Back to Journals » Journal of Inflammation Research » Volume 13
Activity Disease in SLE Patients Affected IFN-γ in the IGRA Results
Authors Maharani W , Ratnaningsih DF, Utami F, Yulianto FA , Dewina A, Hamijoyo L , Atik N
Received 22 April 2020
Accepted for publication 30 July 2020
Published 14 August 2020 Volume 2020:13 Pages 433—439
DOI https://doi.org/10.2147/JIR.S258235
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Winni Maharani,1,2 Dwi Febni Ratnaningsih,3 Fitria Utami,3 Fajar Awalia Yulianto,4 Anneke Dewina,5 Laniyati Hamijoyo,5,6 Nur Atik5,7
1Department of Microbiology, Faculty of Medicine, Universitas Islam Bandung, Bandung, Indonesia; 2Biomedical Sciences Master Program, Faculty of Medicine, Padjadjaran University, Bandung, Indonesia; 3Immunology Laboratory, Faculty of Medicine, Padjadjaran University, Bandung, Indonesia; 4Department of Public Health, Faculty of Medicine, Universitas Islam Bandung, Bandung, Indonesia; 5Lupus Study Centre, Immunology Study Group, Faculty of Medicine, Padjadjaran University, Bandung, Indonesia; 6Department of Internal Medicine, Faculty of Medicine, Padjadjaran University/Hasan Sadikin Hospital, Bandung, Indonesia; 7Department of Biomedical Sciences, Faculty of Medicine, Padjadjaran University, Bandung, Indonesia
Correspondence: Nur Atik
Department of Biomedical Sciences, Faculty of Medicine, Padjadjaran University, Bandung, Indonesia
Tel +62 812-8095-6825
Email [email protected]
Purpose: Highly active systemic lupus erythematosus (SLE) causes a high risk of tuberculosis (TB) infection in SLE patients in Indonesia, a country in which the disease, especially extrapulmonary TB, is endemic. Interferon (IFN)-γ releasing assay (IGRA) can detect latent or previous TB infection. This study sought to determine latent TB infection and levels of IFN-γ, a key player in various inflammation and autoimmune disease, in patients with SLE and relate findings to disease activity.
Patients and Methods: This experimental study included 79 female subjects distributed into three groups of active SLE, quiescent SLE and healthy controls. We used SLE Disease Activity Index– 2000 (SLEDAI-2K) scores to stratify the subjects. Each group underwent IGRA testing using the QuantiFERON-TB Gold Plus kit.
Results: We recruited 59 female patients with SLE. The patients had a median age and disease duration 30 and 5 years, respectively. Statistical analysis using the Kruskal–Wallis test showed that active condition, high SLEDAI-2K score and immunosuppressive therapies affect IGRA results. Specifically, healthy controls (n=20) were most likely to have negative IGRA results (67.09%), whilst 27.27% of active cases (n=33) and 3.85% of quiescent cases (n=26) had indeterminate results (p=0.02). The number of immunosuppressant therapies was significantly negatively correlated with IFN-γ (p=0.004). No difference in IFN-γ concentration was detected amongst the active and other groups (p> 0.05).
Conclusion: High-activity SLE and immunosuppressive therapies cause dysregulation of the immune response, which, in turn, influences IGRA results. Thus, additional testing is necessary to detect TB infection in patients with SLE.
Keywords: SLE, active, quiescent, IFN-γ, IGRA
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disorder with multiple organ involvement that mostly occurs in females of child-bearing age.1 Excess autoantibody production presents as several clinical manifestations and leads to permanent organ damage and even death.2 A natural history of SLE is marked by fluctuating disease activity, including active and quiescent, which may elicit a number of comorbidities; infection is the most common cause of mortality amongst patients with SLE.3,4
The role of cytokines in immunomodulation is well recognised. Autoantibody production as a consequence of SLE leads to an abundance of inflammatory cytokines, and these cytokines may be used as a biomarker of disease activity.5,6 Changes in disease activity (active or quiescent) influence the immune response and levels of several cytokines, including interleukin (IL)-6, IL-4 and IL-17 and interferon (IFN)-γ. Previous studies demonstrated that high-activity disease results in increased IFN-γ levels.7,8
IFN systems (types I, II and III) are related to infection and autoimmune disease. IFNs have diverse cell origins, receptors and functions in infection. Type I IFN consists of IFN-α, β, ε, κ and ω, type-III IFN, or IFN-λ, plays roles in viral and bacterial infection. Type II IFN, or IFN-γ, plays a role in intracellular infection. IFN-α is responsible for the early stages of SLE, whilst IFN-λ could reflect the progress and severity of the disease.9–11
Tuberculosis (TB) is endemic in Indonesia. Indeed, the number of patients with SLE and TB infection, particularly extrapulmonary TB, in the country shows a consistent increase. In our rheumatology clinic outpatients Dr. Hasan Sadikin Hospital Bandung, TB incidence was found in 11.4% of total registered patients.12 High disease activity may cause a high risk of TB infection due to immune response dysregulation and immunosuppressant therapy.13,14 Latent or previous TB infection is usually detected using IFN-γ releasing assay (IGRA). Three FDA-cleared IGRAs, including the T-SPOT.TB test (Oxford Diagnostic Laboratories, Memphis, TN, USA), the QuantiFERON-TB Gold In-Tube (QFT-GIT; Qiagen, Germantown, MD, USA) test and the new-generation QuantiFERON-TB Gold Plus (QFT-Plus; Qiagen) assay, are currently available. These assays comprise specific TB antigens. QFT-GIT, for example, uses ESAT-6, CFP-10 and TB7.7 to elicit IFN-γ from CD4+ T cells, whilst QFT-Plus employs ESAT-6 and CFP-10, which are released from CD4+and CD8+ T cells.15,16
Because of unreliable IGRA findings, this examination is not performed routinely in Indonesia. Previous studies showed that disease activity affects patients’ immune status and could interfere with the body’s response towards infection or inflammation.3,17 IGRA methods can expose cytokine IFN-γ when stimulated by TB antigen; thus, whether disease activity could affect IGRA result must be determined. Our study aims to determine latent TB in patients with SLE. The results of active condition are compared with those of quiescent condition on the basis of SLE Disease Activity Index–2000 (SLEDAI-2K) scores, and IFN-γ levels are measured.
Patients and Methods
Patient Selection and Study Design
In this experimental study, we consecutively enrolled 59 female patients with SLE who presented to the rheumatology outpatient clinic at the Dr. Hasan Sadikin Hospital, Bandung, Indonesia, and met the American College of Rheumatology and/or Systemic Lupus International Collaborating Clinics classification criteria.18 We also recruited 20 female healthy controls using the inclusion criterion no history of autoimmune disease or TB infection. Disease activity in patients with SLE using SLEDAI-2K scores; here a cut-off point of ≥4 was used to indicate active disease on the basis of clinical appearance, haematology, and immunology results.19 Clinical data, including age, disease duration, history of TB and number and dose of all therapies, were collected and analysed. Disease duration was defined as the time from SLE diagnosis until an IGRA was performed (Figure 1). Clinical manifestations of SLE included skin rash, photosensitivity, oral ulcers, arthritis, serositis, nephritis and neurological, haematological and immunological disorders, as previously defined.
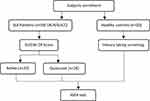 |
Figure 1 Flowchart of the subject enrolment process. |
The study was conducted in compliance with the Declaration of Helsinki and the Research Ethics Committee of Universitas Padjadjaran, Indonesia, approved all procedures performed in this study (136/UN6.KEP/EC/2019). All patients provided written informed consent for joining the study.
IGRA Examination
IGRA was performed using whole blood samples collected from each patient through QuantiFERON-TB Gold Plus (QFT-Plus QIAGEN, USA) kits according to the manufacturer’s instructions. Briefly, 1 mL of blood was directly drawn into each of the QFT-Plus blood collection tubes.16 The kit consists of four blood collection tubes: (i) nil tube (negative control: whole blood without antigens or mitogen), (ii) mitogen tube (positive control: whole blood with phytohemagglutinin); (iii) TB antigen tube 1 (whole blood with peptides of ESAT-6 and CFP-10 proteins stimulating TB-specific antigens to elicit CD4) and (iv) TB antigen tube 2 (whole blood with peptides of ESAT-6 and CFP-10 proteins stimulating TB-specific antigens to elicit CD4 and CD8). The tubes were incubated overnight at 37°C, and the concentrations of IFN-γ (IU/mL) were measured using an enzyme-linked immunosorbent assay kit. Results were considered positive if the concentration of IFN-γ in the antigen-stimulated well was ≥0.35 IU/mL after subtraction the level of the nil well and negative if the IFN-γ concentration in the antigen-stimulated well was ≤0.35 IU/mL after subtracting the level of the nil well and the concentration in the mitogen well was ≥0.35 IU/mL. A result was considered indeterminate if: (1) the blood sample did not respond appropriately to either the positive or negative tube and, thus, could not be interpreted, or (2) the concentration of IFN-γ in the antigen well minus that in the nil well was <0.35 IU/mL but the concentration of the cytokine in the mitogen well was <0.5 IU/mL or (3) the concentration of IFN-γ in the nil well was >0.7 IU/mL and the concentration of the cytokine in the antigen well was >50% greater than that in the nil well.17
Data Analysis
Data analysis was performed using STATA version 11.0 software (Stata Corp, College Station, TX, USA). Most variables were numerical; therefore, the data distribution was examined by analysing differences between means and medians, standard deviation, skewness, kurtosis and normality. Variables of normal distribution are presented as mean±standard deviation with parametric hypothesis testing, whilst variables of non-normal distribution are presented as median with non-parametric hypothesis testing. Categorical variables are presented as proportion (percentages) and analysed using the chi-squared or Fisher’s exact and Kruskal–Wallis test. Correlation analysis was conducted using Spearman’s test.
Results
A total of 59 female patients with SLE were recruited in this study. These patients and 20 female healthy controls underwent the IGRA. According to the SLEDAI-2K criteria, 33 and 26 patients had active and quiescent disease, respectively. The average age of cases in our study was 30 years, and the median disease duration was 5 years. Immunosuppressant and non-immunosuppressant therapies, including methylprednisolone, azathioprine, chloroquine, mycophenolate mofetil, cyclophosphamide, methotrexate, cyclosporine, folic acid and calcium, were given to control disease activity. Two immunosuppressant therapies were frequently prescribed, and methylprednisolone and azathioprine were the most common combination of these therapies. In this research, we also found that 23.73% of subjects have TB history and only 35.6% of subjects have scar sign after BCG vaccination (Table 1).
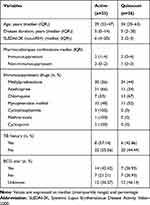 |
Table 1 Characteristics of Systemic Lupus Erythematosus Patients |
Next, we analysed the IGRA results. We found that IGRA was most likely to have negative (67.09%) and indeterminate results (12.66%) amongst groups. Most of the healthy controls (30%) had negative IGRA results, whilst many cases with SLE (27.27%), especially those in the active group, showed indeterminate results (p=0.02), as shown in Table 2.
 |
Table 2 Effects of Disease Activity on IGRA Results |
Previous studies showed that disease activity affects patients’ immune status and could interfere with the body’s response towards infection or inflammation.3,20 IGRA methods can expose cytokine IFN-γ when stimulated by TB antigen; thus, whether disease activity could affect IGRA results must be determined. Disease activity in patients with SLE was defined using SLEDAI-2K scores; here, a cut-off point of ≥4 was used to indicate active disease on the basis of clinical appearance, haematology and immunology results.19 The active group had a median SLEDAI-2K score of 6, whilst the quiescent group had a median SLEDAI-2K score of 2 (p=0.0001; data not shown). Moreover, to evaluate the higher SLEDAI-2K score leads to indeterminate results more frequently, we stratify patients by disease activity. We showed that 50% of indeterminate group has ≥6 SLEDAI-2K score. However, categorized SLEDAI-2K scores were not correlated with IGRA results (p=0.12; Table 3).
 |
Table 3 SLEDAI-2K Scores and IGRA Results |
We further analysed the IFN-γ values and disease activity correlation. However, we found no significant difference in IFN-γ level amongst all groups based on the Kruskal–Wallis test (Table 4). Spearman’s test revealed that disease activity is negatively correlated with IFN-γ (p>0.05).
 |
Table 4 Disease Activity and IFN-γ Results |
Whilst immunosuppressant therapies are administered to control disease activity in SLE patients, such therapies may also result in immune response imbalance.21 This study revealed that a higher number of drug combinations are more likely to yield negative results than a low number of drug combinations, as shown in Table 5. However, Spearman’s test showed that a higher number of drugs are more likely to decrease IFN-γ significantly than a lower number of drugs (p<0.05; Figure 2).
 |
Table 5 Correlation Between Immunosuppressive Drugs and IGRA Results |
 |
Figure 2 Drug combinations and IFN-γ levels (IU/mL). |
Discussion
Female was typically predisposed to developed SLE as shown in this study with normally affects 30-year-old female and has 5-year disease duration, this data is similar with previous studies in our country and anywhere else.12,22 Dysregulation of immune responses in SLE patients is caused by the autoimmunity process and immunosuppressant therapy. Moreover, fluctuating disease activity may exacerbate this condition. Chronic high disease activity can enhance the severity of clinical features and promote irreversible organ damage, thereby increasing morbidity and mortality.21,23,24 Recent cross-sectional study showed that IFN-gamma levels are increased in subjects with active SLE and its levels positively correlates with a more severe phenotype of disease.25 However, imbalances in the immunity of patients due to intrinsic or extrinsic factors may cause T cell regulation and cytokine disturbances, as exhibited by an abnormal T-helper ratio and increases in cytokine level.6,26 Shifts in T cell signalling occur between Th-1 cytokines IL-2 and IFN- γ, Th-2 cytokines IL-4, IL-5 and IL-10 and the Th-17 cytokine IL-17; specifically, some cytokines are over-expressed whilst others are suppressed.7,27,28 These effects would result in increased susceptibility to TB infection amongst SLE patients.
Imbalances in immune response may be observed in a patient’s IGRA results. In the present work, indeterminate results (12.66%) were correlated with an active disease condition (Table 2), in agreement with a prior study.29,30 Previous research demonstrated that high disease activity causes an anomalous T-helper ratio, which leads to inhibited Th-1 signalling and low IFN-γ.27 The results indicate that TB infection may be more difficult to detect in this group compared to other groups.31,32 Many studies have revealed that high SLEDAI scores are related to more severe symptoms, poor haematology and immunology features and a high probability of indeterminate results.30,33 Similarly, our study revealed that, compared with other groups, the active group has the lowest IFN-γ level, which reflects an abnormality of the immune response.
Immunosuppressive agents are commonly used to treat SLE patients, but the effects of these drugs could worsen an abnormal immune response. We found that greater numbers and combinations of immunosuppressive therapies could affect patients’ IGRA results (Table 5).17,34 In addition, a previous study revealed that glucocorticoid therapy alone is likely to cause IGRA with indeterminate results.17,29 Another study reported that immunosuppressive agents negatively affect IGRA results, particularly when used in combination. However, one report demonstrated that immunosuppressive agents, especially with non-glucocorticoid agents, do not influence IGRA results.17,35
This study presents a number of limitations that must be taken into consideration when interpreting the results. We recruited a small number of participants with a wide age range, which could affect the normal distribution of data and accuracy of the statistical analysis. Future studies involving other inflammatory cytokines related to the immune responses of SLE patients in the active or quiescent phase of TB infection are recommended.
Conclusion
In summary, high-activity disease, as reflected by high SLEDAI-2K scores, is correlated with IGRA results. Whilst a number of immunosuppressive drugs are negatively correlated with IFN-γ, neither SLE activity nor SLEDAI-2K score is correlated with IFN-γ level. This finding suggests that detection of TB infection in SLE patients requires additional testing following IGRA.
Acknowledgments
This work was supported by a grant from the Ministry of Research, Technology and Higher Education of the Republic of Indonesia awarded to Nur Atik.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kaul A, Gordon C, Crow MK, et al. Systemic lupus erythematosus. Nat Rev Dis Prim. 2016;2(16039):1–22.
2. Zharkova O, Celhar T, Cravens PD, Satterthwaite AB, Fairhurst A, Davis LS. The SLE review series: working for a better standard of care pathways leading to an immunological disease: systemic lupus erythematosus. Rheumatology. 2017;56:55–66. doi:10.1093/rheumatology/kew427
3. Devlin A, Shmerling R. Systemic Lupus Erythematosus and Infections. Systemic Lupus Erythematosus: Basic, Applied and Clinical Aspects. Elsevier; 2016:403–410.
4. Yang Y, Thumboo J, Tan BH, et al. The risk of tuberculosis in SLE patients from an Asian tertiary hospital. Rheumatol Int. 2017;37(6):1027–1033. doi:10.1007/s00296-017-3696-3
5. Tahernia L, Namazi S, Rezaei N, Ziaee V. Cytokines in systemic lupus erythematosus: their role in pathogenesis of disease and possible therapeutic opportunities. Rheumatol Res. 2017;2(1):1–9. doi:10.22631/rr.2017.69997.1010
6. Jacob N, Stohl W. Cytokine disturbances in systemic lupus erythematosus. Arthritis Res Ther. 2011;13(4):1–11. doi:10.1186/ar3349
7. Talaat RM, Mohamed SF, Bassyouni IH, Raouf AA. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: correlation with disease activity. Cytokine. 2015;72(2):146–153. doi:10.1016/j.cyto.2014.12.027
8. Ohl K, Tenbrock K. Inflammatory cytokines in systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:1–14. doi:10.1155/2011/432595
9. Rönnblom L, Leonard D. Interferon pathway in SLE: one key to unlocking the mystery of the disease. Lupus Sci Med. 2019;6(1):1–11. doi:10.1136/lupus-2018-000270
10. Pollard KM, Cauvi DM, Toomey CB, Morris KV, Kono DH. Interferon-γ and Systemic Autoimmunity. Discov Med. 2013;16(87):123–131.
11. Hagberg N, Rönnblom L. The Interferon System in Lupus Erythematosus [Internet]. Systemic Lupus Erythematosus: Basic, Applied and Clinical Aspects. Elsevier; 2016:153–158.
12. Hamijoyo L, Candrianita S, Rahmadi AR, et al. The clinical characteristics of systemic lupus erythematosus patients in Indonesia: a cohort registry from an Indonesia-based tertiary referral hospital. Lupus. 2019;096120331987849.
13. Bhattacharya PK, Jamil M, Roy A, Talukdar KK. SLE and tuberculosis: a case series and review of literature. J Clin Diagnostic Res. 2017;11(2):OR01–3.
14. Balbi GGM, MacHado-Ribeiro F, Marques CDL, Signorellia F, Levy RA. The interplay between tuberculosis and systemic lupus erythematosus. Curr Opin Rheumatol. 2018;30(4):395–402. doi:10.1097/BOR.0000000000000493
15. Elitza S. T, Hilgart H, Margaret Breen-Lyles EA. Comparison of the QuantiFERON-TB gold plus and QuantiFERON-TB gold in-tube interferon gamma release assays in patients at risk for tuberculosis and in health care workers. J Clin Microbiol. 2018;56(7):1–12.
16. Redelman-Sidi G, Sepkowitz KA. IFN-g release assays in the diagnosis of latent tuberculosis infection among immunocompromised adults. Am J Respir Crit Care Med. 2013;188(4):422–431. doi:10.1164/rccm.201209-1621CI
17. Calabrese C, Overman RA, Dusetzina SB, Hajj-Ali RA. Evaluating indeterminate interferon-γ-release assay results in patients with chronic inflammatory diseases receiving immunosuppressive therapy. Arthritis Care Res. 2015;67(8):1063–1069. doi:10.1002/acr.22454
18. Aringer M, Costenbader K, Daikh D, et al. 2019 European league against rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2019;1–13.
19. Merrill JT. Measuring Disease Activity [Internet]. Systemic Lupus Erythematosus: Basic, Applied and Clinical Aspects. Elsevier; 2016:23–29.
20. Jung J, Suh C. Infection in systemic lupus erythematosus, similarities, and differences with lupus flare. Korean J Intern Med. 2017;32(3):429–438. doi:10.3904/kjim.2016.234
21. Annamayra A, Ekawardhani S, Rahmadi AR, Hamijoyo L, Atik N. The molecular mechanism of corticosteroids for systemic lupus erythematosus patient’s treatment and its adverse effects. Syst Rev Pharm. 2020;11(2):249–254.
22. Atik N, Pratiwi SP, Hamijoyo L. Correlation between C-reactive protein with malondialdehyde in systemic lupus erythematosus patients. Int J Rheumatol. 2020;2020:1–5. doi:10.1155/2020/8078412
23. Fernandez D, Kirou KA. What causes lupus flares? Curr Rheumatol Rep. 2016;18(14):1–10. doi:10.1007/s11926-016-0562-3
24. Ospina FE, Echeverri A, Zambrano D, et al. Distinguishing infections vs flares in patients with systemic lupus erythematosus. Rheumatology (Oxford). 2017;56(1):i46–54. doi:10.1093/rheumatology/kew340
25. Oke V, Gunnarsson I, Dorschner J, et al. High levels of circulating interferons type I, type II and type III associate with distinct clinical features of active systemic lupus erythematosus. Arthritis Res Ther. 2019;21(1):1–11. doi:10.1186/s13075-019-1878-y
26. Rother N, Van der Vlag J. Disturbed T cell signaling and altered Th17 and regulatory T cell subsets in the pathogenesis of systemic lupus erythematosus. Front Immunol. 2015;6(NOV):1–10. doi:10.3389/fimmu.2015.00610
27. Sigdel KR, Duan L, Wang Y, et al. Serum cytokines Th1, Th2, and Th17 expression profiling in active lupus nephritis-IV: from a Southern Chinese Han Population. Mediators Inflamm. 2016;2016:1–10. doi:10.1155/2016/4927530
28. Alunno A, Bartoloni E, Bistoni O, et al. Balance between regulatory T and Th17 cells in systemic lupus erythematosus: the old and the new. Clin Dev Immunol. 2012;2012:1–5. doi:10.1155/2012/823085
29. Cho H, Kim YW, Suh CH, et al. Concordance between the tuberculin skin test and interferon gamma release assay (IGRA) for diagnosing latent tuberculosis infection in patients with systemic lupus erythematosus and patient characteristics associated with an indeterminate IGRA. Lupus. 2016;25(12):1341–1348. doi:10.1177/0961203316639381
30. Jung HJ, Kim TJ, Kim HS, et al. Analysis of predictors influencing indeterminate whole-blood interferon-gamma release assay results in patients with rheumatic diseases. Rheumatol Int. 2014;34(12):1711–1720. doi:10.1007/s00296-014-3033-z
31. Goel N, Wax S, Kao A, Reeve R, Mackey M. Quantiferon testing in a clinical trial of systemic lupus erythematosus. Arthritis Rheumatol. 2017;69(Supplement10).
32. Salomon-escoto K, Hatch S, Pellish R, Salomon-Escoto K, Hatch S, Pellish R. The conundrum of indeterminate QuantiFERON- TB Gold results before anti-tumor necrosis factor initiation. Biol Targets Ther. 2018;12:61–67. doi:10.2147/BTT.S150958
33. Takeda N, Nojima T, Terao C, et al. Interferon-gamma release assay for diagnosing Mycobacterium tuberculosis infections in patients with systemic lupus erythematosus. Lupus. 2011;20:792–800. doi:10.1177/0961203310397966
34. Del M, Arenas M, Hidalgo-tenorio C, Jimenez-gamiz P, Jiménez-alonso J. Diagnosis of latent tuberculosis in patients with systemic lupus erythematosus: T. SPOT. TB versus tuberculin skin test. Biomed Res Int. 2014;2014(ID 291031):1–8.
35. Wong SH, Gao Q, Tsoi KKF, et al. Effect of immunosuppressive therapy on interferon γ release assay for latent tuberculosis screening in patients with autoimmune diseases: a systematic review and meta-analysis. Thorax. 2016;71:64–72. doi:10.1136/thoraxjnl-2015-207811
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
