Back to Journals » Patient Related Outcome Measures » Volume 6
Achieving optimal delivery of follow-up care for prostate cancer survivors: improving patient outcomes
Authors Hudson S, O'Malley D, Miller S
Received 2 December 2014
Accepted for publication 4 February 2015
Published 19 March 2015 Volume 2015:6 Pages 75—90
DOI https://doi.org/10.2147/PROM.S49588
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Robert Howland
Shawna V Hudson,1 Denalee M O’Malley,2 Suzanne M Miller3
1Department of Family Medicine and Community Health, Rutgers Robert Wood Johnson Medical School, Somerset, 2Rutgers School of Social Work, New Brunswick, NJ, 3Cancer Prevention and Control Program, Fox Chase Cancer Center/Temple University Health System, Philadelphia, PA, USA
Background: Prostate cancer is the most commonly diagnosed cancer in men in the US, and the second most prevalent cancer in men worldwide. High incidence and survival rates for prostate cancer have resulted in a large and growing population of long-term prostate cancer survivors. Long-term follow-up guidelines have only recently been developed to inform approaches to this phase of care for the prostate cancer population.
Methods: A PubMed search of English literature through August 2014 was performed. Articles were retrieved and reviewed to confirm their relevance. Patient-reported measures that were used in studies of long-term prostate cancer survivors (ie, at least 2 years posttreatment) were reviewed and included in the review.
Results: A total of 343 abstracts were initially identified from the database search. After abstract review, 105 full-text articles were reviewed of which seven met inclusion criteria. An additional 22 articles were identified from the references of the included articles, and 29 were retained. From the 29 articles, 68 patient-reported outcome measures were identified. The majority (75%) were multi-item scales that had been previously validated in existing literature. We identified four main areas of assessment: 1) physical health; 2) quality of life – general, physical, and psychosocial; 3) health promotion – physical activity, diet, and tobacco cessation; and 4) care quality outcomes.
Conclusion: There are a number of well-validated measures that assess patient-reported outcomes that document key aspects of long-term follow-up with respect to patient symptoms and quality of life. However, there are fewer patient-reported outcomes related to health promotion and care quality within the prevention, surveillance, and care coordination components of cancer survivorship. Future research should focus on development of additional patient-centered and patient-related outcomes that enlarge the assessment portfolio.
Keywords: prostate cancer, patient-reported outcomes, follow-up care, cancer survivorship, systematic review
Introduction
Prostate cancer is the second most commonly diagnosed cancer globally.1 As of January 1, 2012, the prostate cancer survivor population in the US approached 2.8 million survivors, approximately 43% of the entire male population of Americans with a history of cancer.2 Approximately 62% are aged 70 and older3 with multiple comorbid conditions. Further, the population of prostate cancer survivors has been steadily growing in the past 10 years4 and increases by an estimated 200,000 men annually.2 Prostate cancer rates vary widely worldwide with the highest incidence rates in North America and Europe attributed in large part to the diagnostic practice of prostate-specific antigen (PSA) testing at the population level.
In the last decade, both prostate cancer screening and optimal treatments for early-stage prostate cancer have been the subject of intense debate.5–7 In 2011, in response to accumulating data, both the US Preventive Services Task Force and the European Association of Urology recommended against the use of PSA-based testing for cancer screening, indicating with moderate-to-high certainty that this service was not beneficial and discouraged its clinical use in the general population.6,8 In part, this response was due to lack of sensitivity of PSAs to discriminate indolent, slow-growing prostate cancers from those associated with increased morbidity and mortality,1,9,10 as well as the psychosocial toll caused by false positives. The absence of evidence demonstrating clear benefits regarding the timing and types of treatments available on outcome11 has increased patient and provider uncertainty regarding whether men should be screened and if diagnosed, what treatments to pursue.12
Regardless of existing screening recommendations, the number of prostate cancer survivors continues to increase.2,13 In the US, over 90% of prostate cancers are diagnosed as localized, early-stage cancers, and the 5-year relative survival rate for patients with local disease is nearly 100%.14,15 Treatment approaches range from noninvasive expectant approaches (active surveillance or watchful waiting) to radiologic therapies (radiotherapy, external beam radiologic therapy, brachytherapy, cryoablation), aggressive surgical procedures (open radical prostatectomy, laparoscopic prostatectomy with or without robotic assistance), and hormonal treatments (primary androgen deprivation therapy). Most localized prostate cancer patients choose to undergo active treatment rather than expectant strategies; the most common treatment pathways include either radical prostatectomy or radiotherapy.16 Regardless of treatment type,17–20 the survival benefits are high, including active surveillance,21 though the late and long-term effects associated with each treatment vary. Symptomology in the majority of cases includes physical complications (ie, urinary, bowel, and sexual dysfunction), which are associated with long-term psychosocial morbidity.22,23 The type of symptom profile may require different medical and follow-up care strategies and interventions24 as well as a risk-based care approach.25,26
Until recently, clinical guidelines for the care of men with prostate cancer from leading organizations such as the National Comprehensive Cancer Center Network, American Society of Clinical Oncology and American Urological Association, and American Society for Radiation Oncology focused almost solely on prostate cancer screening and treatment with little attention to the survivorship phase of care.27 However, as patients move from active treatment into cancer survivorship and follow-up care, there are a number of physical and psychosocial factors related to prostate cancer treatment that continue to need management. In July 2014, the American Cancer Society released a set of prostate cancer survivorship guidelines to guide this phase of care.13 A multidisciplinary group of experts was convened to evaluate and consolidate findings into clinical recommendations to address follow-up care based on 1) level of evidence, 2) consistency across studies, 3) dose response (for radiologic therapy findings), 4) racial disparities with potential to impact survivorship, and 5) second primary cancers.13 The resulting guidelines cover health promotion (ie, informational needs, obesity, physical activity, nutrition, and smoking cessation), surveillance for recurrence, screening for second primary cancers, assessment and management of physical and psychosocial (ie, anemia, cardiovascular/metabolic effects, distress, anxiety/depression, fracture risk/osteoporosis, urinary dysfunction, and vasomotor symptoms), and care coordination.
Given the need to systematically assess prostate cancer patient-centered and patient-reported outcomes that are relevant to the post-acute treatment phase of follow-up care, it is critical to evaluate resources for assessing these outcomes. Many measures that are used currently have been developed to assess patient-reported outcomes for the active treatment phase or the earlier phases of survivorship. While there are a number of systematic reviews10,11,28–31 that address prostate cancer from the screening and treatment perspectives, there are none, to our knowledge, that focus on the posttreatment and follow-up care phases. Therefore, the goal of this systematic review is to examine the current research literature focused on extended follow-up care to 1) document relevant studies and measures that could be used to delineate the prostate cancer patient-centered and patient-reported outcomes available, 2) assess the state of the science in terms of these measures, and 3) document the gaps in the literature where additional appropriate survivorship measures need to be developed.
Methods
We searched the PubMed database which comprises more than 24 million citations for biomedical literature from MEDLINE, life science journals, and online books (http://www.ncbi.nlm.nih.gov/pubmed). The initial search was conducted in February 2014 and updated in August 2014 after the issuance of the American Cancer Society Prostate Cancer Survivorship Care Guidelines.13 We used Endnote X7 reference software to conduct searches and manage references and the article database. Time frame for the search was not limited. Terms used to generate articles for analysis in the searches included prostate cancer and the following: “follow-up”, “surveillance”, “monitoring”, “late effects”, “long-term effects”, “survivorship”, “quality of life”, “health-related quality of life”, “care coordination”, “care management”, “management”, “sexual side effects”, and “incontinence”.
All references and abstracts were downloaded into the Endnote X7 reference software library by two undergraduate research interns. All potentially eligible study abstracts were reviewed by two undergraduate research interns who were trained and supervised by SVH and DMO, a cancer prevention and control researcher and oncology social worker who had highly relevant research and clinical expertise. The coders received intensive training which consisted of reading a subset of abstracts and articles together, coding the articles for relevance based on criteria established by SVH and DMO, discussing interpretations of code definitions, and refining code definitions to maximize coder agreement. Once coders were clear about the inclusion and exclusion parameters, the coders were given access to all of the initial abstracts. All abstracts were reviewed independently by the two coders for relevance and fit with the review inclusion criteria: 1) original research; 2) focused on posttreatment follow-up care rather than active treatment or continued treatment of prostate cancer; 3) patient treatment was at least 2 years ago; and 4) focus on patient outcomes such as therapy, surgical, and/or quality of life (QOL). Articles were excluded from the analysis if they 1) were not published in English, 2) were solely qualitative and did not evaluate patient treatment outcomes, 3) assessed surgical equipment, 4) focused solely on screening, and/or 5) were not focused on treatment follow-up. Disagreements in classification of the articles were discussed and resolved by SVH and DMO.
After the research interns reviewed each reference and abstract to select for review, they classified articles based on relevance and type of outcome. Using the groups feature in EndnoteX7, references were classified and coded as irrelevant if they included no patient outcomes, focused on surgical methods or outcomes or treatment toxicity, focused on survival outcomes, and/or focused on patients who had finished active treatment less than 2 years ago. Full text of articles that were assessed as relevant based on the initial review of their abstracts was uploaded into Endnote X7 for further review and analysis by SVH and DMO. A second round of review were conducted for the remaining selected for full-text review. The same exclusion criteria were applied to the full text analyses. A total of seven articles remained that fit the inclusion criteria. In July 2014, the American Cancer Society issued Prostate Cancer Survivorship Care Guidelines.13 The bibliography of this document as well as four additional systematic reviews32–35 cited in the guideline on prostate cancer treatment was mined for additional relevant articles resulting in the final analytic dataset.
Results
Figure 1 presents the process used to identify, screen, and select articles for inclusion in this review. The search terms initially yielded a total number of 343 references. The research interns reviewed each reference and abstract to select for review. Articles were organized by relevance and type of outcome. A total of 128 references were excluded based on the abstract review because of their lack of focus on patient outcomes or a limited focus on surgical outcomes or reporting of new surgical techniques, prostate cancer survival outcomes, active treatment toxicity, or focus on the active treatment period for prostate cancer. Several articles were classified in multiple categories; therefore, the number of articles reported in each subgroup exceeded the total number that were excluded. A total of 105 full-text articles were gathered and uploaded into Endnote X7. After the full text review, an additional 98 were excluded because the review of the text indicated that the focus of the articles was on patients in active prostate cancer treatment, were clinical reviews, or were epidemiological studies of the treatment profile of prostate cancer patients. At the conclusion of full text review, seven articles were retained. The American Cancer Society Prostate Cancer Survivorship Care Guidelines were also examined as a source of references in August 2014.13 The bibliography of this document as well as four additional systematic reviews32–35 cited in the guideline on prostate cancer treatment was mined for additional relevant articles resulting in the final analytic dataset. Review of the bibliographies yielded an additional 22 articles that met inclusion criteria for extraction. Therefore, a total of 29 articles were included in this review.
Most studies used survey and patient self-report measures to assess patient-centered and patient-reported outcomes for prostate cancer survivor populations (Table 1). Only four studies surveyed partners or spouses.36–39 Studies were conducted using multiple design strategies including using population-based cohorts24,40–46 and clinical-based37–39,47–58 populations as well as one community-based study36 and one multinational clinical trial.59 A number of studies used cohort designs and assessed outcomes in either cross-sectional or prospective, longitudinal study designs. Most studies focused on prostate cancer patients (72%, n=21) in contrast with 28% (n=8) that included prostate cancer patients as a subgroup within a larger study of cancer patients.
  | Table 1 Articles retained for their patient-reported measures related to prostate cancer follow-up care (N=29) |
For the purpose of this review, patient-centered and patient-reported outcome measures from the studies have been categorized into several groups based on the initial sorting of studies (Table 2). We identified four main areas of assessment: 1) physical health including treatment outcomes from surgery, radiologic therapies, hormone, or expectant management treatments; 2) QOL – general, physical (ie, urinary, bowel, sexual), and psychosocial (ie, mental health/emotional well-being and couples/partner focused); 3) health promotion – physical activity and diet; and 4) patient-reported care quality outcomes. Approximately 75% of studies used validated, multi-item scales that had been previously validated in existing literature to assess patient-reported outcomes.
  | Table 2 Identified patient-reported measures related to prostate cancer follow-up care (n=68 discrete measures) |
Physical health
Physical health is an important patient-centered outcome for prostate cancer survivors, many of whom struggle with comorbid conditions as well as late and long-term effects of the disease and its treatment.13 In the studies reviewed, there were 20 measures identified to assess physical health among prostate cancer survivors. The most commonly cited measures were the Medical Outcomes Study Short Form versions of the SF-3646,51,55,56,59 including a Dutch version60 and the SF-1236,37,39,48,51 to assess physical health, emotional health, and general health. The Expanded Prostate Cancer Index Composite (EPIC-26)24,37,60,53,61 followed as the second most cited measure. With the exception of two self-reported health single-item measures,43,45 the scales used in these studies were well-documented, well-validated scales that are used often in research studies. Of note, however, is that few studies included measures that provide data on or document comorbid health conditions.
Quality of life
QOL was the construct with the most robust measurement in terms of patient-centered and patient-reported outcomes. Three domains of QOL were measured across the studies – general, physical, and psychosocial.
General QOL was most often measured using the SF-3646,51,55,56,59,60 and SF-12.36,37,39,48,51 Additional general measures included the European Organization for Research and Treatment of Cancer core Questionnaire (EORTC QLQ-30; a global QOL scale),56 the Functional Assessment of Cancer Therapy – General,37,38 the Functional Assessment of Cancer Therapy – Prostate (FACT-P),37,38 the Patient Oriented Prostate Utility Scales (PORPUS),52 and a Dutch version of the Quality of Life – Cancer Survivors (QOL-CS).60
Physical QOL measures were found to relate to three key areas of physical functioning associated with prostate cancer (ie, urinary, bowel, sexual). Urinary and bowel issues were often measured in tandem. The most commonly used measures were the Expanded Prostate Cancer Index Composite (EPIC-26; urinary, 12 items; bowel, 14 items)24,37,42,48,61,62 followed by the University of California, Los Angeles Prostate Cancer Index (urinary function/bother, six items; bowel function/bother, five items)24,54,55,63 and the American Urological Association Symptom Index (bladder storage and urinary symptoms).55,56,64 Sexuality was addressed in terms of sexual function and sexual bother. The International Index of Erectile Function (IIEF-15)47,52,57,58 and a five-item abridged version of the IIEF-15 entitled the Sexual Health Inventory for Men (SHIM)47,57,58 were used most often to assess sexual function and bother. In addition to these measures, subscales of the Expanded Prostate Cancer Index Composite (EPIC-26)24,37,42,61 and the UCLA Prostate Cancer Index54,55,63 were also used to assess sexual functioning.
Psychosocial QOL measures including mental health and emotional well-being and couples-/partner-focused QOL were assessed fairly comprehensively across the studies. A total of 20 measures were included in the studies that assessed mental health/emotional well-being. Again, the Medical Outcomes Study Short Form versions of the SF-3646,55,56 and the SF-1237,48 were the most widely used measures to address emotional health. A total of eight measures of couples-related QOL were found in studies. The most often cited measures were the Spouse Expanded Prostate Cancer Index Composite (S-EPIC),36,37,39 a six-item scale that was referenced in three studies, and the four-item Dyadic Adjustment Scale (DAS-4).36,39
Health promotion
The American Cancer Society prostate cancer survivorship guidelines have established health promotion guides for prostate cancer survivors around nutrition, physical activity, alcohol consumption, and tobacco use. They counsel that survivors should achieve and maintain a healthy weight through diet and physical activity. We found several studies that addressed these health promotion and behavioral outcomes.45,50,59 Physical and dietary assessments used in the studies were validated.45,50,59 The tobacco use items were fielded as part of the Centers for Disease Control’s Behavioral Risk Factor Surveillance System (BRFSS) Survey.45
Care quality outcomes
In contrast with the other areas, the care quality measures were not as well developed or disseminated. There were nine measures documented in studies. Two were confirmed as multi-item scales.46,65 The others were mainly single-item measures and author constructed. There were little data available on the psychometric properties of these measures.
In summary, the areas of physical health and QOL have a number of well-established, validated measures to choose from for patient-centered and patient-reported outcomes research. There are, however, far fewer resources available for assessing health promotion and care quality patient-centered and patient-reported outcomes for prostate cancer survivorship and long-term follow-up.
Discussion
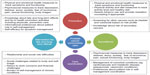
To our knowledge, this paper presents the first synthesis of the existing literature on prostate cancer patient-centered and patient-reported outcomes for follow-up care. In this review, we identified 68 patient-reported outcome measures that have been used in studies of prostate cancer follow-up care. While we reviewed a number of measures available for assessing both QOL and physical health in terms of functioning and symptom management, there were far fewer measures that addressed other key components of cancer survivorship. Figure 2 presents a schema of the recommended components of prostate cancer follow-up care with summaries of the content areas for which there are current measures, as well as highlights areas for which there are fewer resources.
In the areas of prevention and surveillance, the current review identified a number of measures (eg, SF-36, SF-12, EPIC-26) in the domains of physical health and QOL that allow patients to comprehensively track symptoms with regard to multiple dimensions of physical and emotional health. As well, several health promotion measures related to diet, nutrition, physical activity,45,50,59 and tobacco use were identified.45,50,59 However, lacking in this individualized tracking of symptoms and activities was an organizing framework for collating patient-centered and patient-related outcomes data into a comprehensive, health services approach to health promotion. This is one of the foremost challenges to providing optimal prostate cancer follow-up care. As currently constructed, patient-centered and patient-related outcomes provide comprehensive measures of discrete systems (eg, bowel and urinary) and acute and chronic treatment-related symptoms. To be optimally effective, these outcomes need to be examined and explored in context with comorbid diseases including conditions that patients are managing in addition to a risk-stratified cancer survivorship context.25,26 Such an approach would focus on prostate cancer survivors’ cancer-related risk profiles as well as other health considerations that put them at risk for new and secondary cancers and might exacerbate ongoing late and anticipated long-term treatment effects. A risk-stratified approach to management of these and other comorbid conditions would prioritize various screenings for prevention and health promotion. This is an area in which development of additional patient-centered and patient-reported outcomes that focus on providing such risk stratification would benefit both patients and their providers.
In the survivorship domains of surveillance, care coordination, and intervention for late and long-term effects, relevant QOL measures were identified. Psychosocial measures that address patient issues related to depression, distress, worry, anxiety, fear of recurrence, sexual functioning, and body image were documented. Similarly, physical symptom tracking related to various treatment modalities was well documented. However, management of comorbid conditions, which is a particular concern for the prostate cancer survivorship experience, was largely unaddressed by the current measures. There are unfolding health conditions that plague prostate cancer survivors, including cardiovascular and metabolic disease, osteoporosis, and vasomotor conditions.13 Many of these conditions, as well as those that pre-date their prostate cancer, require patients to play an active role in self-management to supplement clinician monitoring. To be effective and active in their care, patients need to 1) be knowledgeable about late and long-term effects and how they are tied to health promotion activities such as general physicals and prevention care, 2) have a sense of self-efficacy to manage lingering and new symptoms that are related to previous prostate cancer treatments, and 3) be able to process health promotion information to engage effectively in a self-management role in their cancer survivorship.66,67 For example, the American Cancer Society prostate cancer survivorship guideline13 suggests that screening for bladder and colorectal cancer be implemented for certain subpopulations of prostate cancer survivors based on their treatment profile. Data from our previous studies of cancer survivorship in this population66,67 suggest that survivors’ knowledge about the risks of secondary cancers, as well as late and long-term effects of cancer treatment, is variable with few men having a comprehensive understanding of the range of issues. Additional measures are needed to assess and address these central components of cancer survivorship.
Two final areas in which there are needs for further development of patient-centered and patient-related outcomes include the changes in social roles that come about as part of extended prostate cancer survivorship and assessing care quality from a cancer survivorship perspective. While there are measures of social role and dyadic relationship difficulties in the current literature, social challenges related to body image and self-image, their impact on returning to work, and the financial burden of prostate cancer survivorship are not comprehensively addressed. Similarly, available care quality measures related to follow-up care and cancer survivorship are not as well developed or disseminated as the physical functioning and QOL measures. These are critical gaps in the measurement portfolio that require future research.
The purpose of this review was to identify studies and measures that utilize prostate cancer patient-centered and patient-reported outcomes, to assess the evidence base supporting these measures, and to document the gaps in the literature. Therefore, it is beyond the scope of this study to recommend or advocate for the use of specific measures. We cannot objectively assess which measures may be better or ill suited for future research in long-term survivors for two reasons. First, one size does not fit all. Depending on the study context and level of focus on patient, provider, clinical practice, and/or health system targets, a survey instrument or clinical measure that performs well in one setting as a patient-centered or patient-reported outcome measure may be ill suited in another. Second, the empirical measures identified in this study suggest that while some areas (eg, symptoms from treatment and associated distress) are well developed, other significant domains of the patient experience of survivorship are lacking. The empirical database of patient-centered and patient-reported outcomes among prostate survivors needs to be further developed before an effective evaluation of which measures are better suited for future research can be effectively conducted.
There are four limitations of this review that should be noted. First, the focus on prostate cancer survivorship is novel as an area of focus with respect to patient-centered and patient-related outcome measures. The concept of extended survivorship and examining the emerging and growing needs of survivors who are not currently receiving active treatment for prostate cancer is novel. Therefore, it was difficult to identify studies for this review. Prior studies and reviews centered on active treatment and the transition period immediately following active treatment as key foci for intervention. We chose to limit our search of studies to those that included populations that were at least 2 years post-active treatment so that studies reported comprised long-term survivor populations. Most of the studies that met these criteria were studies that were included in the American Cancer Society Prostate Cancer Survivorship Care Guidelines. It is possible that had we chosen a different benchmark that additional studies may have been included in the review, and the resulting domain mapping of content areas may have been different. Second, only English language studies were included in the review; therefore, there is a possibility that there may have been additional studies that were not conducted in English that may be relevant. Third, the majority of the samples reviewed comprised primarily White and Caucasian men. While there was one study that focused on racial differences,44 it is not clear that the patient-centered and patient-reported outcomes were the most relevant to ethnic and racial minority men. Finally, there is the possibility that an article or abstract may have been inadvertently excluded during abstract reviews. However, our method of having abstracts triaged by two pairs of research assistant reviewers followed by additional reviews from two senior investigators significantly decreased this possibility.
Conclusion
The studies reviewed reflect variation in setting, design, and measurement of patient-centered and patient-reported outcomes and are informative about the state of the science in prostate cancer follow-up care. A major conclusion is that there is little research focused on long-term follow-up care and patient-centered outcomes for prostate cancer patients; therefore, future research needs to focus on adding to the available measurement and evidence bases of patient-centered and patient-reported outcomes. While the current measures are largely well established and well validated in the physical health areas of prevention and surveillance as well as QOL, there are fewer measures that comprehensively address factors that impact survivorship care, notably: 1) patient knowledge about late and long-term treatment effects and ability to link this knowledge to health promotion activities including physicals and prevention care; 2) patient informational preferences regarding patient role in self-management; 3) self-efficacy expectations about symptom management skills; 4) the challenge of self-management of chronic conditions; and 5) social challenges related to body image and self-image as well as work concerns and financial challenges. Development of these tools will provide providers, patients, and other health care stakeholders with basic patient-related outcomes to better track long-term cancer survivorship progress and strategies.
Acknowledgments
This study was partially supported through a career development award to Dr Hudson (K01 CA131500) from the National Cancer Institute and a Doctoral Training Grant in Oncology Social Work (DSW 13-279-01) from the American Cancer Society awarded to Ms O’Malley. It was additionally supported by National Cancer Institute grants R01 CA176838 and R01 CA158019 awarded to Drs Hudson and Miller and Cancer Center Support Grants P30 CA072720 and P30 CA06927 to the Rutgers Cancer Institute of New Jersey and Fox Chase Cancer Center. We thank our research interns Andrew Barra, Herman Chadha, Cara Kubinak, and Veronica Vargas for their assistance and attention to detail while screening abstracts.
Disclosure
None of the authors have any potential conflicts of interest to disclose.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | |
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistic, 2009. Cancer J Clin. 2009;59:225–249. | |
Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–241. | |
Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010. | |
Basch E, Oliver TK, Vickers A, et al. Screening for prostate cancer with prostate-specific antigen testing: American Society of Clinical Oncology provisional clinical opinion. J Clin Oncol. 2012;30(24):3020–3025. | |
Heidenreich A, Bellmunt J, Bolla M, et al; European Association of Urology. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59(1):61–71. | |
Bill-Axelson A, Holmberg L, Ruutu M, et al; SPCG-4 Investigators. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364(18):1708–1717. | |
Moyer VA. Screening for prostate cancer: US preventive services task force recommendation statement. Ann Intern Med. 2012;157(2):120–134. | |
Ilic D, O’Connor D, Green S, Wilt T. Screening for prostate cancer: a Cochrane systematic review. Cancer Causes Control. 2007;18(3):279–285. | |
Ilic D, O’Connor D, Green S, Wilt TJ. Screening for prostate cancer: an updated Cochrane systematic review. BJU Int. 2011;107(6):882–891. | |
Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435–448. | |
Aizer AA, Paly JJ, Michaelson MD, et al. Medical oncology consultation and minimization of overtreatment in men with low-risk prostate cancer. J Oncol Pract. 2014;10(2):107–112. | |
Skolarus TA, Wolf AM, Erb NL, et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin. 2014;64(4):225–249. | |
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. | |
Howlander N, Noone A, Krapcho M, Neyman N, Aminou R, Alterkuse S. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations). Bethesda, MD: National Institutes of Health; 2012. | |
Cooperburg MR, Broering JM, Carroll PR. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J Natl Cancer Inst. 2009;101(12):878–887. | |
Alicikus ZA, Yamada Y, Zhang Z, et al. Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer. 2011;117(7):1429–1437. | |
D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy or external beam radiation therapy for patients with clinically localized prostate carcinoma in the prostate specific antigen era. Cancer. 2002;95(2):281–286. | |
Birkhahn M, Penson DF, Cai J, et al. Long-term outcome in patients with a Gleason score ≤6 prostate cancer treated by radical prostatectomy. BJU Int. 2011;108(5):660–664. | |
Lowrance WT, Elkin EB, Jacks LM, et al. Comparative effectiveness of prostate cancer surgical treatments: a population based analysis of postoperative outcomes. J Urol. 2010;183(4):1366–1372. | |
Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009; 302(11):1202–1209. | |
Eton DT, Lepore SJ, Helgeson VS. Early quality of life in patients with localized prostate carcinoma: an examination of treatment-related, demographic, and psychosocial factors. Cancer. 2001;92(6):1451–1459. | |
Katz A. Quality of life for men with prostate cancer. Cancer Nurs. 2007;30(4):302–308. | |
Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368(5):436–445. | |
McCabe MS, Bhatia S, Oeffinger KC, et al. American Society of Clinical Oncology statement: achieving high-quality cancer survivorship care. J Clin Oncol. 2013;31(5):631–640. | |
McCabe MS, Partridge AH, Grunfeld E, Hudson MM. Risk-based health care, the cancer survivor, the oncologist, and the primary care physician. Semin Oncol. 2013;40(6):804–812. | |
Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, version 3.2012 featured updates to the NCCN guidelines. J Natl Compr Cancer Network. 2012;10(9):1081–1087. | |
Chou R, Croswell JM, Dana T, et al. Screening for prostate cancer: a review of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2011;155(11):762–771. | |
Djulbegovic M, Beyth RJ, Neuberger MM, et al. Screening for prostate cancer: systematic review and meta-analysis of randomised controlled trials. BMJ. 2010;341:c4543. | |
Haseen F, Murray LJ, Cardwell CR, O’Sullivan JM, Cantwell MM. The effect of androgen deprivation therapy on body composition in men with prostate cancer: systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):128–139. | |
Dall’Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012; 62(6):976–983. | |
Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):242–274. | |
Cappelleri JC, Rosen RC. The Sexual Health Inventory for Men (SHIM):a 5-year review of research and clinical experience. Int J Impot Res. 2005;17(4):307–319. | |
Singh J, Trabulsi EJ, Gomella LG. The quality-of-life impact of prostate cancer treatments. Curr Urol Rep. 2010;11(3):139–146. | |
Weber BA, Sherwill-Navarro P. Psychosocial consequences of prostate cancer: 30 years of research. Geriatr Nurs. 2005;26(3):166–175. | |
Harden J, Sanda MG, Wei JT, et al. Survivorship after prostate cancer treatment: spouses’ quality of life at 36 months. Oncol Nurs Forum. 2013;40(6):567–573. | |
Northouse LL, Mood DW, Montie JE, et al. Living with prostate cancer: patients’ and spouses’ psychosocial status and quality of life. J Clin Oncol. 2007;25(27):4171–4177. | |
Northouse LL, Mood DW, Schafenacker A, et al. Randomized clinical trial of a family intervention for prostate cancer patients and their spouses. Cancer. 2007;110(12):2809–2818. | |
Harden JK, Sanda MG, Wei JT, et al; PROSTQA Consortium Study Group. Partners’ long-term appraisal of their caregiving experience, marital satisfaction, sexual satisfaction, and quality of life 2 years after prostate cancer treatment. Cancer Nurs. 2013;36(2):104–113. | |
Chen RC, Clark JA, Talcott JA. Individualizing quality-of-life outcomes reporting: how localized prostate cancer treatments affect patients with different levels of baseline urinary, Bowel, and Sexual Function. J Clin Oncol. 2009;27(24):3916–3922. | |
Fowler FJ Jr, Barry MJ, Lu-Yao G, Wasson J, Roman A, Wennberg J. Effect of radical prostatectomy for prostate cancer on patient quality of life: results from a Medicare survey. Urology. 1995;45(6):1007–1015. | |
Darwish-Yassine M, Berenji M, Wing D, et al. Evaluating long-term patient-centered outcomes following prostate cancer treatment: findings from the Michigan Prostate Cancer Survivor study. J Cancer Surviv. 2014;8(1):121–130. | |
Eakin EG, Youlden DR, Baade PD, et al. Health status of long-term cancer survivors: results from an Australian population-based sample. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1969–1976. | |
Johnson TK, Gilliland FD, Hoffman RM, et al. Racial/ethnic differences in functional outcomes in the 5 years after diagnosis of localized prostate cancer. J Clin Oncol. 2004;22(20):4193–4201. | |
Linsky A, Nyambose J, Battaglia TA. Lifestyle behaviors in Massachusetts adult cancer survivors. J Cancer Surviv. 2011;5(1):27–34. | |
McInnes DK, Cleary PD, Stein KD, Ding L, Mehta CC, Ayanian JZ. Perceptions of cancer-related information among cancer survivors: a report from the American Cancer Society’s Studies of Cancer Survivors. Cancer. 2008;113(6):1471–1479. | |
Raina R, Agarwal A, Goyal KK, et al. Long-term potency after iodine-125 radiotherapy for prostate cancer and role of sildenafil citrate. Urology. 2003;62(6):1103–1108. | |
Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772–2780. | |
Burnet KL, Parker C, Dearnaley D, Brewin CR, Watson M. Does active surveillance for men with localized prostate cancer carry psychological morbidity? BJU Int. 2007;100(3):540–543. | |
Avery KN, Donovan JL, Gilbert R, et al. Men with prostate cancer make positive dietary changes following diagnosis and treatment. Cancer Causes Control. 2013;24(6):1119–1128. | |
Reeve BB, Chen RC, Moore DT, et al. Impact of comorbidity on health-related quality of life after prostate cancer treatment: combined analysis of two prospective cohort studies. BJU Int. 2014;114(6b):E74–E81. | |
Santa Mina D, Guglietti CL, Alibhai SM, et al. The effect of meeting physical activity guidelines for cancer survivors on quality of life following radical prostatectomy for prostate cancer. J Cancer Surviv. 2014;8(2):190–198. | |
Schroeck FR, Krupski TL, Sun L, et al. Satisfaction and regret after open retropubic or robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2008;54(4):785–793. | |
Gacci M, Simonato A, Masieri L, et al. Urinary and sexual outcomes in long-term (5+ years) prostate cancer disease free survivors after radical prostatectomy. Health Qual Life Outcomes. 2009;7(1):94. | |
Gore JL, Kwan L, Lee SP, Reiter RE, Litwin MS. Survivorship beyond convalescence: 48-month quality-of-life outcomes after treatment for localized prostate cancer. J Natl Cancer Inst. 2009;101(12):888–892. | |
Roeloffzen EM, Hinnen KA, Battermann JJ, et al. The impact of acute urinary retention after iodine-125 prostate brachytherapy on health-related quality of life. Int J Radiat Oncol Biol Phys. 2010;77(5):1322–1328. | |
Raina R, Lakin MM, Agarwal A, Ausmundson S, Montague DK, Zippe CD. Long-term intracavernous therapy responders can potentially switch to sildenafil citrate after radical prostatectomy. Urology. 2004;63(3):532–537. | |
Raina R, Lakin MM, Agarwal A, et al. Long-term effect of sildenafil citrate on erectile dysfunction after radical prostatectomy: 3-year follow-up. Urology. 2003;62(1):110–115. | |
Demark-Wahnefried W, Morey MC, Sloane R, et al. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J Clin Oncol. 2012;30(19):2354–2361. | |
Mols F, Thong MS, Vreugdenhil G, van de Poll-Franse LV. Long-term cancer survivors experience work changes after diagnosis: results of a population-based study. Psychooncology. 2009;18(12):1252–1260. | |
Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56(6):899–905. | |
Szymanski KM, Wei JT, Dunn RL, Sanda MG. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology. 2010;76(5):1245–1250. | |
Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Brook RH. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36(7):1002–1012. | |
Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549–1557. Discussion 1564. | |
Sanson-Fisher R, Girgis A, Boyes A, et al; Supportive Care Review Group. The unmet supportive care needs of patients with cancer. Cancer. 2000;88(1):226–237. | |
Hudson SV, Miller SM, Hemler J, et al. Adult cancer survivors discuss follow-up in primary care: ‘not what i want, but maybe what i need’. Ann Fam Med. 2012;10(5):418–427. | |
Hudson SV, Miller SM, Hemler J, et al. Cancer survivors and the patient-centered medical home. Transl Behav Med. 2012;2(3):322–331. | |
Pirl WF, Siegel GI, Goode MJ, Smith MR. Depression in men receiving androgen deprivation therapy for prostate cancer: a pilot study. Psychooncology. 2002;11(6):518–523. | |
Zakowski SG, Harris C, Krueger N, et al. Social barriers to emotional expression and their relations to distress in male and female cancer patients. Br J Health Psychol. 2003;8(3):271–286. | |
van Dis F, Mols F, Vingerhoets AJM, Ferrell B, van de Poll-Franse L. A validation study of the Dutch version of the Quality of Life – Cancer Survivor (QOL-CS) questionnaire in a group of prostate cancer survivors. Qual Life Res. 2006;15(10):1607–1612. | |
Galbraith ME, Hays L, Tanner T. What men say about surviving prostate câncer: complexities represented in a decade of comments. Clin J Oncol Nurs. 2012;16(1):65–72. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.