reduced amastigotes survival by the induced production of disease-resolving Th1 (IL-12, TNF-α) cytokines and decreased release of disease-promoting Th2 (IL-10, TGF-β) cytokines. It was observed that the production of IL-12 and TNF-α was increased in Leishmania-infected macrophages after treatment as compared with the untreated controls and untreated uninfected controls. Although the level of reduction of IL-10 and TGF-β was not very high in infected macrophages following treatment, this might be explained by the sharp induction of IL-12 and TNF-α (anti-Leishmania cytokine).
Conclusion
In conclusion, we have shown for the first time that a benzenesulfonamide, 2NB, possesses leishmanicidal activity against L. donovani in both promastigote and intracellular amastigote forms at concentrations that are not toxic to the host. The lethal effects are associated with induction of disease-resolving Th1 cytokine response along with the generation of ROS and NO. 2NB also increases the efficacy of the AmB and reverses the AmB-resistant property of the resistant L. donovani in combination with AmB. Therefore, 2NB compounds or its analogs may in future be used alone or in combination with conventional drugs as an alternate chemotherapy for VL.
Acknowledgments
The work is supported by ICMR, India. We would like to thank Dr Mark JI Paine, Liverpool School of Tropical Medicine, UK, for critically reviewing this manuscript. MRD and BP are the joint first authors.
Disclosure
The authors report no conflicts of interest in this work.
References
Chappuis F, Sundar S, Hailu A, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–882. | ||
Dawit G, Girma Z, Simenew K. A review on biology. Epidemiology and public health significance of leishmaniasis. J Bacteriol Parasitol. 2013;4:2. | ||
Tiuman TS, Santos AO, Ueda-Nakamura T, et al. Recent advances in leishmaniasis treatment. Int J Infect Dis. 2011;15:e525–e532. | ||
Dube A, Singh N, Sundar S, Singh N. Refractoriness to the treatment of sodium stibogluconate in Indian kalaazar field isolates persists in in-vitro and in-vivo experimental models. Parasitol Res. 2005;96:216–223. | ||
Ritmeijer K, Dejenie A, Assefa Y. A comparison of miltefosine and sodium stibogluconate for treatment of visceral leishmaniasis in an Ethiopian population with high prevalence of HIV infection. Clin Infect Dis. 2006;43:357–364. | ||
Sundar S, Jha TK, Thakur CP, Sinha PK, Bhattacharya SK. Injectable paromomycin for visceral leishmaniasis in India. N Engl J Med. 2007;356:2571–2581. | ||
Athanasiou LV, Batzias GC, Saridomichelakis MN, et al. Pharmacokinetics and tolerability of aminosidine after repeated administrations using an optimal dose regimen in healthy dogs and in dogs with leishmaniasis. Vet Parasitol. 2014;205:365–370. | ||
Walker RG, Thomson G, Malone K, et al. High throughput screens yield small molecule inhibitors of Leishmania CRK3: CYC6 cyclin-dependent kinase. PLoS Negl Trop Dis. 2011;5:e1033. | ||
Purkait B, Kumar A, Nandi N, et al. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob Agents Chemother. 2012;56:1031–1041. | ||
Drews J. Drug discovery: a historical perspective. Science. 2000;287:1960–1964. | ||
Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors and their therapeutic potential. Expert Opin Ther Pat. 2000;10:575–600. | ||
Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev. 2003;23:146–189. | ||
Ogden RC, Flexner CW. Protease Inhibitors in AIDS Therapy. New York: Marcel Dekker; 2001. | ||
Supuran CT, Scozzafava A, Mastrolorenzo A. Bacterial proteases: current therapeutic use and future prospects for the development of new antibiotics. Expert Opin Ther Pat. 2001;11:221–259. | ||
Bridger, Gary James, et al. assignee. Chemokine receptor binding heterocyclic compounds. United States US Patent No. 7022717 B2. 2006 Apr, 4. | ||
Bashir R, Ovais S, Yaseen S, et al. Synthesis of some new 1, 3, 5-trisubstituted pyrazolines bearing benzene sulfonamide as anticancer and anti-inflammatory agents. Bioorg Med Chem Lett. 2011;21:4301–4305. | ||
Senekji H. The effect of sulfanilamide and trypaflavin on cultures of Leishmania tropica. J Infect Dis. 1940;66:111–112. | ||
Sabin AB, Warren J. Therapeutic effectiveness of certain sulfonamides on infection by an intracellular protozoon (Toxoplasma). Exp Biol Med. 1942;51:19–23. | ||
Rodaniche EC, Kirsner JB. The effect of sulfonamide compounds on the growth of Entamoeba histolytica in culture. J Parasitol. 1942;28:441–449. | ||
Andrews KT, Fisher GM, Sumanadasa SD, et al. Antimalarial activity of compounds comprising a primary benzene sulfonamide fragment. Bioorg Med Chem Lett. 2013;23:6114–6117. | ||
Sanderson L, Yardley V, Croft SL. Activity of anti-cancer protein kinase inhibitors against Leishmania spp. J Antimicrob Chemother. 2014;69:1888–1891. | ||
Fournel M, Trachy-Bourget MC, Yan PT, et al. Sulfonamide anilides, a novel class of histone deacetylase inhibitors, are antiproliferative against human tumors. Cancer Res. 2002;62:4325–4330. | ||
Purkait B, Singh R, Wasnik K, et al. Up-regulation of silent information regulator 2 (Sir2) is associated with amphotericin B resistance in clinical isolates of Leishmania donovani. J Antimicrob Chemother. 2015;70:1343–1356. | ||
Smith DA, Jones RM. The sulfonamide group as a structural alert: a distorted story? Curr Opin Drug Discov Devel. 2008;11:72–79. | ||
Yardley V, Croft SL. A comparison of the activities of three amphotericin B lipid formulations against experimental visceral and cutaneous leishmaniasis. Int J Antimicrob Agents. 2000;13:243–248. | ||
Das S, Rani M, Pandey K, et al. Combination of paromomycin and miltefosine promotes TLR4-dependent induction of antileishmanial immune response in vitro. J Antimicrob Chemother. 2012;67:2373–2378. | ||
Sharma A, Madhubala R. Ubiquitin conjugation of open reading frame F DNA vaccine leads to enhanced cell-mediated immune response and induces protection against both antimony-susceptible and-resistant strains of Leishmania donovani. J Immunol. 2009;183:7719–7731. | ||
Kar S, Ukil A, Das PK. Signaling events leading to the curative effect of cystatin on experimental visceral leishmaniasis: involvement of ERK1/2, NF-κB and JAK/STAT pathways. Eur J Immunol. 2009;39:741–751. | ||
Henry RJ. The mode of action of sulfonamides. Bacteriol Rev. 1943;7:175. | ||
Madigan MT. Brock Biology of Microorganisms. 11th ed. SciELO Barcelona; 2005. | ||
Peters W, Richards WH. Antimalarial Drugs. 1: Biological Background, Experimental Methods, and Drug Resistance. 2: Current Antimalarials and New Drug Developments. (Handbook of Experimental Pharmacology Volume 68 Nos. 1 & 2). Springer-Verlag: Berlin Heidelberg; 1984. | ||
Grossman PL, Remington JS. The effect of trimethoprim and sulfamethoxazole on Toxoplasma gondii in vitro and in vivo. Am J Trop Med Hyg. 1979;28:445–455. | ||
McDougald LR. Chemotherapy of coccidiosis. In: Long PL, editor. The Biology of the Coccidia. Baltimore, MD: University Park Press; 1982. pp. 373–427. | ||
Hardy L, Matthews W, Nare B, Beverley SM. Biochemical and genetic tests for inhibitors of Leishmania pteridine pathways. Exp Parasitol. 1997;87:158–170. | ||
Syrjanen L, Vermelho AB, de Almeida Rodrigues I, et al. Cloning, characterization, and inhibition studies of a β-carbonic anhydrase from Leishmania donovani chagasi, the protozoan parasite responsible for leishmaniasis. J Med Chem. 2013;56:7372–7381. | ||
Basu R, Bhaumik S, Basu JM, Naskar K, De T, Roy S. Kinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and-resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: evidence for mixed Th1-and Th2-like responses in visceral leishmaniasis. J Immunol. 2005;174:7160–7171. | ||
Das L, Datta N, Bandyopadhyay S, Das PK. Successful therapy of lethal murine visceral leishmaniasis with cystatin involves up-regulation of nitric oxide and a favorable T cell response. J Immunol. 2001;166:4020–4028. | ||
Roach T, Kiderlen AF, Blackwell JM. Role of inorganic nitrogen oxides and tumor necrosis factor alpha in killing Leishmania donovani amastigotes in gamma interferon-lipopolysaccharide-activated macrophages from Lshs and Lshr congenic mouse strains. Infect Immun. 1991;59:3935–3944. | ||
Gantt KR, Goldman TL, McCormick ML, et al. Oxidative responses of human and murine macrophages during phagocytosis of Leishmania chagasi. J Immunol. 2001;167:893–901. | ||
Awasthi A, Mathur RK, Saha B. Immune response to Leishmania infection. Indian J Med Res. 2004;119:238–258. | ||
Rogers KA, DeKrey GK, Mbow ML, Gillespie RD, Brodskyn CI, Titus RG. Type 1 and type 2 responses to Leishmania major. FEMS Microbiol Lett. 2002;209:1–7. | ||
Souza AS, Giudice A, Pereira JM, et al. Resistance of Leishmania (Viannia) braziliensis to nitric oxide: correlation with antimony therapy and TNF-α production. BMC Infect Dis. 2010;10:209. | ||
Anderson CF, Lira R, Kamhawi S, Belkaid Y, Wynn TA, Sacks D. IL-10 and TGF-β control the establishment of persistent and transmissible infections produced by Leishmania tropica in C57BL/6 mice. J Immunol. 2008;180:4090–4097. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.