Back to Journals » Open Access Journal of Clinical Trials » Volume 6
Acti-Tape™ (elastic therapeutic tape) as compared with a knee guard in providing support to the knee joint: an open-label, randomized, crossover study
Authors Hui HK, Karne N, Sonawane N
Received 27 November 2013
Accepted for publication 22 January 2014
Published 28 April 2014 Volume 2014:6 Pages 29—36
DOI https://doi.org/10.2147/OAJCT.S58252
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Hoong Keong Hui,1 Narayan J Karne,2 Navneet Sonawane3
1Nutriworks Ltd, Kowloon, Hong Kong; 2Karne Hospital, Pune, India; 3Vedic Lifesciences Pvt Ltd, Mumbai, India
Study design: Randomized, open-label, crossover, controlled study.
Background: Elastic taping methods are used to provide support to the musculoskeletal system in athletes. Acti-Tape™ (an elastic therapeutic tape) has been marketed for the last 2–3 years and has shown good results in providing support to the joints. This pilot study was planned to collect data on the clinical outcomes and to assess if a single tape application of Acti-Tape over the knee joint could provide benefits similar to a traditionally used knee guard.
Methods: Thirteen subjects aged 30–65 years visiting an orthopedic center in Pune, India who were suffering from osteoarthritis were randomly assigned to either Acti-Tape (n=6) or a knee guard (n=7) in the first intervention period (6 days) and were crossed over to the other group in the second intervention period (6 days) after a washout of 1 day. Main outcome measures were change from day 0 to day 6 in pain visual analog score (VAS); timed up and go (TUG), medial step down (MSD), and unilateral anterior reach (UAR) tests; and subject's preference.
Results: Data for all the 13 subjects were pooled and analyzed by Student's t-test as treatment-by-period interaction was not significant by analysis of variance (P>0.05). The changes (mean ± standard deviation) after using Acti-Tape and a knee guard, respectively, were pain VAS, –10±5.4 versus (vs) –11.5±5.83; TUG, –0.62±1.33 vs –0.46±1.56; UAR, 0.15±1.07 vs 0.75±0.44; and MSD, 1.08±095 vs 0.85±1.14. These were statistically significant with both devices for pain VAS, UAR, and MSD, but not for TUG. Between the treatments however, no statistically significant difference was seen. Eleven of 13 (85%) subjects preferred Acti-Tape for future use (P<0.05 by McNemar’s χ2 test). No safety concerns were reported by the subjects.
Conclusion: Single tape application of Acti-Tape over the knee joint improves clinical outcomes similar to that of the knee guard. The patients preferred Acti-Tape to knee guard for future use.
Keywords: elastic taping methods, pain VAS, knee support, enhanced mobility
Introduction
Incessant wear and tear of the joint leads to early degeneration of the joint cartilage. This generally occurs in the weight bearing joints like the knee and hip joint and is most commonly seen in those who are overweight, the elderly, sportsmen, and athletes.1 Other causes for the degeneration of cartilage include trauma to the knee, meniscus tears, and ligament damage.2 Symptoms of degeneration of joint cartilage typically include pain, along with stiffness and tenderness.3 Treatment modality comprises analgesics, nonsteroidal anti-inflammatory drugs (NSAIDs), knee braces, and surgery. The goal of treatment is to relieve pain and restore maximum utility of the affected joint.4 Knee braces of various kinds are used to provide external support. Knee guards are known to provide compression to the joint and reduce pain at the site of application in subjects with osteoarthritis of the knee, especially while climbing stairs.5 Simple open patellar knee guards are more preferred by patients for long term and regular use.
Elastic taping methods have been used since the 1970s to provide support to the musculoskeletal system, especially for sportsmen.5 Acti-Tape™ (Nutriworks Ltd, Kowloon, Hong Kong) has its origins in similar therapeutic sports tapes for rehabilitation of injuries, providing support to joints and muscles and enhancing sports performance. Nowadays, aside from application in rehabilitation, newer taping methodologies have been developed that address benefits associated with preventive health maintenance, reducing edema, providing support, and pain relief. Acti-Tape has been marketed for the last 2–3 years and is used by patients for providing support to the muscles and joints. Many users have indicated in customer feedback the relief seen in their movements involving the knee joint. However, to the best of our knowledge, there is no study reporting a comparison of these two methods. Taping methods have also not been studied in subjects with knee pain due to degenerative changes.
This pilot study was planned to collect data on the clinical outcomes and assess if a single tape application of Acti-Tape over the knee joint could provide benefits similar to a traditionally used knee guard.
Methods
The study was reviewed and approved by the Independent Ethics Committee, Pune, India, and conducted according to the principles of good clinical research practice and the Declaration of Helsinki. Voluntary written informed consent was obtained from all subjects prior to initiation of any study-related procedure.
Subjects
Potential subjects were screened from the patients attending the orthopedic center in Karne Hospital, Pune, India, between March 5, 2013 and April 15, 2013. Male and female subjects aged 30–65 years with mild to moderate degenerative changes of the knee joint as confirmed by American Rheumatism Association functional class II and III and Kellgren–Lawrence grade II and III were recruited in the study if they had a pain score of ≥50 on a 100 mm visual analog scale (VAS).6,7
Subjects with symptoms due to any form of arthritis other than osteoarthritis were excluded. Other exclusions were subjects with arthroscopy of either knee in the past year, administration of intra-articular or oral steroids in the past 3 months or intra-articular hyaluronic acid in the last 9 months, parenteral use of NSAIDs, or requiring immediate surgery for the knee. Subjects with a history of major chronic hepatic, cardiovascular, neurological, or immunosuppressive conditions; infections; psychiatric conditions; risk of deep vein thrombosis, as depicted by a score of ≥3 on the Well’s questionnaire;8 pregnant or lactating women; or those who have recently participated in any clinical trial were also excluded.
Subjects with localized trauma or dermatological conditions affecting the lower limb, or history of skin irritation on application of bandages/tapes that could prohibit the use of tape application at the knee, were excluded.
Study design
A randomized, open-label, controlled, crossover design was chosen for the study. The subjects were randomized to receive either Acti-Tape followed by a knee guard for 6 days each or vice versa. A washout period of 1 day was observed between the two intervention periods, during which the subjects did not use any joint support. A one-day gap between treatments was considered adequate because no carryover of effect was expected. The outcome measures were recorded on days 0, 3, and 6 for the first intervention period, and on days 8, 11, and 14 for the second intervention period. For analysis, day 8 was considered as day 0, and day 14 as day 6, for the second period. Change was considered as the day 6 score minus day 0 score.
Interventions
Acti-Tape is an elastic therapeutic tape manufactured by Nutriworks Ltd and made up of a cotton (95%) and spandex (5%) mix with a patterned adhesive layer that has a specified elasticity of ≥50%. For the study, the tape was applied as a single strip just below the patella with the knee bent at 90°. The ends were rounded off to prevent easy accidental peeling (Figure 1). The knee guard used for the study was of a tubular form with an open patella and adjustable size, supplied by Gold Medal Sports Wholesalers Co, Tsuen Wan, Hong Kong. It is made up of foam rubber (90%) and nylon (10%). It is worn over the knee so as to align the patella opening with the knee cap and is strapped in place firmly by adjusting the velcro strips. The knee guard is made up of a neoprene-type material with a specified elasticity.
  | Figure 1 Instructions for application of Acti-Tape™ (Nutriworks Ltd, Kowloon, Hong Kong). |
Randomization
The master randomization chart with blocks of four was generated using statistical software (StatsDirect version 2.7.9; StatsDirect Ltd, Altrincham, UK). At day 0, subjects were randomized in the ratio 1:1 to receive either Acti-Tape or a knee guard by the study coordinator based on the next available number as per the randomization chart. No blinding procedures were applicable, as this was an open-label study.
Monitoring
Regular monitoring visits were carried out at the site to ensure the data quality and compliance to protocol and International Conference on Harmonisation Good Clinical Practice. For Acti-Tape, the subjects’ compliance to treatment was confirmed by the used tapes brought to the site. A clinical research co-coordinator appointed at the site contacted the subjects via telephone during the study to ensure that they adhered to the treatment completely.
Outcome measures
Outcome measures included pain VAS score, timed up and go (TUG) test, unilateral anterior reach (UAR) test, medial step down (MSD) test, and adverse events (AEs) assessed at day 0, day 3, and day 6 for the first intervention period, and day 8, day 11, and day 14 for the second intervention period. Subject’s preference was assessed at day 14 after the completion of both periods.
Pain VAS score
Subjects rated the severity of pain at the selected knee joint on a 100 mm VAS at each visit by placing a mark on the line at a point that represented the intensity of pain.
Timed up and go test
In this test the subjects were asked to sit correctly on a chair. A marker was placed on the floor 3 meters away from the chair. On the word “go” the subject stood up and walked on the line on the floor and then turned around and walked back to the chair. Subjects were asked to walk at a regular pace. This was repeated three times and the best time to complete the activity was recorded.9,10
Unilateral anterior reach test
Each individual (with hands positioned on the hips) was asked to extend a leg out as far as possible (while balancing on the opposite leg) over a standard tape measure while keeping the anterior foot close to the floor without touching. The distance between the toe of the balancing foot and the heel of the extended foot was measured. Three trials were given per assessment and the best score or the greatest distance was recorded.10
Medial step down test
An 11.4 cm step was used for performing this test. Each subject stepped down medially from the step until the heel of the front foot of the unaffected leg lightly touched the floor and then returned to starting position. The subjects were instructed to repeat this movement and the numbers of repetitions performed comfortably by each subject were recorded. That is, if the subject complained of any kind of pain in the knee joint, underwent any kind of physical stress, or felt uncomfortable he/she was allowed to discontinue the test. The number of step down movements were recorded. The subjects were not permitted to take any support during this test.10
Adverse events
AEs, especially skin irritation (inflammation, swelling, or redness) at the site of application, were recorded and graded by the investigator on a scale of 0–3 based on increasing severity, where 0 represented absence of the symptoms and 3 represented severe symptoms.
Subject’s preference
At the end of both intervention periods, the subjects were asked the following questions to assess their preference: 1) Between the tape and the knee guard, which one do you think is easy to use and more user friendly? and 2) Between the tape and knee guard, which one would you prefer for future use?
Statistics
As this was a pilot study, no statistical method was applied for calculation of sample size. An arbitrarily chosen sample size of twelve was considered appropriate to assess the effect of Acti-Tape. Considering a dropout rate of 10%, 14 subjects were planned to be recruited into the study to achieve 12 completed cases. Mean change in VAS score, TUG test, UAR test, MSD test, and vital parameters from day 0 to day 6 for the first intervention period and from day 8 to day 14 for the second intervention period were summarized as means and standard deviation by treatments and periods. When the interaction of period and treatments was not significant, the data were pooled for the treatments and overall effects. Within-group differences were analyzed using paired t-test, and between-group differences by Student’s t-test. McNemar’s paired χ2 test was used for analyzing categorical data from the subject’s preference. The level of significance was P≤0.05.
All the recruited subjects completed the entire study as per the protocol specified requirements. Analysis of safety and efficacy was done on the entire per protocol population.
Results
Disposition of subjects
The details of the subjects recruited and completing the study are represented in Figure 2. A total number of 20 subjects were contacted. Out of these, three did not wish to come for frequent follow-up visits as required for the study and four were not willing to provide informed consent. The remaining subjects were randomized and then assigned either to Acti-Tape (n=6) or a knee guard (n=7) for a period of 6 days (from day 0 to day 6). Day 7 was considered as washout day, after which the subjects were crossed over: ie, the subjects who were on Acti-Tape were now given a knee guard and vice versa. All the 13 subjects completed the study and their data analyzed.
  | Figure 2 Disposition of subjects. |
Baseline characteristics
The demographics and key baseline characteristics for the subjects recruited in the study are presented in Table 1.
  | Table 1 Baseline characteristics |
Efficacy
Table 2 presents the changes in the efficacy variables according to the treatments and intervention periods. No statistically significant differences were seen between the treatments and periods for any variable except for VAS score. VAS change was found to vary between periods for knee guard (P<0.05) but not for Acti-Tape. Hence, the data were pooled for all the subjects and analyzed using Student’s t-test. Table 3 presents the pooled data for all subjects according to the treatments.
Pain VAS score
Mean score reduced from 68.08±10.89 to 58.38±10.89 after using Acti-Tape and from 60.77±14.41 to 49.62±10.89 after using knee guard from baseline to end of treatment. This was found to be statistically significant (P<0.01) in both the groups.
Timed up and go test
A minimal reduction was seen in the mean time taken to complete the TUG test while using Acti-Tape or a knee guard from baseline to end of treatment, but was not statistically significant.
Unilateral anterior reach test
There was a statistically significant increase seen in the mean distance, as measured by the UAR test when using Acti-Tape (11.78±2.71 to 12.08±2.78; P<0.01), as well as when using a knee guard (10.92±2.75 to 11.67±2.67; P<0.05) from baseline to end of treatment.
Medial step down test
There was a statistically significant increase seen in the number of steps taken, as assessed by the MSD test from baseline to end of treatment when the subjects used Acti-Tape (10.46±3.40 to 11.54±2.99; P<0.01), as well as when they used a knee guard (10.69±2.66 to 11.54±2.63; P<0.05). An overall reduction of 16.61% and 17.75% was seen in the mean pain VAS score after using Acti-Tape and a knee guard, respectively. The changes seen in the timed up and go test, unilateral anterior reach test, and the medial step down tests were 4.06%, 3.65%, and 12.89%, respectively, after using Acti-Tape, and 2.12%, 7.61%, and 8.99%, respectively, after using a knee guard (Figure 4).
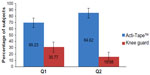  | Figure 3 Subject’s preference. |
Investigator’s global assessment
The investigator rated the improvement as good, fair, and poor, respectively, in 53.85%, 38.46%, and 7.69% of the subjects after using Acti-Tape, and good and fair, respectively, in 46.15% and 53.85% after using a knee guard. None was rated poor in the knee guard group.
Subject’s preference
A total of 69.23% of the subjects gave preference toward Acti-Tape with regard to its ease of application and comfort, whereas 30.77% of the subjects preferred the knee guard over Acti-Tape. A total of 84.62% of the subjects preferred Acti-Tape for future use, whereas 15.38% of the subjects preferred a knee guard over Acti-Tape for future use (Figure 3). The differences for future use were statistically significant (P=0.05) and in favor of Acti-Tape when analyzed by McNemar’s paired chi-square test.
Safety
Only one AE occurred in the entire study. A subject experienced redness over the left knee at the site where the knee guard was applied. This AE was mild in intensity and was resolved effectively. The investigator related the causality to the knee guard.
Discussion
Taping methods are used for providing support to muscles and joints. However, not much evidence exists in the scientific literature to support their use in managing musculoskeletal disorders.11 A few studies have assessed various taping methods in patients with plantar heel pain,12 shoulder pain,13 shoulder impingement,14,15 patellofemoral pain syndrome,16 and ankle proprioception,17 but have shown mixed results.
A clinical study conducted on 42 patients suffering with rotator cuff tendonitis/impingement using a kinesiotaping method demonstrated improvement in range of motion of shoulder joint as compared with a sham tape.13 This study found kinesiotape to be no more efficacious than sham tape at decreasing shoulder pain intensity or disability. However, positive effects of kinesiotape application were seen in the studies for plantar heel pain,14 shoulder impingement,14,15 and patellofemoral pain syndrome.16
The current study also did not show statistically significant changes when compared across the groups. This may be due to the small sample size. However, when the 95% confidence intervals for the change from baseline to end of treatment for both Acti-Tape and the knee guard were compared, it was seen that they overlapped each other for all the variables. Thus, it can be said that the effect of both Acti-Tape and the knee guard was similar; in other words, Acti-Tape provided benefits similar to those provided by a knee guard. However, the patients’ overall preference for future use, implying a combination of adequate relief and convenience, favored Acti-Tape significantly (P<0.05), which seems clinically important.
As in any open label study, this study may also have an element of both observer and responder bias as the assessments were performed with Acti-Tape or knee guard applied at the knee joint. In order to ensure reduction of this bias, objective study assessments (TUG, UAR, MSD) were used and were performed by a trained investigator.
A post hoc analysis for calculating the power of the study was carried out, considering the overall subject preference as the primary variable. Considering the null hypothesis that there is no difference in the preferences for the two, and a significance level of 0.05, the current study with 11 preferences for Acti-Tape and 2 for the knee guard had a power of 0.80 (80%). However, regarding the measured efficacy variables, standard deviation was high and the power ranged from 50% to 60%. Hence, if these variables are to be considered as the primary variables in the next study, it would require a much larger sample size to achieve statistical significance.
Acti-Tape being a non-prescription product, for the current study we decided to see if this could be used as an alternative to a commonly used knee guard. The results of this study are encouraging enough to warrant further exploration of the benefits in an adequately-powered, larger study using measured efficacy variables.
Conclusion
The present study demonstrates that Acti-Tape improves clinical outcomes of the joint similarly to a knee guard in subjects with degenerative changes of the knee joint. Acti-Tape was also preferred over a knee guard for future use by the subjects. Thus, Acti-Tape could be a good alternative to improve the quality of life of patients with painful knee joints and deserves further exploration.
Author contributions
Hoong Keong Hui and Navneet Sonawane were involved with the conception and design of the study and the analysis and interpretation of data. Narayan J Karne collected patient data. Navneet Sonawane drafted the manuscript, which was revised and approved by the other authors.
Disclosure
This study is registered on Clinical Trials Registry – India: CTRI/2013/02/003402. This study has been conducted by Vedic Lifesciences Pvt Ltd with the financial support of Nutriworks Ltd. Vedic Lifesciences Pvt Ltd is an independent research organization that is in no way related to Nutriworks Ltd nor has any financial interests in the results of the study. Hoong Keong Hui is the Director of Nutriworks Limited. The authors have no other conflicts of interest in this work.
References
Who gets osteoarthritis? [webpage on the Internet]. Atlanta, GA: Arthritis Foundation. Available from: http://www.arthritis.org/who-gets-osteoarthritis.php. Accessed January 23, 2014. | |
Osteoarthiritis health center [webpage on the Internet]. WebMD. Available from: http://www.webmd.com/osteoarthritis/guide/osteoarthritis-causes. Accessed January 23, 2014. | |
Degenerative joint disease [webpage on the Internet]. HealthCentral. Available from: http://www.healthcentral.com/encyclopedia/408/577.html. Accessed January 23, 2014. | |
What treatments are there for osteoarthritis of the knee? [webpage on the Internet]. Arthritis Research UK. Available from: http://www.arthritisresearchuk.org/arthritis-information/conditions/osteoarthritis-of-the-knee/treatments.aspx. Accessed January 23, 2014. | |
Kevin Lee. Do knee guards really help? [webpage on the Internet]. AsiaOne; Feb 2011. Available from: http://yourhealth.asiaone.com/content/do-knee-guards-really-help/. Accessed June 14, 2013. | |
Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F. The American College of Reumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992;35(5):498–502. | |
Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. | |
Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA. 2006;295(2):199–207. | |
Kulkarni MP, Shakeel A, Shinde BS, Rosenbloom RA. Efficacy and safety of E-OA-07 in moderate to severe symptoms of osteoarthritis: a double blind randomized placebo-controlled study. Am J Ther. 2011;18(2):170–177. | |
Kraemer WJ, Ratamess NA, Maresh CM, et al. A cetylated fatty acid topical cream with menthol reduces pain and improves functional performance in individuals with arthritis. J Strength Cond Res. 2005;19(2):475–480. | |
Morris D, Jones D, Ryan H, Ryan CG. The clinical effects of Kinesio® Tex taping: A systematic review. Physiother Theory Pract. 2013;29(4):259–270. | |
Radford JA, Landorf KB, Buchbinder R, Cook C. Effectiveness of low-Dye taping for the short-term treatment of plantar heel pain: a randomised trial. BMC Musculoskelet Disord. 2006;7:64. | |
Thelen MD, Dauber JA, Stoneman PD. The clinical efficacy of kinesio tape for shoulder pain: a randomized, double-blinded, clinical trial. J Orthop Sports Phys Ther. 2008;38(7):389–395. | |
Kaya E, Zinnuroglu M, Tugcu I. Kinesio taping compared to physical therapy modalities for the treatment of shoulder impingement syndrome. Clin Rheumatol. 2011;(30):201–207. | |
Host HH. Scapular taping in the treatment of anterior shoulder impingement. Phys Ther. 1995;75(9):803–812. | |
Lowry CD, Cleland JA, Dyke K. Management of patients with patellofemoral pain syndrome using a multimodal approach: a case series. J Othop Sports Phys Ther. 2008;38(11):691–702. | |
Halseth T, McChesney W, DeBeliso M, Vaughn R, Lien J. The effects of kinesiotaping on proprioception at the ankle. J Sports Sci Med. 2004;3:1–7. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.