Back to Journals » Journal of Pain Research » Volume 11
Activation of spinal dorsal horn P2Y13 receptors can promote the expression of IL-1β and IL-6 in rats with diabetic neuropathic pain
Authors Zhou R, Xu T, Liu XH, Chen YS, Kong D, Tian H, Yue MX, Huang DJ, Zeng JW
Received 18 October 2017
Accepted for publication 10 January 2018
Published 27 March 2018 Volume 2018:11 Pages 615—628
DOI https://doi.org/10.2147/JPR.S154437
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Rui Zhou,* Tao Xu,* XiaoHong Liu, YuanShou Chen, DeYing Kong, Hong Tian, Mingxia Yue, Dujuan Huang, Junwei Zeng
Department of Physiology, Zunyi Medical College, Zunyi, People’s Republic of China
*These authors contributed equally to this work
Objective: The dorsal horn P2Y13 receptor is involved in the development of pain behavior induced by peripheral nerve injury. It is unclear whether the expression of proinflammatory cytokines interleukin (IL)-1β and IL-6 at the spinal dorsal horn are influenced after the activation of P2Y13 receptor in rats with diabetic neuropathic pain (DNP).
Methods: A rat model of type 1 DNP was induced by intraperitoneal injection of streptozotocin (STZ). We examined the expression of P2Y13 receptor, Iba-1, IL-1β, IL-6, JAK2, STAT3, pTyr1336, and pTyr1472 NR2B in rat spinal dorsal horn.
Results: Compared with normal rats, STZ-diabetic rats displayed obvious mechanical allodynia and the increased expression of P2Y13 receptor, Iba-1, IL-1β, and IL-6 in the dorsal spinal cord that was continued for 6 weeks in DNP rats. The data obtained indicated that, in DNP rats, administration of MRS2211 significantly attenuated mechanical allodynia. Compared with DNP rats, after MRS2211 treatment, expression of the P2Y13 receptor, Iba-1, IL-1β, and IL-6 were reduced 4 weeks after the STZ injection. However, MRS2211 treatment did not attenuate the expression of the P2Y13 receptor, Iba-1, IL-1β, and IL-6 at 6 weeks after the STZ injection. MRS2211 suppressed JAK2 and STAT3 expression in the early stage, but not in the later stage. Moreover, pTyr1336 NR2B was significantly decreased, whereas pTyr1472 NR2B was unaffected in the dorsal spinal cord of MRS2211-treated DNP rats.
Conclusion: Intrathecal MRS2211 produces an anti-nociceptive effect in early-stage DNP. A possible mechanism involved in MRS2211-induced analgesia is that blocking the P2Y13 receptor downregulates levels of IL-1β and IL-6, which subsequently inhibit the activation of the JAK2/STAT3 signaling pathway. Furthermore, blocking the activation of the P2Y13 receptor can decrease NR2B-containing NMDAR phosphorylation in dorsal spinal cord neurons, thereby attenuating central sensitization in STZ-induced DNP rats.
Keywords: diabetic neuropathic pain, dorsal horn, P2Y13 receptor
Introduction
Neuropathic pain is a complex, chronic pain state that is usually caused by nerve injury or dysfunction of the nervous system in a large number of patients. It is well known that diabetic neuropathic pain (DNP) is caused by peripheral neuropathy and neuroimmune activation in the central nervous system.1,2 In an animal model of DNP, rats exhibited increase in levels of proinflammatory cytokines in the dorsal spinal cord – a critical region involved in the development of DNP.3 Subsequent studies showed that changes in proinflammatory cytokines in the dorsal spinal cord are paralleled by pain behavior in DNP rats.3,4 Elevated levels of spinal cord proinflammatory cytokines, including interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) were observed in rats with diabetic neuropathy.3–7 Emerging evidence recently revealed that chronic administration of minocycline – an inhibitor of microglia activation – suppressed levels of proinflammatory cytokines and attenuated mechanical allodynia in diabetic rats.2,8 Although microglial activation has been implicated in the development of DNP, there is poor understanding of the mechanism underlying microglial activation and accumulation of proinflammatory cytokines in the dorsal spinal cord of diabetic animals.
It is well known that microglia – small cells with elaborate thin processes – are resident macrophages of the central nervous system. Following a peripheral noxious stimulus, resting microglia are activated through a series of cellular and molecular changes.9–11 Microglial activation at the spinal cord contributes to the mechanical allodynia and thermal hyperalgesia induced by diabetes.8,12,13 Spinal microglial cells are endowed with some neurotransmitter receptors, which may participate in the initiation and maintenance of pain hypersensitivity. Recent evidences suggest the upregulation of spinal P2Y6, P2Y12, P2Y13, and P2Y14 receptors following nerve injury and that these receptors may be involved in the development and maintenance of neuropathic pain.14,15 Our earlier studies have shown that the activation of P2Y12 and P2Y13 receptors induce release of IL-1β and IL-6 from cultured dorsal spinal cord microglial cells in vitro conditions.16 In addition, cell culture experiments reveal that the P2Y13 receptor subtype participates in adenosine diphosphate (ADP)-evoked Ca2+ mobilization in dorsal spinal cord microglia cells.17 However, it is unclear whether the spinal P2Y13 receptor plays a crucial role in inflammatory responses in the dorsal spinal cord of rats with DNP. This study was conducted with an aim to define the function role of the dorsal spinal cord P2Y13 receptor in streptozotocin (STZ)-induced DNP and to evaluate increased levels of spinal cord proinflammatory cytokines in laboratory rats.
Materials and methods
Animals
All experiments were conducted using Sprague–Dawley (SD) rats (weight 180–200 g) in accordance with the National Institutes of Health guidelines in a manner that minimized animal suffering and animal numbers. All experiments were carried out in accordance with People’s Republic of China animal welfare legislations and were approved by the Zunyi Medical College Committee for Ethics in the Care and Use of Laboratory Animals.
Animal models of diabetes-induced neuropathic pain
Diabetes was induced by a single intraperitoneal (ip) injection of STZ at 50 mg/kg in SD rats.18 Then, 60 min after STZ administration, animals were allowed free access to food and water. To maintain cleanliness and prevent infection due to excessive urination, animal bedding was changed frequently.19 Fourteen days later, tail-vein blood samples were obtained to detect fasting blood glucose (FBG) levels with a portable blood glucose meter (ACCU-CHEK Integra, Roche Diagnostics GmbH, Mannheim, Germany) in the morning. Rats that displayed a blood glucose level of at least 16.7 mM were considered to be diabetic and included into the study.20 Animals were considered to have neuropathic pain when they exhibited mechanical allodynia. Control rats received the same volume/kg of ip citrate buffer.
Implantation of intrathecal catheter
Lumbosacral intrathecal catheters were constructed and implanted as described in a previous study.21 Under anesthesia with pentobarbital sodium (40 mg/kg, ip), rats were fixed and a 2-cm longitudinal incision was made between vertebrae L5 and L6. Polyethylene catheters (PE-10) were pushed through the intervertebral space until a sudden movement of tail or the hind limb was observed and thereafter passed gently 2 cm cranially to reach the level of the lumbar enlargement. The tip of the catheter was fixed to the neck area of the rats. Correct intrathecal placement was confirmed by injection of lidocaine (2%, 10 µL) through the catheter. The catheter was judged to be intrathecal if paralysis and dragging of bilateral hind limbs occurred within 30 s of lidocaine injection. Rats with signs of motor weakness were excluded from the experiment. All rats were housed individually after implantation of the intrathecal catheter and allowed to recover for 5 days before the STZ injection.
Drug application
STZ was purchased from Sigma (CAS No: 18883-66-4; St. Louis, MO, USA) and dissolved in citrate buffer (pH 4.5) for each period of administration. MRS2211 (P2Y13 receptor antagonist) was purchased from Abcam (ab120445, La Jolla, CA, USA) and dissolved in 0.01M PBS.
Behavioral testing
Tests of the mechanical withdrawal threshold (MWT) were carried out to assess the response of the paw to mechanical stimulus. Mechanical hypersensitivity was determined with an electronic von Frey plantar aesthesiometer (IITC, Wood Dale, IL, USA). Baseline values were obtained prior to the STZ injection. Before the initial Von Frey measurements, rats were given 15 min to habituate to the test environment. A rigid tip was applied against the mid-plantar surface of the left hind paw. The paw withdrawal threshold was automatically recorded by the device, and the cut-off force was set at 60 g. The rigid tip was presented perpendicular to the plantar surface, and brisk withdrawal or paw flinching were considered as positive responses. Simultaneously, the digital number presented on the monitor was recorded as the MWT. Three successive stimuli were applied. The MWT for individual animals was represented by the mean values (calculated from the three successive stimuli).
Western blot
Rats were terminally anesthetized with pentobarbital sodium (50 mg/kg). The dorsal side of the lumbar L3–L5 spinal cord was rapidly dissected and rinsed in cold PBS, and then homogenized in 1 mL ice-cold chilled radioimmunoprecipitation (RIPA) lysis buffer containing 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 µg/mL aprotinin, 100 µg/mL phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 1 μM batimastat (BB-94), and 1% protease inhibitor cocktail (Roche, Basel, Switzerland). The protein concentration was determined using a Bio-Rad Protein Assay kit (category number [cat no] 5000002; Bio-Rad Laboratories, Inc., Hercules, CA, USA). Pre-stained protein marker was purchased from Thermo Scientific (cat. no: 26616; Rockford, IL, USA). Total proteins were diluted in 4× loading buffer and incubated at 100°C for 5 min. Samples (80 µg) were then loaded onto a loading gel and separated on a Bis–Tris gel (12% separation gel and 4% stacking gel for P2Y13, Iba-1, IL-1β, IL-6; 8% separation gel and 4% stacking gel for janus kinase-2 (JAK2) and activators of transcription 3 (STAT3); and 6% separation gel and 4% stacking gel for phosphT1472 NR2B and phosphT1336NR2B). Separated proteins were transferred onto a nitrocellulose membrane (conditions, P2Y13, Iba-1, IL-1β, and IL-6: 20 V for 1 h; JAK2 and STAT3: 220 mA for 2 h; and phosphT1472 NR2B and phosphT1336NR2B: 300 mA for 4 h). Nonspecific sites were blocked for 1 h at room temperature in fat-free milk solution (10% in 0.1% Tween-Tris-buffered saline [TTBS]). Membranes were then incubated overnight at 4°C with the following polyclonal antibodies: rabbit anti-P2Y13 Receptor (cat no ab108444; 1:200; Abcam), goat anti-Iba-1 (cat no ab5076; 1:500; Abcam), rabbit anti-IL-1β (cat no ab9787; 1:250; Abcam), goat anti-IL-6 (cat no ab9770; 1:500; Abcam), anti-JAK2 (rabbit monoclonal, 1:200, cat no 3230, Cell Signaling Technology, Danvers, MA, USA), anti-STAT3 (rabbit monoclonal, 1:200; cat no 9139, Cell Signaling Technology), rabbit anti-phosphT1472 NR2B (1:500; Abcam; cat no ab3856), and rabbit anti-phosphT1336NR2B (cat no ab193286; 1:500; Abcam). In addition, mouse monoclonal β-actin antibody (cat no NB600-501; 1:1,000; Novus Biologicals, Littleton, CO, USA) was used. The secondary antibodies (goat anti-rabbit IgG: cat no A0208; goat anti-mouse IgG: cat no A0216, Beyotime Institute of Biotechnology, Haimen, Jiangsu, People’s Republic of China) were diluted to 1:1,000 and incubated for 1.5 h at room temperature. Subsequently, the membranes were developed using the enhanced chemiluminescence (ECL) reagent Beyo ECL plus (Beyotime Institute of Biotechnology). Images of the blots were captured using a ChemiDoc XRS system (Bio-Rad Laboratories, Inc.). The image was scanned, and band intensity was semi-quantified using Quantity One v4.52 (Bio-Rad Laboratories, Inc.).
ELISA
Levels of IL-1β and IL-6 in the spinal cord were measured by ELISA. The dorsal side of the lumbar L3–L5 spinal cord was dissected out, ground with a grinder, and homogenized with an ultrasonic tissue homogenizer. Samples were centrifuged and the supernatants were collected for cytokine analysis. IL-1β and IL-6 production were evaluated using ELISA kits (Hushang Biological Technology Co., Ltd., Shanghai, People’s Republic of China). OD values at 490 nm were recorded using a microplate reader (NK3; Ladsystems, Helsinki, Finland). The average levels of IL-1β and IL-6 were calculated on the basis of the standard curves as directed by manufacturer’s instructions for the kit.
Statistical analysis
All data are presented as mean ± SD. Statistical analysis was conducted with one-way analysis of variance followed by application of Dunnett’s multiple comparison test or Student’s t-test by using SPSS18.0 (SPSS Inc., Chicago, IL, USA). Differences at the P<0.05 level were considered statistically significant.
Results
Changes of fasting blood glucose and body weight in diabetic rats
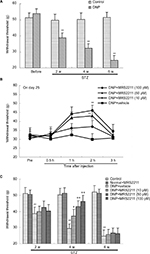
The administration of STZ experimentally induced type-1 diabetes mellitus in the rats. After injection of STZ, all rats in different groups showed polydipsia, polyphagia, and polyuria. Control rats received the same volume/kg of ip citrate buffer. Body weights of the rats in the STZ-diabetic group were substantially lower than those in the normal control group (Figure 1, 2 weeks: P<0.05; 4 and 6 weeks: P<0.01 vs the control group in the same week), whereas the FBG was significantly increased in the STZ-diabetic group (>16.7 mM; P<0.01 vs the control group in the same week). MRS2211 (a specific P2Y13 antagonist) was administered twice daily via the intrathecal route (100 pM, 10 μL) from Day 15 after STZ injection for 28 consecutive days. We noticed there was no statistically significant difference between STZ-induced diabetic rats and MRS2211 (100 pM)-treated diabetic rats with regard to body weight and FBG at any point during the 4 weeks of treatment with MRS2211 (Figure 1). It appears that the administration of MRS2211 via the intrathecal route has no effect on body weight and blood glucose levels in STZ-induced diabetic rats.
Blockage of P2Y13 receptors attenuates mechanical allodynia in diabetic rats
In animal models of STZ-induced type 1 diabetes, diabetic peripheral neuropathy manifests as hyperalgesia in the early stages (<4 weeks), followed by hypoalgesia from 8 weeks after diabetes development.22,23 Some previous studies reported that diabetes duration of 2 weeks was sufficient to induce mechanical hypersensitivity and thermal allodynia in STZ-induced diabetic rats.24–26 Furthermore, we observed that, 2 weeks after STZ injection, rats showed a significant decrease in mechanical allodynia as compared to the control group. The MWT in the vehicle-treated DNP rats was significantly decreased at every time point (Figure 2A, P<0.01). Subsequently, MRS2211 was administered consecutively from Day 15 after STZ injection for 28 days.
Previous research from Kou et al showed that STZ-induced diabetic rats exhibit hyperglycemia, decreased body weight gain, mechanical allodynia as well as impaired locomotor activity at Day 28, supporting the use of STZ-induced diabetic rats as an animal model for studying the mechanisms underlying early-stage (<4 weeks) type 1 diabetic polyneuropathy.27 In addition, 4 weeks after STZ injection, diabetic rats exhibited behaviors indicative of neuropathic pain, and this hypersensitivity persisted for up to 6 weeks.28 However, STZ-induced diabetic animals exhibit hypoalgesia and approximately 38% loss of intraepidermal nerve fibers at 6 weeks after STZ injection.29 Moreover, Grotle et al indicated that at 6 weeks after STZ treatment, the pressor and cardioaccelerator responses to contraction were significantly attenuated in diabetic rats, suggesting that afferent activity decreases to some extent.30 Our experiments show that these diabetic rats exhibit mechanical allodynia at 6 weeks after induction (Figure 2A, 4 weeks: P<0.05; 2, 4, and 6 weeks: P<0.01). This result is in agreement with the previous observation that Paulson et al reported.28 It appears then that changes at 6 weeks after STZ induction could be defined as the later stages of DNP. MRS2211 at 10, 50, and 100 pM exert anti-hyperalgesic effects in DNP rats but not in control rats. Compared to the vehicle-treated DNP rats, MRS2211 produced a dose-dependent reversal of the mechanical nociceptive threshold, with maximal reversal observed at 2 h after the MRS2211 injection in DNP rats (Figure 2B, 10 pM: P<0.05; 50 and 100 pM: P<0.01). MRS2211 10 pM induced a slight but significant analgesic effect in DNP rats. Furthermore, we noted that intrathecal injection of MRS2211 (10, 50, or 100 pM) attenuated diabetes-evoked mechanical pain hypersensitivity mainly at 4 weeks, (Figure 2C, 10 pM: P<0.05; 50 and 100 pM: P<0.01) but not at 6 weeks, after the STZ injection. Therefore, MRS2211 appears to exert an anti-hyperalgesic effect mainly in the early stage, but not in the later stage, of the development of DNP.
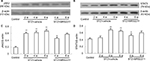
Blockage of P2Y13 receptors attenuates the expression of P2Y13 receptor, Iba-1, IL-1β, and IL-6 in the dorsal spinal cord of DNP rats
Increased expression of the P2Y13 receptor in the dorsal spinal cord has been observed in rats with spared nerve injury or chronic constriction injury.31 It is clear that the accumulation of proinflammatory cytokines in the dorsal spinal cord plays an important role in the development of DNP.32 Therefore, we detected the protein expression of the P2Y13 receptor, Iba-1, IL-1β, and IL-6 using Western blotting. We found that, after STZ injection, the protein expression of the P2Y13 receptor, Iba-1, IL-1β, and IL-6 in the ipsilateral spinal cord was significantly increased at 2, 4, and 6 weeks after the STZ injection. Compared with the control group, expression of the P2Y13 receptor sharply increased and peaked at 2 weeks after the STZ injection (Figure 3A and E: P<0.01). In vehicle-treated DNP rats, the increased expression of Iba-1 lasted for 6 weeks (Figure 3B and F: P<0.01). In addition, IL-1β and IL-6 expression sharply increased to peak at 4 weeks as compared to vehicle-treated DNP rats, and the increased expression was sustained for 6 weeks (Figure 3C and G: P<0.01; Figure 3D and H: P<0.01). The administration of MRS2211 (100 pM) did not change the expression of spinal P2Y13 receptor, Iba-1, IL-1β, and IL-6 in control rats. Four weeks after the STZ injection, MRS2211 (100 pM) significantly suppressed the increased expression of P2Y13 (P<0.01), Iba-1 (P<0.01), IL-1β (P<0.05), and IL-6 (P<0.01) in the dorsal spinal cord of diabetic rats. However, at 6 weeks after the STZ injection, MRS2211 (100 pM) did not affect the increased expression of P2Y13, Iba-1, IL-1β, and IL-6 in the dorsal spinal cord of diabetic rats. These observations suggest that MRS2211 exerts an inhibitory effect on the increased expression of P2Y13, Iba-1, IL-1β, and IL-6 in the dorsal spinal cord, mainly in early-stage, but not in later-stage, DNP.
Blockage of P2Y13 receptors attenuates the concentration of IL-1β and IL-6 in the dorsal spinal cord tissue of DNP rats
In the present study, Western blot analysis demonstrated that prolonged administration of MRS2211 via the intrathecal route significantly attenuated the increased expression of IL-1β and IL-6 in DNP pathogenesis. To further ascertain the role of the P2Y13 receptor in the increased expression of IL-1β and IL-6, we assessed concentrations of IL-1β and IL-6 in the dorsal spinal cord tissue of DNP rats by using ELISA. As shown in Figure 4, compared with the control group, concentrations of IL-1β and IL-6 in the spinal cord were significantly increased at 2, 4, and 6 weeks after STZ injection (IL-1β: P<0.01; IL-6: P<0.01). The administration of MRS2211 (100 pM) did not alter concentrations of IL-1β and IL-6 in the dorsal spinal cord of control rats. Intrathecal administration of MRS2211 (100 pM) suppressed the increase in the concentration of IL-1β and IL-6 in the spinal dorsal horn, mainly at 4 weeks (IL-1β: P<0.05; IL-6: P<0.05) although not at 6 weeks after STZ injection in DNP rats. These observations further confirm that blockage of P2Y13 receptors could attenuate concentrations of IL-1β and IL-6 in the dorsal spinal cord of DNP rats. Nevertheless, MRS2211 showed only a slight inhibitory effect on the increased expression of IL-1β.
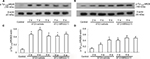
Blockage of P2Y13 receptors attenuates the expression of JAK2/STAT3 in the dorsal spinal cord of rats with DNP
It is clear that, after peripheral nerve injury, changes in microglial reactivity in the dorsal spinal cord are associated with early activation of JAK signal transducers and the STAT3 signaling pathway whose blockade attenuates local inflammation and development of neuropathic pain.33,34 IL-6 is one of the most prevalent cytokines that mediates its effects via the phosphorylation of STAT3.35 In addition, Dominguez et al reported that intrathecal administration of IL-6 to naive rats rapidly activated microglial JAK/STAT3 and induced downstream changes that closely resemble controlled cortical impact (CCI)-evoked alterations.36 Thus, we have reason to speculate the possible role of the P2Y13 receptor in IL-6 production as well as the elevated activation of JAK2/STAT3 signaling in the development of DNP. Thereafter, we observed the effect of MRS2211 (100 pM) on the expression of JAK2 and STAT3 in the dorsal spinal cord of DNP rats. As shown in Figure 5A and C, compared with the control group, the expression of JAK2 in the dorsal spinal cord was significantly increased at 2, 4, and 6 weeks after STZ injection (P<0.01). The expression of STAT3 in the dorsal spinal cord was significantly increased at 4 and 6 weeks after STZ injection (Figure 5B and D, 4 and 6 weeks: P<0.01). At 4 weeks after STZ injection, intrathecal administration of MRS2211 (100 pM) not only significantly inhibited the increased expression of JAK2 but also suppressed the increased expression of STAT3 (Figure 5, JAK2: P<0.05; STAT3: P<0.05) in the spinal dorsal horn of diabetic rats. However, at 6 weeks after STZ injection, the expression of JAK2 and STAT3 were not impaired by MRS2211 administration. Thus, it seems that the inhibitory effect of MRS2211 on the expression of JAK2/STAT3 is mainly present in the early stage, but not in later stages, of DNP.
Blockage of P2Y13 receptors attenuates the expression of p-NR2B in the dorsal spinal cord of rats with DNP
A growing body of evidence indicates that NMDA receptors are composed of NR1 and NR2 A–D subunits that mediate excitatory synaptic transmission and have an important role in neuronal development, plasticity, and disease.37–39 NR2B phosphorylation at tyrosine1472 in the dorsal spinal cord is associated with remifentanil-induced postoperative hyperalgesia.40 In addition, increased expression of pTyr1336 NR2B is involved in spinal nerve ligation (SNL)-induced neuropathic pain.41 Dang et al reported that expression of p-NR2B in the dorsal spinal cord was significantly increased in a rat model of type II DNP.42 Furthermore, we observed the effect of MRS2211 on the phosphorylation of tyrosine residues (pTyr1336 and pTyr1472) in the NR2B at the dorsal spinal cord of DNP rats. Surprisingly, we found that, at 4 weeks after the STZ injection, intrathecal administration of MRS2211 significantly inhibited NR2B phosphorylation at tyrosine1336, but not at tyrosine1472 (Figure 6, pTyr1336: P<0.05). At 6 weeks after the STZ injection, pTyr1336 and pTyr1472 expression remained unimpaired by MRS2211 treatment. These observations suggest that the inhibitory effect of MRS2211 on the expression of pTyr1336 is more obvious in the early stages, but not in the later stages, of DNP pathogenesis.
Discussion
A growing body of literature strongly supports the involvement of extracellular nucleotides and their receptors in modulating the origination and maintenance of neuropathologic pain.43 The P2Y13 receptor – one of the most recently cloned metabotropic P2Y nucleotide receptors – is involved in spinal nociceptive transmission and nocifensive behavior after nerve injury.44,45 In mouse dorsal root ganglia (DRG), less-intense P2Y13 receptor immunoreactivity in larger-diameter neurons suggests that these receptors may contribute to functional properties of large A-fiber nociceptors. An ADPBS (P2Y receptor agonist) of the P2Y13 receptor can inhibit nociceptive signaling in isolated DRG neurons and reduce behavioral hyperalgesia in vivo.44 However, the P2Y13 receptor expressed in rat spinal dorsal horn microglial cells is upregulated and involved in the phosphorylation of ROCK/p38 MAPK after spared nerve injury.45 In our experiments, the selective P2Y13 receptor antagonist MRS2211 considerably alleviated mechanical allodynia, mainly in early-stage but not later-stage DNP – which suggests that P2Y13 receptor antagonists may be candidates for early pain management in DNP.
Kobayashi et al reported that a robust increase in P2Y13 receptor mRNA was observed in rat dorsal spinal cord microglial cells after spared nerve injury.14 MRS2211 has been reported as a selective antagonist of the P2Y13 receptor.46–48 In the rat peripheral nerve injury model, intrathecal administration of MRS2211 attenuated nerve injury-induced spinal p38 MAPK phosphorylation and neuropathic pain behavior.45 It is well known that, in different models of neuropathic pain, the phosphor-P38MAPK in rat dorsal spinal cord was found exclusively in microglia, but not in neurons or astrocytes.49–51 Thus, it is evident that the dorsal spinal cord microglia is a primary site that contributes to MRS2211-mediated analgesia. In our experiments, intrathecal administration of MRS2211 has no effect on blood glucose levels in STZ-induced diabetic rats, which suggests that blood glucose levels are not associated with P2Y13 receptor-mediated microglial activation in the dorsal spinal cord. This result is in agreement with a previous observation that blood glucose levels in naive and STZ-treated rats were not affected after intrathecal injection of minocycline (a selective inhibitor of microglial activation).8
The P2Y13 receptor couples to Gi/o and is activated by ADP. High ADP concentrations cause an increase in cyclic adenosine monophosphate (cAMP) production, suggesting that the P2Y13 receptor can, in some instances, couple to Gs (stimulatory G protein).52 In sensory neurons, ADP acts at the Gi-coupled P2Y13 receptors for modulation of nociceptor sensitivity.44 In the rat peripheral nerve injury model, extracellular ADP stimulates microglial activation leading to rearrangement of the actin-containing cytoskeletal structure through P2Y13 receptors; moreover, Rho-associated coiled-coil forming protein kinase (ROCK) acts as an intracellular mediator of these cytoskeletal modifications in spinal microglia.45 Soulet et al characterized a Gi-dependent pathway leading to cell proliferation through phosphoinositide-3 kinase and a Gi-independent pathway responsible for cytoskeletal changes through Rho and Rho-kinase.53 These findings alert us to the possibility that the Gi-independent pathway may be involved in the P2Y13 receptor-related signaling pathway in dorsal horn microglial cells. In cultured dorsal microglial cells, P2Y13 receptor-mediated ROCK/P38MAPK/NF-kB signaling leads to the increased expression and release of IL-1β and IL-6.16 For this reason, although our experiment does not directly address the role of the Gi-independent ROCK/P38MAPK/NF-kB pathway in the production and release of inflammatory cytokines, the present finding alerts us to the possibility that the Gi-independent ROCK/P38MAPK/NF-kB pathway may be involved in P2Y13 receptor-evoked production and release of inflammatory cytokines in the dorsal horn of DNP rats.
Some reports reveal that IL-1β and IL-6 are upregulated in the lumbar spinal cord following nerve injury.54,55 The exogenous intrathecal administration of IL-1β or IL-6 induced obvious pain behaviors in rats,56,57 whereas the blockade of spinal IL-1β or IL-6 signaling relieved nerve injury-induced neuropathic pain,55,58–60 supporting important roles of spinal IL-1β and IL-6 in the development of neuropathic pain. Further research showed that IL-1β was localized to microglia and minocycline inhibited carrageenan-induced increases in spinal IL-1β expression.61 Moreover, a study by Rojewska et al suggests that minocycline decreases the expression of IL-1β and IL-6 within the spinal cord following sciatic nerve injury.62 Similarly, in our study, significantly increased IL-1β and IL-6 protein expression in the dorsal horn was observed in STZ-treated rats. Treatment with MRS2211 significantly attenuated mechanical allodynia and inhibited the increased expression of IL-1β and IL-6 in diabetic rats; this opens up the possibility that the increased expression of IL-1β and IL-6 is mediated, at least in part, by the P2Y13 receptor. In addition, ELISA results show that MRS2211 treatment significantly downregulated the concentration of these two proinflammatory cytokines in the dorsal spinal cord tissue, which largely confirms that MRS2211 can induce analgesia – probably and partly due to its anti-inflammatory effect.
In the present study, intrathecal administration of MRS2211 suppressed an increase in the expression of IL-6 in the spinal dorsal horn. On the other hand, MRS2211 showed only a slight inhibitory effect on the increased expression of IL-1β. This is probably because the cellular localization of IL-1β and IL-6 in the dorsal spinal cord is not similar. For example, fluorocitrate or minocycline could decrease the IL-1β expression in a rat model of neuropathic pain induced by resiniferatoxin – implying that astrocytes and microglia could be a source of IL-1β.63 In CCI rats, greater IL-1β and GFAP co-expression is observed in the dorsal spinal cord, which indicates that chronic neuropathic pain activated astrocytes to release more IL-1β.64,65 Thus, IL-6 immunoreactivity localized to dorsal horn neurons, astrocytes, and microglial cells.66 Minocycline attenuated pain behavior, and a decrease in IL-6 concentration was observed in dorsal spinal cord microglial cells.67 Furthermore, in rats with complete Freund’s adjuvant (CFA)-induced inflammatory pain, inhibition of P38 activation by SB203580 suppressed rat dorsal spinal cord IL-6, but not IL-1β, production to the normal level.68 Thus, it seems microglial cells are a primary source for the increased production of IL-6. Moreover, we noted that MRS2211 administration significantly reduced Iba-1 expression in the dorsal spinal cord of DNP rats. These experimental results indicate MRS2211 could efficiently decrease microglial activity, which results in downregulation of IL-1β and IL-6 production from microglial cells. However, the production of IL-1β from dorsal spinal cord astrocytes may not be impaired by MRS2211.
It is well known that IL-6–stimulation-induced activation of JAK2/STAT3 has also been observed in many different cell types.69–72 IL-6 is critically involved in the development and maintenance of central sensitization in different models of chronic pain. Minocycline attenuated pain behavior, and a decrease in IL-6 concentration was observed in dorsal spinal cord microglial cells.67 Furthermore, the JAK2/STAT3 signaling pathway has been shown to play an important regulatory role in microglial reactivity to various stimuli and mediates proinflammatory responses in microglia in response to various insults.73–75 In the present study, in early-stage DNP in STZ-induced DNP rats, the increased expression of IL-6 and JAK2/STAT3 in the dorsal spinal cord could be significantly suppressed by MRS2211. Dominguez et al suggested that intrathecal injection of anti-rat IL-6 antibodies obviously prevented SNL-induced accumulation of phospho-STAT3 in dorsal spinal cord microglia.34 Moreover, STAT3 pathway blockade with AG490 (JAK2 inhibitor) attenuated both mechanical allodynia and thermal hyperalgesia in SNL rats.34 Therefore, our results raise a possibility that MRS2211 treatment can reduce P2Y13 receptor activation with a subsequent inhibition of IL-6 expression, resulting in decreased expression levels of JAK2 and STAT3 in the dorsal spinal cord tissue.
More evidence indicates that IL-6 immunoreactivity was detected in microglia and astrocytes in the spinal dorsal horn.76,77 Furthermore, morphological results from Qi et al showed that almost all of the IL-6-immunoreactivity was positive for Iba1 in the dorsal horn.68 On the other hand, the IL-6 receptor partly co-localized with NeuN, Iba-1, and GFAP–positive-cells in the dorsal horn.68 Lin et al suggest that blockade of IL-6 function markedly suppressed the activation of astrocytes and microglia in the dorsal horn and reduced the upregulation of glutamate receptor subunits NR2B in the dorsal horn in rats with monosodium iodoacetate-induced osteoarthritis.78 These observations alert us to the possibility that microglial P2Y13 receptor signaling may lead to IL-6 production, which in turn may prompt microglial activation through the JAK2/STAT3 signaling pathway.
It is clear that the activation of glial cells, the production of inflammatory cytokines, and the expression p-NR2B in the spinal dorsal horn increase significantly during the pathogenesis of peripheral nerve injury-induced neuropathic pain.79 Spinal IL-6 can prompt a state of spinal hyperexcitability.80 Inhibition of IL-6 receptor activation suppressed Fos expression and inhibited the upregulation of glutamate receptor subunits NR2B in dorsal horn neurons in rats with monosodium iodoacetate-induced osteoarthritis in dorsal horn neurons.78 Similarly, we found that, in early-stage DNP in STZ-induced DNP rats, the increased expression of IL-6 and NR2B phosphorylation at tyrosine1472 in the dorsal spinal cord can be significantly suppressed by MRS2211. We could preliminarily speculate that MRS2211 treatment can reduce P2Y13 receptor activation with a subsequent inhibition of IL-6 expression, resulting in decreased expression levels of NR2B phosphorylation at tyrosine1336 in the dorsal spinal cord tissue of DNP rats.
Molet et al suggest that early JAK/STAT3 activation in spinal cord microglia is associated with functional alteration of dorsal horn neurons and participates in spinal cord tissue plasticity and remodeling that occurs after peripheral nerve injury.33 In rat spinal cord slices, the JAK2/STAT3 inhibitor AG490 attenuated leptin-induced enhancement of NMDA currents of dorsal horn neurons.81 Moreover, the anti-nociceptive effect of triptolide (one of the major active components of tripterygium extracts) was associated with the inhibition of SNL-induced JAK2/STAT3 signaling pathway activation and inhibited the upregulation of the NR2B-containing spinal N-methyl D-aspartate receptor (NMDAR).82 It seems that JAK2/STAT3 activation may be, at least partly, associated with increased expression levels of NR2B phosphorylation at tyrosine1336. Of course, we noted that MRS2211 only induced a moderate inhibitory effect on the expression of JAK2, STAT3, and NR2B phosphorylation at tyrosine1336, which means that P2Y13 receptor activation may partially participate in the DNP-induced increase in the expression of JAK2, STAT3, and NR2B phosphorylation at tyrosine1336. Interestingly, we found that NR2B phosphorylation at tyrosine1336 was significantly decreased in the spinal cord, whereas NR2B phosphorylation at tyrosine1472 was unaffected in the dorsal spinal cord of MRS2211-treated DNP rats. It appears that NR2B phosphorylation of Tyr1336 and of Tyr1472 is regulated by respective signaling pathways.
In summary, these results presented here strongly suggest that the intrathecal selective P2Y13 receptor antagonist MRS2211 produces an anti-nociceptive effect in early-stage DNP. A possible mechanism mediating the analgesic effects of MRS2211 is that blocking the P2Y13 receptor downregulates levels of IL-1β and IL-6, which subsequently inhibits the activation of the JAK2/STAT3 signaling pathway. In addition, blocking P2Y13 receptor activation can decrease NR2B-containing NMDAR phosphorylation in dorsal spinal cord neurons, thereby attenuating central sensitization in STZ-induced DNP rats. Our results suggest that blocking the P2Y13 receptor may be a potential treatment strategy for the treatment of NP in early-stage DNP.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (grant numbers 31460266 and 31640040), the Program for New Century Excellent Talents in University (NCET-13-1070), and the Scientific Research Foundation for Excellent Talents in Guizhou Province (grant number 2012–93). The authors are very grateful to the other staff of the Department of Physiology, especially He Li and Yang Junna, for their support in creating statistical charts.
Disclosure
The authors report no conflicts of interest in this work.
References
Ortmann KL, Chattopadhyay M. Decrease in neuroimmune activation by HSV-mediated gene transfer of TNFα soluble receptor alleviates pain in rats with diabetic neuropathy. Brain Behav Immun. 2014;41:144–151. | ||
Pabreja K, Dua K, Sharma S, Padi SS, Kulkarni SK. Minocycline attenuates the development of diabetic neuropathic pain: possible anti-inflammatory and anti-oxidant mechanisms. Eur J Pharmacol. 2011;661(1–3):15–21. | ||
Lu Y, Lin B, Zhong J. The therapeutic effect of dexmedetomidine on rat diabetic neuropathy pain and the mechanism. Biol Pharm Bull. 2017;40(9):1432–1438. | ||
Ni GL, Cui R, Shao AM, Wu ZM. Salidroside ameliorates diabetic neuropathic pain in rats by inhibiting neuroinflammation. J Mol Neurosci. 2017;63(1):9–16. | ||
Deng XT, Wu MZ, Xu N, Ma PC, Song XJ. Activation of ephrinB-EphB receptor signalling in rat spinal cord contributes to maintenance of diabetic neuropathic pain. Eur J Pain. 2017;21(2):278–288. | ||
Mert T, Gisi G, Celik A, Baran F, Uremis MM, Gunay I. Frequency-dependent effects of sequenced pulsed magnetic field on experimental diabetic neuropathy. Int J Radiat Biol. 2015;91(10):833–842. | ||
Bishnoi M, Bosgraaf CA, Abooj M, Zhong L, Premkumar LS. Streptozotocin-induced early thermal hyperalgesia is independent of glycemic state of rats: role of transient receptor potential vanilloid 1(TRPV1) and inflammatory mediators. Mol Pain. 2011;7:52. | ||
Talbot S, Chahmi E, Dias JP, Couture R. Key role for spinal dorsal horn microglial kinin B1 receptor in early diabetic pain neuropathy. J Neuroinflammation. 2010;7(1):36. | ||
Echeverry S, Shi XQ, Yang M, et al. Spinal microglia are required for long-term maintenance of neuropathic pain. Pain. 2017;158(9):1792–1801. | ||
Fiore NT, Austin PJ. Are the emergence of affective disturbances in neuropathic pain states contingent on supraspinal neuroinflammation? Brain Behav Immun. 2016;56:397–411. | ||
Miyamoto K, Kume K, Ohsawa M. Role of microglia in mechanical allodynia in the anterior cingulate cortex. J Pharmacol Sci. 2017;134(3):158–165. | ||
Zychowska M, Rojewska E, Piotrowska A, Kreiner G, Mika J. Microglial inhibition influences XCL1/XCR1 expression and causes analgesic effects in a mouse model of diabetic neuropathy. Anesthesiology. 2016;125(3):573–589. | ||
Cheng KI, Wang HC, Chuang YT, et al. Persistent mechanical allodynia positively correlates with an increase in activated microglia and increased P-p38 mitogen-activated protein kinase activation in streptozotocin-induced diabetic rats. Eur J Pain. 2014;18(2):162–173. | ||
Kobayashi K, Yamanaka H, Yanamoto F, Okubo M, Noguchi K. Multiple P2Y subtypes in spinal microglia are involved in neuropathic pain after peripheral nerve injury. Glia. 2012;60(10):1529–1539. | ||
Barragán-Iglesias P, Pineda-Farias JB, Cervantes-Durán C, et al. Role of spinal P2Y6 and P2Y11 receptors in neuropathic pain in rats: possible involvement of glial cells. Mol Pain. 2014;10:29. | ||
Liu PW, Yue MX, Zhou R, et al. P2Y12 and P2Y13 receptors involved in ADPβs induced the release of IL-1β, IL-6 and TNF-α from cultured dorsal horn microglia. J Pain Res. 2017;10:1755–1767. | ||
Zeng J, Wang G, Liu X, et al. P2Y13 receptor-mediated rapid increase in intracellular calcium induced by ADP in cultured dorsal spinal cord microglia. Neurochem Res. 2014;39(11):2240–2250. | ||
Field MJ, McCleary S, Hughes J, Singh L. Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain. 1999;80(1–2):391–398. | ||
Ali G, Subhan F, Abbas M, Zeb J, Shahid M, Sewell RD. A streptozotocin-induced diabetic neuropathic pain model for static or dynamic mechanical allodynia and vulvodynia: validation using topical and systemic gabapentin. Naunyn Schmiedebergs Arch Pharmacol. 2015;388(11):1129–1140. | ||
Meng Y, Wang W, Kang J, Wang X, Sun L. Role of the PI3K/AKT signalling pathway in apoptotic cell death in the cerebral cortex of streptozotocin-induced diabetic rats. Exp Ther Med. 2017;13(5):2417–2422. | ||
Pogatzki EM, Zahn PK, Brennan TJ. Lumbar catheterization of the subarachnoid space with a 32-gauge polyurethane catheter in the rat. Eur J Pain. 2000;4(1):111–113. | ||
Pabbidi RM, Yu SQ, Peng S, Khardori R, Pauza ME, Premkumar LS. Influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Mol Pain. 2008;4:9. | ||
Khomula EV, Viatchenko-Karpinski VY, Borisyuk AL, Duzhyy DE, Belan PV, Voitenko NV. Specific functioning of Cav3.2 T-type calcium and TRPV1 channels under different types of STZ-diabetic neuropathy. Biochim Biophys Acta. 2013;1832(5):636–649. | ||
Shi L, Zhang HH, Xiao Y, Hu J, Xu GY. Electroacupuncture suppresses mechanical allodynia and nuclear factor κ B signaling in streptozotocin-induced diabetic rats. CNS Neurosci Ther. 2013;19(2):83–90. | ||
Hwang HS, Yang EJ, Lee SM, Lee SC, Choi SM. Antiallodynic effects of electroacupuncture combined with MK-801 treatment through the regulation of p35/p25 in experimental diabetic neuropathy. Exp Neurobiol. 2011;20(3):144–152. | ||
Rondón LJ, Privat AM, Daulhac L, et al. Magnesium attenuates chronic hypersensitivity and spinal cord NMDA receptor phosphorylation in a rat model of diabetic neuropathic pain. J Physiol. 2010;588(Pt 21):4205–4215. | ||
Kou ZZ, Li CY, Hu JC, et al. Alterations in the neural circuits from peripheral afferents to the spinal cord: possible implications for diabetic polyneuropathy in streptozotocin-induced type 1 diabetic rats. Front Neural Circuits. 2014;8:6. | ||
Paulson PE, Wiley JW, Morrow TJ. Concurrent activation of the somatosensory forebrain and deactivation of periaqueductal gray associated with diabetes-induced neuropathic pain. Exp Neurol. 2007;208(2):305–313. | ||
Drel VR, Pacher P, Vareniuk I, et al. A peroxynitrite decomposition catalyst counteracts sensory neuropathy in streptozotocin-diabetic mice. Eur J Pharmacol. 2007;569(1–2):48–58. | ||
Grotle AK, Garcia EA, Huo Y, Stone AJ. Temporal changes in the exercise pressor reflex in type 1 diabetic rats. Am J Physiol Heart Circ Physiol. 2017;313(4):H708–H714. | ||
Niu J, Huang D, Zhou R, et al. Activation of dorsal horn cannabinoid CB2 receptor suppresses the expression of P2Y12 and P2Y13 receptors in neuropathic pain rats. J Neuroinflammation. 2017;14(1):185. | ||
Lees JG, Fivelman B, Duffy SS, Makker PG, Perera CJ, Moalem-Taylor G. Cytokines in neuropathic pain and associated depression. Mod Trends Pharmacopsychiatry. 2015;30:51–66. | ||
Molet J, Mauborgne A, Diallo M, et al. Microglial Janus kinase/signal transduction and activator of transcription 3 pathway activity directly impacts astrocyte and spinal neuron characteristics. J Neurochem. 2016;136(1):133–147. | ||
Dominguez E, Rivat C, Pommier B, Mauborgne A, Pohl M. JAK/STAT3 pathway is activated in spinal cord microglia after peripheral nerve injury and contributes to neuropathic pain development in rat. J Neurochem. 2008;107(1):50–60. | ||
Wang D, Tang S, Zhang Q. Maslinic acid suppresses the growth of human gastric cells by inducing apoptosis via inhibition of the interleukin-6 mediated Janus kinase/signal transducer and activator of transcription 3 signaling pathway. Oncol Lett. 2017;13(6):4875–4881. | ||
Dominguez E, Mauborgne A, Mallet J, Desclaux M, Pohl M. SOCS3-mediated blockade of JAK/STAT3 signaling pathway reveals its major contribution to spinal cord neuroinflammation and mechanical allodynia after peripheral nerve injury. J Neurosci. 2010;30(16):5754–5766. | ||
Gu X, Zhou L, Lu W. An NMDA receptor-dependent mechanism underlies inhibitory synapse development. Cell Rep. 2016;14(3):471–478. | ||
Monaco SA, Gulchina Y, Gao WJ. NR2B subunit in the prefrontal cortex: a double-edged sword for working memory function and psychiatric disorders. Neurosci Biobehav Rev. 2015;56:127–138. | ||
Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8(6):413–426. | ||
Sun Y, Zhang W, Liu Y, Liu X, Ma Z, Gu X. Intrathecal injection of JWH015 attenuates remifentanil-induced postoperative hyperalgesia by inhibiting activation of spinal glia in a rat model. Anesth Analg. 2014;118(4):841–853. | ||
Morel V, Pickering G, Etienne M, et al. Low doses of dextromethorphan have a beneficial effect in the treatment of neuropathic pain. Fundam Clin Pharmacol. 2014;28(6):671–680. | ||
Dang JK, Wu Y, Cao H, et al. Establishment of a rat model of type II diabetic neuropathic pain. Pain Med. 2014;15(4):637–646. | ||
Trang T, Beggs S, Salter MW. ATP receptors gate microglia signaling in neuropathic pain. Exp Neurol. 2012;234(2):354–361. | ||
Malin SA, Molliver DC. Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol Pain. 2010;6:21. | ||
Tatsumi E, Yamanaka H, Kobayashi K, Yagi H, Sakagami M, Noguchi K. RhoA/ROCK pathway mediates p38 MAPK activation and morphological changes downstream of P2Y12/13 receptors in spinal microglia in neuropathic pain. Glia. 2015;63(2):216–228. | ||
von Kügelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110(3):415–432. | ||
Ortega F, Pérez-Sen R, Delicado EG, Teresa Miras-Portugal M. ERK1/2 activation is involved in the neuroprotective action of P2Y13 and P2X7 receptors against glutamate excitotoxicity in cerebellar granule neurons. Neuropharmacology. 2011;61(8):1210–1221. | ||
Gao ZG, Ding Y, Jacobson KA. P2Y(13) receptor is responsible for ADP-mediated degranulation in RBL-2H3 rat mast cells. Pharmacol Res. 2010;62(6):500–505. | ||
Choi DC, Lee JY, Lim EJ, Baik HH, Oh TH, Yune TY. Inhibition of ROS-induced p38MAPK and ERK activation in microglia by acupuncture relieves neuropathic pain after spinal cord injury in rats. Exp Neurol. 2012;236(2):268–282. | ||
Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45(1):89–95. | ||
Ding Y, Shi W, Xie G, Yu A, Wang Q, Zhang Z. CX3CR1 mediates nicotine withdrawal-induced hyperalgesia via microglial P38 MAPK signaling. Neurochem Res. 2015;40(11):2252–2261. | ||
Abbracchio MP, Burnstock G, Boeynaems JM, et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58(3):281–341. | ||
Soulet C, Sauzeau V, Plantavid M, et al. Gi-dependent and -independent mechanisms downstream of the P2Y12 ADP-receptor. J Thromb Haemost. 2004;2(1):135–146. | ||
Liu M, Liao K, Yu C, Li X, Liu S, Yang S. Puerarin alleviates neuropathic pain by inhibiting neuroinflammation in spinal cord. Mediators Inflamm. 2014;2014:485927. | ||
Wei XH, Na XD, Liao GJ, et al. The up-regulation of IL-6 in DRG and spinal dorsal horn contributes to neuropathic pain following L5 ventral root transection. Exp Neurol. 2013;241:159–168. | ||
Sung CS, Cherng CH, Wen ZH, et al. Minocycline and fluorocitrate suppress spinal nociceptive signaling in intrathecal IL-1β-induced thermal hyperalgesic rats. Glia. 2012;60(12):2004–2017. | ||
Zhou YQ, Liu Z, Liu ZH, et al. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation. 2016;13(1):141. | ||
Kleibeuker W, Gabay E, Kavelaars A, et al. IL-1 beta signaling is required for mechanical allodynia induced by nerve injury and for the ensuing reduction in spinal cord neuronal GRK2. Brain Behav Immun. 2008;22(2):200–208. | ||
Gui WS, Wei X, Mai CL, et al. Interleukin-1β overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol Pain. 2016;12. pii: 1744806916646784. | ||
Vazquez E, Kahlenbach J, Segond von Banchet G, König C, Schaible HG, Ebersberger A. Spinal interleukin-6 is an amplifier of arthritic pain in the rat. Arthritis Rheum. 2012;64(7):2233–2242. | ||
Choi HS, Roh DH, Yoon SY, et al. Microglial interleukin-1β in the ipsilateral dorsal horn inhibits the development of mirror-image contralateral mechanical allodynia through astrocyte activation in a rat model of inflammatory pain. Pain. 2015;156(6):1046–1059. | ||
Rojewska E, Makuch W, Przewlocka B, Mika J. Minocycline prevents dynorphin-induced neurotoxicity during neuropathic pain in rats. Neuropharmacology. 2014;86:301–310. | ||
Lei Y, Sun Y, Lu C, Ma Z, Gu X. Activated glia increased the level of proinflammatory cytokines in a resiniferatoxin-induced neuropathic pain rat model. Reg Anesth Pain Med. 2016;41(6):744–749. | ||
Wang J, Gao Y, Chen S, et al. The effect of repeated electroacupuncture analgesia on neurotrophic and cytokine factors in neuropathic pain rats. Evid Based Complement Alternat Med. 2016;2016:8403064. | ||
Zhang GH, Lv MM, Wang S, et al. Spinal astrocytic activation is involved in a virally-induced rat model of neuropathic pain. PLoS One. 2011;6(9):e23059. | ||
Ko JS, Eddinger KA, Angert M, et al. Spinal activity of interleukin 6 mediates myelin basic protein-induced allodynia. Brain Behav Immun. 2016;56:378–389. | ||
Moini-Zanjani T, Ostad SN, Labibi F, Ameli H, Mosaffa N, Sabetkasaei M. Minocycline effects on IL-6 concentration in macrophage and microglial cells in a rat model of neuropathic pain. Iran Biomed J. 2016;20(5):273–279. | ||
Qi J, Chen C, Meng QX, Wu Y, Wu H, Zhao TB. Crosstalk between activated microglia and neurons in the spinal dorsal horn contributes to stress-induced hyperalgesia. Sci Rep. 2016;6:39442. | ||
Kim BK, Tran HY, Shin EJ, et al. IL-6 attenuates trimethyltin-induced cognitive dysfunction via activation of JAK2/STAT3, M1 mAChR and ERK signaling network. Cell Signal. 2013;25(6):1348–1360. | ||
Zhu L, Cheng X, Ding Y, et al. Bone marrow-derived myofibroblasts promote colon tumorigenesis through the IL-6/JAK2/STAT3 pathway. Cancer Lett. 2014;343(1):80–89. | ||
Kurosaka M, Machida S. Interleukin-6-induced satellite cell proliferation is regulated by induction of the JAK2/STAT3signalling pathway through cyclin D1 targeting. Cell Prolif. 2013;46(4):365–373. | ||
Jang HJ, Lee SJ, Lee S, Jung K, Lee SW, Rho MC. Acyclic triterpenoids from Alpinia katsumadai Inhibit IL-6-induced STAT3 activation. Molecules. 2017;22(10). pii: E1611. | ||
Li WY, Li FM, Zhou YF, et al. Aspirin down regulates hepcidin by inhibiting NF-κB and IL6/JAK2/STAT3 pathways in BV-2 microglial cells treated with lipopolysaccharide. Int J Mol Sci. 2016;17(12). pii: E1921. | ||
Chen S, Dong Z, Cheng M, et al. Homocysteine exaggerates microglia activation and neuroinflammation through microglia localized STAT3 overactivation following ischemic stroke. J Neuroinflammation. 2017;14(1):187. | ||
Wang C, Ding H, Tang X, Li Z, Gan L. Effect of liuweibuqi capsules in pulmonary alveolar epithelial cells and COPD through JAK/STAT pathway. Cell Physiol Biochem. 2017;43(2):743–756. | ||
Guptarak J, Wanchoo S, Durham-Lee J, et al. Inhibition of IL-6 signaling: a novel therapeutic approach to treating spinal cord injury pain. Pain. 2013;154(7):1115–1128. | ||
Ebisawa S, Andoh T, Shimada Y, Kuraishi Y. Yokukansan improves mechanical allodynia through the regulation of interleukin-6 expression in the spinal cord in mice with neuropathic pain. Evid Based Complement Alternat Med. 2015;2015:870687. | ||
Lin Y, Liu L, Jiang H, Zhou J, Tang Y. Inhibition of interleukin-6 function attenuates the central sensitization and pain behavior induced by osteoarthritis. Eur J Pharmacol. 2017;811:260–267. | ||
Nie H, Weng HR. Glutamate transporters prevent excessive activation of NMDA receptors and extrasynaptic glutamate spillover in the spinal dorsal horn. J Neurophysiol. 2009;101(4):2041–2051. | ||
König C, Morch E, Eitner A, et al. Involvement of spinal IL-6 trans-signaling in the induction of hyperexcitability of deep dorsal horn neurons by spinal tumor necrosis factor-alpha. J Neurosci. 2016;36(38):9782–9791. | ||
Tian Y, Wang S, Ma Y, Lim G, Kim H, Mao J. Leptin enhances NMDA-induced spinal excitation in rats: a functional link between adipocytokine and neuropathic pain. Pain. 2011;152(6):1263–1271. | ||
Tang J, Li ZH, Ge SN, et al. The inhibition of spinal astrocytic JAK2-STAT3 pathway activation correlates with the analgesic effects of triptolide in the rat neuropathic pain model. Evid Based Complement Alternat Med. 2012;2012:185167. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.