Back to Journals » OncoTargets and Therapy » Volume 14
Actin-Like Protein 8 Promotes the Progression of Triple-Negative Breast Cancer via Activating PI3K/AKT/mTOR Pathway
Authors Fan S, Yan S, Yang Y, Shang J, Hao M
Received 10 November 2020
Accepted for publication 8 March 2021
Published 12 April 2021 Volume 2021:14 Pages 2463—2473
DOI https://doi.org/10.2147/OTT.S291403
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tohru Yamada
Shaoxia Fan,* Shen Yan,* Yang Yang, Jian Shang, Min Hao
Breast and Thyroid Surgery, Dongying People’s Hospital, Dongying, Shandong, 257091, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yang Yang
Breast and Thyroid Surgery, Dongying People’s Hospital, No. 317, Dongying, Shandong, 257091, People’s Republic of China
Email [email protected]
Objective: The purpose of this study was to investigate the function of actin-like protein 8 (ACTL8) on triple-negative breast cancer (TNBC) and its potential mechanisms.
Methods: In our study, ACTL8 expression and the prognostic values of ACTL8 were evaluated via the dataset from the Cancer Genome Atlas (TCGA). At the same time, the expression of ACTL8 in TNBC cells was measured by Western blot and qRT-PCR. Then, the effects of ACTL8 on the growth and metastasis of TNBC were investigated by using 5-ethynyl-20-deoxyuridine (EdU), colony formation, flow cytometry, wound healing and transwell assays. Mechanistically, Western blot was performed to confirm the interaction between ACTL8 and phosphatidylinositol 3′-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling pathway in TNBC.
Results: ACTL8 expression was upregulated in TNBC and associated with the poor prognosis of TNBC. Silencing ACTL8 suppressed the proliferation, migration and invasion, also promoted the apoptosis in MDA-MB-231 and BT-549 cells. Moreover, we found that silencing ACTL8 could inhibit the activation of PI3K/AKT/mTOR signaling pathway in MDA-MB-231 and BT-549 cells. Meanwhile, the impact of silencing ACTL8 on the proliferation, apoptosis, migration and invasion was enhanced by PI3K/AKT/mTOR pathway inhibitor (Wortmannin) and reversed by PI3K/AKT/mTOR pathway activator (740Y-P).
Conclusion: Our data demonstrated that ACTL8 may facilitate the proliferation, migration and invasion, while inhibiting apoptosis through activating PI3K/Akt/mTOR signaling pathway in TNBC.
Keywords: TNBC, ACTL8, proliferation, migration, invasion, PI3K/AKT/mTOR pathway
Introduction
Breast cancer, one of the most common malignant diseases in women worldwide, is a histologically and clinically heterogeneous disease, which is divided into four main subtypes: luminal A (Lum A), luminal B (Lum B), triple-negative/basal-like and human epidermal growth factor receptor 2+ (HER2) type.1–4 Triple-negative breast cancer (TNBC) is the most malignant subtype of breast cancer and characterized by the lack of estrogen receptor (ER), progesterone receptor (PR) and HER2, which accounts for 15–20% of all breast cancer cases.5–7 Because of the lack of effective therapeutic targets, TNBC patients have a poorer prognosis and a higher recurrence risk than those with other subtypes of breast cancer.8 Thus, it is urgent to explore novel molecular targets for therapy of TNBC patients.
Cancer-testis antigens (CTAs) are a class of tumor-associated antigens that are specifically expressed in placental tissue, many types of cancer tissues and testicles tissue, but expressed at a low level in normal tissues.9 Actin-like protein 8 (ACTL8), a member of CTA family, has been reported to contribute to the progression of multiple cancers, such as colorectal cancer,10 head and neck squamous cell carcinoma11 and non-small cell lung cancer.12 Moreover, studies have shown that ACTL8 is highly expressed in TNBC.13 However, the effects of ACTL8 on TNBC and its related mechanisms have not yet been reported.
The phosphatidylinositol 3′-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling pathway controls a variety of cellular functions such as proliferation, growth, survival, motility and metabolism.14 Scholars have confirmed that the PI3K/AKT/mTOR pathway is closely related to the initiation and progress of various cancers.15,16 Moreover, more and more studies have also indicated that PI3K/AKT/mTOR pathway can play an important role in TNBC.17 In fact, many PI3K/AKT/mTOR pathway regulators are acquiring a growing interest in TNBC treatment.18,19
In the present study, we investigated the role of ACTL8 in TNBC progression and explore how it achieves the function. TCGA dataset and our results confirmed that ACTL8 expression was significantly upregulated in TNBC tissues and cells. Moreover, ACTL8 may facilitate the proliferation, migration and invasion, while inhibiting apoptosis through activating PI3K/Akt/mTOR signaling pathway in TNBC, indicating that ACTL8 may be considered a novel prognostic marker and therapeutic target for TNBC.
Materials and Methods
Cell Culture and Transfection
The following cell lines were supplied by the American Type Culture Collection (ATCC, Manassas, VA, USA): human TNBC cell clines (MDA-MB-231, MDA-MB-453, MDA-MB-468, BT-549) and normal mammary epithelial cell line (MCF-10A). MCF-10A cells were grown in MEBM BulletKit (Lonza, Basel, Switzerland), and other cell lines were cultured in RPMI 1640 medium (Gibco, USA) with 10% fetal bovine serum (FBS, Gibco, Germany), 100 U/mL penicillin (Invitrogen, USA) and 100 mg/mL streptomycin (Invitrogen, USA). The cells were cultured in a humidified temperature at 37°C with 5% CO2. To knockdown ACTL8, small interfering RNA (siRNA) targeting the back-splice junction site of ACTL8 (si-ACTL8) and siRNA negative control (si-NC) were synthesized by GeneChem (Shanghai, China). The BT-549 and MDA-MB-231 cells were transfected with 50 nM siRNA or si-NC using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen, USA) according to the manufacturer’s instructions.
5-Ethynyl-20-Deoxyuridine (EdU) Assay
The proliferation ability of BT-549 and MDA-MB-231 cells was detected by Cell-LightTM EdU DNA Cell Proliferation Kit (RiboBio, Guangzhou, China) following the manufacturer’s protocols. In brief, the transfected BT-549 and MDA-MB-231cells were cultured with 50 μM EdU for 2 h and then stained with 4′,6-diamidino-2phenylindole (DAPI, Sigma, USA). In the end, the EdU-positive cells were observed by using a fluorescence microscope (Zeiss LSM710, Oberkochen, Germany).
Colony Formation Assay
A colony formation assay was performed to assess colony formation of BT-549 and MDA-MB-231 cells. Simply, BT-549 and MDA-MB-231 cells (500 cells/well) transiently transfected with si-ACTL8 and si-NC were seeded into 6-well plates and maintained in RPMI 1640 medium for 2 weeks at 37°C in a 5% CO2 incubator. After that, BT-549 and MDA-MB-231 cells on the plates were washed three times with PBS, fixed using 4% paraformaldehyde and then stained with 0.1% crystal violet dye (Beyotime, Shanghai, China). Finally, the number of clones was counted under a light microscope.
Flow Cytometry
The apoptosis ability of BT-549 and MDA-MB-231 cells was evaluated by using the Annexin V-fluorescein isothiocyanate (FITC) cell apoptosis assay kit (Biovision, USA) according to the manufacturer’s guidelines. Briefly, the transfected BT-549 and MDA-MB-231 cells were collected and double stained with fluorescein Annexin V-FITC and propodium iodide (PI). Finally, the apoptotic BT-549 and MDA-MB-231 cells were analyzed on a flow cytometer (BD Biosciences, USA).
Wound Healing Assay
The transfected BT-549 and MDA-MB-231 cells (1 × 105 cells/well) were seeded into a 6-well plate. Upon reaching 100% confluence, the cell monolayer was lightly and quickly scratched with a sterile pipette tip. After the debris was removed, the cells were cultured in serum-free medium for 24 h. Finally, the scratch wounds were observed and imaged under an inverted microscope.
Transwell Assay
The ability of migration and invasion in BT-549 and MDA-MB-231 cells was detected using transwell chambers (Corning, Corning, NY, USA). In brief, the transfected BT-549 and MDA-MB-231 cells were suspended and inoculated into the upper chamber coated with Matrigel (Millipore, Billerica, USA) for invasion assay or without Matrigel for migration assay. Subsequently, 500 μL complete medium was added to the lower chamber. After 24 hours of incubation at 37°C, the BT-549 and MDA-MB-231 cells that moved to the lower surface of the membrane were fixed 4% paraformaldehyde for 10 min and stained by 0.1% crystal violet solution for 10 min. Finally, the migrated and invaded cells were photographed with an inverted microscope.
Quantitative Real Time-PCR (qRT-PCR)
Total RNA from cells was isolated with TRIZOL reagent (Invitrogen, USA) and their quality was measured by microplate reader. Total RNA was reverse-transcribed into complementary DNAs using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech, China). Then, the qRT-PCR was carried out using TransStart TipTop Green qPCR SuperMix (TransGen Biotech, China) on an ABI StepOne PlusTM RT-PCR System (ABI, Carlsbad, CA) using the following primers: ACTL8 (sense): 5′-GCCACGTGCTCACAGAGTAG-3′, (anti-sense): 5′-CTCAGCTGCACACTGCAAAC-3′; GAPDH (sense): 5′-GAAGGTGAAGGTCGGAGTC-3′, (anti-sense): 5′-GAAGATGGTGATGGGATTTC-3′.
Western Blot Analysis
Total protein was extracted from cells with the RIPA lysis buffer (Pierce, Waltham, MA, USA). The protein concentration was determined with a bicinchoninic acid protein assay kit (Beyotime, Jiangsu, China). After that, 20 mg of protein mixed with 2× SDS loading buffer was loaded per lane, separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to the polyvinylidene difluoride membranes (Millipore, USA). Blocking was then carried on with 5% skimmed milk and the membranes were incubated with anti-ACTL8 (1:500, cat. no. ab184562, Abcam, UK), anti-Bcl-2 (1:1000, cat. no. ab185002, Abcam, UK), anti-Bax (1:1000, cat. no. ab32503, Abcam, UK), anti-cleaved caspase-3 (1:1000, cat. no. ab2302, Abcam, UK), anti-PI3K (1:1000, cat. no. 4257, Cell Signaling, USA), anti-phosphorylated-PI3K (p-PI3K, 1:500, cat. no. 17366, Cell Signaling, USA), anti-AKT (1:1000, cat. no. 2902, Cell Signaling, USA), anti- p-AKT (1:300, cat. no. 9614, Cell Signaling, USA), anti-mTOR (1:1000, cat. no. 2983, Cell Signaling, USA), anti-p-mTOR (1:500, cat. no. 5536, Cell Signaling, USA) and anti-GAPDH (1:1000, cat. no. 8884, Cell Signaling, USA) at 4°C overnight. The membranes were subsequently incubated with the HRP-conjugated secondary antibody (1:5000, Proteintech) for 2 h at room temperature. Finally, the protein bands were visualized with ECL detection system (Thermo, Waltham, MA, USA).
Statistical Analysis
Numerical data were presented as mean ± SD. Statistical analysis was performed using Student’s t-test or one-way ANOVA test. All statistical analyses were performed using SPSS 23.0 software (Chicago, IL). Differences were considered significant when P < 0.05.
Results
ACTL8 is Upregulated in TNBC and Associated with the Poor Prognosis of TNBC
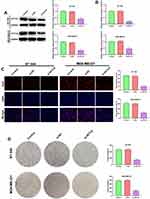
Based on TCGA dataset analysis, ACTL8 expression was markedly upregulated in breast cancer patients, and the upregulation was greater in TNBC when compared with other subtypes (Figure 1A). Then, we also analyzed the expression of ACTL8 in human TNBC cell lines (MDA-MB-231, MDA-MB-453, MDA-MB-468, BT-549) and normal mammary epithelial cell line (MCF-10A) by Western blot and qRT-PCR. The results showed that the mRNA and protein expressions of ACTL8 were significantly upregulated in MDA-MB-453 and MDA-MB-468 cells, especially in MDA-MB-231 and BT-549 cells (Figure 1B and C). In addition, Kaplan-Meier survival curve analysis based on TCGA data revealed that the high expression of ACTL8 was correlated with the poor prognosis (Figure 1D). All data indicated that ACTL8 was upregulated in TNBC and associated with the poor prognosis of TNBC.
Silencing ACTL8 Suppresses the Proliferation in MDA-MB-231 and BT-549 Cells
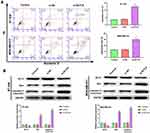
As seen in Figure 2A and B, the transfection with si-ACTL8 significantly decreased the ACTL8 expression in MDA-MB-231 and BT-549 cells. In addition, the results of EdU assay showed that silencing ACTL8 dramatically inhibited the proliferation in MDA-MB-231 and BT-549 cells relative Control and si-NC groups (Figure 2C). We also investigated the effect of ACTL8 on cell clone by colony formation assay and found that silencing ACTL8 markedly reduced the ability of cell clone formation in MDA-MB-231 and BT-549 cells (Figure 2D). Collectively, these results confirmed that silencing ACTL8 may suppress the proliferation in MDA-MB-231 and BT-549 cells.
Silencing ACTL8 Facilitates the Apoptosis in MDA-MB-231 and BT-549 Cells
As shown in Figure 3A, silencing ACTL8 significantly increased the apoptosis in MDA-MB-231 and BT-549 cells in comparison with Control and si-NC groups. In addition, apoptosis-related proteins, such as Bcl-2, Bax and cleaved caspase-3, were determined by Western blot. Silencing ACTL8 notably decreased the expression of Bcl-2, but increased the expression of Bax and cleaved caspase-3 in MDA-MB-231 and BT-549 cells when compared with Control and si-NC groups (Figure 3B). These findings indicated that silencing ACTL8 may facilitate the apoptosis in MDA-MB-231 and BT-549 cells.
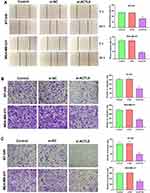
Silencing ACTL8 Suppresses the Migration and Invasion Abilities in MDA-MB-231 and BT-549 Cells
The roles of ACTL8 on the migration and invasion in MDA-MB-231 and BT-549 cells were determined by wound healing assay and transwell assay. From the wound healing assay, we found that silencing ACTL8 significantly decreased the healing capacity of MDA-MB-231 and BT-549 cells compared with Control and si-NC groups (Figure 4A). The results of the transwell assay in Figure 4B and C confirmed that the numbers of invasive and migrated cells were markedly repressed after ACTL8 silencing. These findings suggested that silencing ACTL8 may suppress the migration and invasion abilities in MDA-MB-231 and BT-549 cells.
Silencing ACTL8 Inhibits the Activation of PI3K/AKT/mTOR Signaling Pathway in MDA-MB-231 and BT-549 Cells
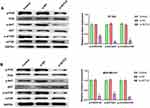
To assess the effect of ACTL8 on PI3K/AKT/mTOR signaling pathway in MDA-MB-231 and BT-549 cells, we measured the related proteins of PI3K/AKT/mTOR signaling pathway by Western blot. As shown in Figure 5A and B, silencing ACTL8 significantly reduced the phosphorylation level of PI3K, AKT and mTOR in MDA-MB-231 and BT-549 cells when compared with Control and si-NC groups. Moreover, silencing ACTL8 did not cause changes in the expression level of PI3K, AKT and mTOR in MDA-MB-231 and BT-549 cells. All these results indicated that silencing ACTL8 may inhibit the activation of PI3K/AKT/mTOR signaling pathway in MDA-MB-231 and BT-549 cells.
PI3K/AKT/mTOR Signaling Pathway Involved in ACTL8 Modulated the Proliferation, Apoptosis, Migration and Invasion in MDA-MB-231 Cells
As shown in Figure 6A, the phosphorylation level of PI3K, AKT and mTOR in MDA-MB-231 cells was significantly decreased in si-ACTL8 + Wortmannin group and remarkably increased in si-ACTL8 + 740Y-P group when compared with si-ACTL8 group. The results of EdU and transwell assays revealed that the proliferation, migration and invasion of MDA-MB-231 cells in si-ACTL8 group were markedly higher than those in si-ACTL8 + Wortmannin group, but notably lower in si-ACTL8 + 740Y-P group (Figure 6B, E and F). In addition, when compared with si-ACTL8 group, MDA-MB-231 cell apoptosis was significantly elevated in si-ACTL8 + Wortmannin group, but dramatically reduced in si-ACTL8 + 740Y-P group (Figure 6C). Moreover, Western blot results confirmed that the expression of Bax and cleaved caspase-3 in MDA-MB-231 cells was significantly increased in si-ACTL8 + Wortmannin group compared with si-ACTL8 group, but markedly decreased in si-ACTL8 + 740Y-P group (Figure 6D). Meanwhile, when compared with si-ACTL8 group, Bcl-2 expression was dramatically decreased in si-ACTL8 + Wortmannin group, but notably increased in si-ACTL8 + 740Y-P group (Figure 6D). Collectively, these results confirmed that PI3K/AKT/mTOR signaling pathway may be involved in ACTL8 modulated the proliferation, apoptosis, migration and invasion in MDA-MB-231 cells.
Discussion
At present, due to the lack of therapeutic targets, chemotherapy is still the main treatment strategy for TNBC. Recurrence rate of TNBC is also high within 1–3 years despite adjuvant chemotherapy.20 Therefore, it is necessary to search novel therapeutic targets for TNBC. In this study, we confirmed that ACTL8 may facilitate the proliferation, migration and invasion, as well as inhibit apoptosis through activating PI3K/Akt/mTOR signaling pathway in TNBC.
In recent years, CTA family members are increasingly recognized as promising targets for cancer immunotherapy.21,22 As a member of the CTA family, ACTL8 is reported to be highly expressed in multiple tumors.23 Moreover, Yao et al have also demonstrated that ACTL8 is highly enriched in TNBC.13 The analysis of TCGA dataset showed that ACTL8 expression was markedly upregulated in TNBC patients, and the upregulation was greater in TNBC when compared with other subtypes. In addition, the high expression of ACTL8 was found in TNBC cells and the patients with high ACTL8 expression had short survival time. Increasing evidence has confirmed that ACTL8 may play an important role in many cancers. For example, the downregulated expression of ACTL8 may decrease the capacity of migration and invasion in colorectal cancer.10 Li et al have reported that ACTL8 promotes the proliferative, migrating and invading capabilities of human endometrial cancer cells.23 In addition, Ma et al have indicated that ACTL8 may facilitate the proliferation, colony-formation, migration and invasion in lung adenocarcinoma cells.12 Our data showed that silencing ACTL8 inhibited proliferation, colony formation, migration and invasion, and promoted apoptosis in MDA-MB-231 and BT-549 cells. These outcomes supported the idea that ACTL8 might function as an oncogene in TNBC.
PI3K/AKT/mTOR signaling pathway is a cell cycle-related pathway that is key in the regulation of cell proliferation, growth, survival, motility and metabolism.24 Moreover, the abnormal activation of PI3K/AKT/mTOR signaling pathway is frequently found in many kinds of cancers.25 Recently, the PI3K/AKT/mTOR pathway has been considered to be an important pathway in breast cancer in recent years.26 Increasing evidences are demonstrating that the aberration of the PI3K/AKT/mTOR pathway is one of the most common genomic abnormalities in various subtypes of breast cancer.27 Therefore, how to better inhibit PIK3/AKT/mTOR pathway has gradually become a hot topic in the research and treatment of breast cancer including TNBC. In the present study, we found that silencing ACTL8 significantly inhibit the activation of PI3K/AKT/mTOR pathway in TNBC cells, indicating that ACTL8 might promote the progress of TNBC by activating PI3K/AKT/mTOR pathway. Then, to further verify the above hypothesis, we used PI3K/AKT/mTOR pathway inhibitor (Wortmannin) and PI3K/AKT/mTOR pathway activator (740Y-P) in the subsequent experiments. Our data revealed that the impact of silencing ACTL8 on the proliferation, apoptosis, migration and invasion was enhanced by Wortmannin and reversed by 740Y-P, suggesting that ACTL8 may facilitate the proliferation, migration and invasion, while inhibiting apoptosis through activating PI3K/Akt/mTOR signaling pathway in TNBC. Of course, our study also has some limitations. In the future experiments, we will further explore how ACTL8 affects the PI3K/AKT/mTOR pathway and whether ACTL8 affects other pathways in TNBC.
Conclusion
ACTL8 expression is higher in TNBC tissues and cells, in comparison with controls, and associated with the poor prognosis of TNBC. ACTL8 may facilitate the proliferation, migration and invasion, while inhibiting apoptosis through activating PI3K/Akt/mTOR signaling pathway in TNBC, which suggests that ACTL8 might be considered a novel prognostic marker and therapeutic target for TNBC.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Shaoxia Fan and Shen Yan are co-first authors for this study.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Yaffe MJ, Jong RA. Adjunctive ultrasonography in breast cancer screening. Lancet. 2016;387(10016):313–314. doi:10.1016/S0140-6736(15)00787-4
2. Braunstein LZ, Taghian AG, Niemierko A, et al. Breast-cancer subtype, age, and lymph node status as predictors of local recurrence following breast-conserving therapy. Breast Cancer Res Treat. 2017;161(1):173–179. doi:10.1007/s10549-016-4031-5
3. Polyak K. Heterogeneity in breast cancer. J Clin Invest. 2011;121(10):3786–3788. doi:10.1172/JCI60534
4. Guiu S, Michiels S, André F, et al. Molecular subclasses of breast cancer: how do we define them? The IMPAKT 2012 working group statement. Ann Oncol. 2012;23(12):2997–3006. doi:10.1093/annonc/mds586
5. Abramson VG, Lehmann BD, Ballinger TJ, Pietenpol JA. Subtyping of triple-negative breast cancer: implications for therapy. Cancer. 2015;121(1):8–16. doi:10.1002/cncr.28914
6. Lehmann BD, Pietenpol JA. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J Pathol. 2014;232(2):142–150. doi:10.1002/path.4280
7. Giuli MV, Giuliani E, Screpanti I, Bellavia D, Checquolo S. Notch signaling activation as a hallmark for triple-negative breast cancer subtype. J Oncol. 2019;2019:8707053. doi:10.1155/2019/8707053
8. Kalimutho M, Parsons K, Mittal D, López JA, Srihari S, Khanna KK. Targeted therapies for triple-negative breast cancer: combating a stubborn disease. Trends Pharmacol Sci. 2015;36(12):822–846. doi:10.1016/j.tips.2015.08.009
9. Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4:1.
10. Han Q, Sun M-L, Liu W-S, et al. <p>Upregulated expression of ACTL8 contributes to invasion and metastasis and indicates poor prognosis in colorectal cancer. Onco Targets Ther. 2019;12:1749–1763. doi:10.2147/OTT.S185858
11. Li B, Zhu J, Meng L. High expression of ACTL8 is poor prognosis and accelerates cell progression in head and neck squamous cell carcinoma. Mol Med Rep. 2019;19(2):877–884. doi:10.3892/mmr.2018.9716
12. Ma S, Wang X, Zhang Z, Liu D. Actin-like protein 8 promotes cell proliferation, colony-formation, proangiogenesis, migration and invasion in lung adenocarcinoma cells. Thorac Cancer. 2020;11(3):526–536. doi:10.1111/1759-7714.13247
13. Yao J, Caballero OL, Yung WK, et al. Tumor subtype-specific cancer-testis antigens as potential biomarkers and immunotherapeutic targets for cancers. Cancer Immunol Res. 2014;2(4):371–379. doi:10.1158/2326-6066.CIR-13-0088
14. Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–619. doi:10.1038/nrg1879
15. Corti F, Nichetti F, Raimondi A, et al. Targeting the PI3K/AKT/mTOR pathway in biliary tract cancers: a review of current evidences and future perspectives. Cancer Treat Rev. 2019;72:45–55. doi:10.1016/j.ctrv.2018.11.001
16. Gasparri ML, Besharat ZM, Farooqi AA, et al. MiRNAs and their interplay with PI3K/AKT/mTOR pathway in ovarian cancer cells: a potential role in platinum resistance. J Cancer Res Clin Oncol. 2018;144(12):2313–2318. doi:10.1007/s00432-018-2737-y
17. Costa RLB, Han HS, Gradishar WJ. Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: a review. Breast Cancer Res Treat. 2018;169(3):397–406. doi:10.1007/s10549-018-4697-y
18. Lei S, Fan P, Wang M, et al. Elevated estrogen receptor β expression in triple negative breast cancer cells is associated with sensitivity to doxorubicin by inhibiting the PI3K/AKT/mTOR signaling pathway. Exp Ther Med. 2020;20(2):1630–1636. doi:10.3892/etm.2020.8809
19. Yue X, Li M, Chen D, Xu Z, Sun S. UNBS5162 induces growth inhibition and apoptosis via inhibiting PI3K/AKT/mTOR pathway in triple negative breast cancer MDA-MB-231 cells. Exp Ther Med. 2018;16(5):3921–3928. doi:10.3892/etm.2018.6675
20. Chen X, Iliopoulos D, Zhang Q, et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature. 2014;508(7494):103–107. doi:10.1038/nature13119
21. Balafoutas D, Zur Hausen A, Mayer S, et al. Cancer testis antigens and NY-BR-1 expression in primary breast cancer: prognostic and therapeutic implications. BMC Cancer. 2013;13(1):271. doi:10.1186/1471-2407-13-271
22. Dhodapkar MV, Osman K, Teruya-Feldstein J, et al. Expression of cancer/testis (CT) antigens MAGE-A1, MAGE-A3, MAGE-A4, CT-7, and NY-ESO-1 in malignant gammopathies is heterogeneous and correlates with site, stage and risk status of disease. Cancer Immun. 2003;3:9.
23. Li L, Cheng GH, Chen C, Ma DM, Deng XC. Actin‑like protein 8 executes a promoting function in the malignant progression of endometrial cancer: identification of a promising biomarker. Biosci Biotechnol Biochem. 2020;84(6):1160–1167. doi:10.1080/09168451.2020.1736508
24. Sharma VR, Gupta GK, Sharma AK, Batra N, Sharma DK, Joshi A. PI3K/Akt/mTOR intracellular pathway and breast cancer: factors, mechanism and regulation. Curr Pharm Des. 2017;23(11):1633–1638. doi:10.2174/1381612823666161116125218
25. Reddy D, Ghosh P, Kumavath R. Strophanthidin Attenuates MAPK, PI3K/AKT/mTOR, and Wnt/β-Catenin signaling pathways in human cancers. Front Oncol. 2019;9:1469. doi:10.3389/fonc.2019.01469
26. Li ZQ, Qu M, Wan HX, Wang H, Deng Q, Zhang Y. FOXK1 promotes malignant progression of breast cancer by activating PI3K/AKT/mTOR signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(22):9978–9987. doi:10.26355/eurrev_201911_19564
27. Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550–562. doi:10.1038/nrc2664
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.