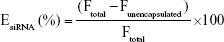Back to Journals » International Journal of Nanomedicine » Volume 12
Achieving long-term stability of lipid nanoparticles: examining the effect of pH, temperature, and lyophilization
Authors Ball RL, Bajaj P, Whitehead KA
Received 23 September 2016
Accepted for publication 12 November 2016
Published 30 December 2016 Volume 2017:12 Pages 305—315
DOI https://doi.org/10.2147/IJN.S123062
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Rebecca L Ball,1 Palak Bajaj,1,2 Kathryn A Whitehead1,2
1Department of Chemical Engineering, 2Department of Biomedical Engineering, Carnegie Mellon University, Pittsburgh, PA, USA
Abstract: The broadest clinical application of siRNA therapeutics will be facilitated by drug-loaded delivery systems that maintain stability and potency for long times under ambient conditions. In the present study, we seek to better understand the stability and effect of storage conditions on lipidoid nanoparticles (LNPs), which have been previously shown by our group and others to potently deliver RNA to various cell and organ targets both in vitro and in vivo. Specifically, this study evaluates the influence of pH, temperature, and lyophilization on LNP efficacy in HeLa cells. When stored under aqueous conditions, we found that refrigeration (2°C) kept LNPs the most stable over 150 days compared to storage in the -20°C freezer or at room temperature. Because the pH of the storage buffer was not found to influence stability, it is suggested that the LNPs be stored under physiologically appropriate conditions (pH 7) for ease of use. Although aggregation and loss of efficacy were observed when LNPs were subjected to freeze–thaw cycles, their stability was retained with the use of the cryoprotectants, trehalose, and sucrose. Initially, lyophilization of the LNPs followed by reconstitution in aqueous buffer also led to reductions in efficacy, most likely due to aggregation upon reconstitution. Although the addition of ethanol to the reconstitution buffer restored efficacy, this approach is not ideal, as LNP solutions would require dialysis prior to use. Fortunately, we found that the addition of trehalose or sucrose to LNP solutions prior to lyophilization facilitated room temperature storage and reconstitution in aqueous buffer without diminishing delivery potency.
Keywords: lipid nanoparticles, nanoparticle stability, lyophilization, lyoprotectants, nanoparticle storage, siRNA delivery
Introduction
Lipidoid nanoparticles (LNPs), made of lipid-like compounds called “lipidoids”, have shown great promise in delivering siRNA and mRNA into various cell types in vitro and in vivo.1–7 Studies in our laboratory and others have demonstrated that these materials mediate potent gene knockdown without inducing toxicity.1,3,5 One concern for translation of the delivery vehicles into a clinical setting is stability, which would require the identification of conditions appropriate for long-term storage of the nanoparticles. Currently, the best conditions for LNP storage, including pH, temperature, and physical state (eg, in solution, lyophilized powder), are poorly understood. In addition to aiding in translation, identifying the conditions suitable for LNP storage may also prove useful to the experimentalist who wishes to use the same batch of nanoparticles for a series of experiments.
Generic LNPs are formulated by combining lipidoids, cholesterol, distearoyl-sn-glycerol-3-phosphocholine (DSPC), polyethylene glycol (PEG), and siRNA.8 Upon mixing aqueous and organic solutions under reduced pH conditions, nanoparticles form spontaneously due to hydrophobic/hydrophilic interactions as well as attractive electrostatic interactions between the positively charged amine groups on the lipidoids and the negative charge of the siRNA phosphate backbone. Although these interactions result in robust particle formation over shorter time periods, it is not known how to retain the stability of the hydrophobic and electrostatic LNP interactions during storage. Because of this, to the best of our knowledge, experimentalists from our laboratory and others must freshly prepare LNPs for each experiment. This is both inconvenient and can lead to modest yet undesirable variability in results due to the difficulty in precisely reproducing LNP formulations. Freshly preparing LNPs for each experiment is time-consuming and would not be practical in a clinical setting. Identifying a method to store LNPs for long term could save time, cost, and improve consistency from trial to trial.
Similar drug delivery systems, such as cationic liposome complexes (lipoplexes), are known to become unstable in solution and form aggregates during long-term storage at room temperature.9,10 Additionally, commercially available liposomes such as DOXIL® have demonstrated instabilities in aqueous solution, including encapsulated solute leakage and aggregation.11,12 To combat these challenges, freeze-drying, also known as lyophilization, is most commonly used as a long-term storage method and has been proven to extend the shelf life of lipid-based systems, proteins, and many other biopharmaceuticals.9,12–15 In addition, several other methods have been investigated for long-term storage of lipid nanoparticles, which include freezing, spray drying, and supercritical fluid technology.16–18
To determine how to best store LNPs to retain efficacy while maximizing convenience, we evaluated LNP stability in different pH solutions, temperature conditions, and under both aqueous and lyophilized conditions. Throughout our study, LNP stability was evaluated by monitoring siRNA entrapment, LNP size, and gene silencing efficacy in HeLa cells. Herein, we provide recommendations for LNP storage and reconstitution based on our improved understanding of the LNP response to varied preservation techniques.
Materials and methods
Materials
Cholesterol, trehalose, and sucrose were purchased from Sigma-Aldrich (St Louis, MO, USA), while DSPC and 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (14:0 PEG2000-PE) were obtained from Avanti Polar Lipids. HeLa cells were purchased from American Type Culture Collection (Manassas, VA, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), trypsin, penicillin/streptomycin, phosphate-buffered saline (PBS), and fetal bovine serum (FBS) were obtained from Thermo Fisher Scientific. Anti-firefly luciferase siRNA for luciferase (siLuc) was purchased from Dharmacon (Lafayette, CO, USA).
LNP formulation
LNPs were prepared as previously described.1 The lipidoid was synthesized by Michael addition of alkyl amines and alkyl acrylates. The lipidoid 306O13 was used for all experiments. The lipidoids, cholesterol, DSPC, PEG2000-DMG, and siRNA were dissolved in ethanol. The solution was then mixed at a molar ratio of 50:38.5:10:1.5 in 90% ethanol and 10% 10 nM sodium citrate (by volume). The final weight ratio of the lipidoid:siRNA was kept at 5:1 by diluting the siRNA in 10 nM sodium citrate. Rapid pipette mixing was used for spontaneous formation of the LNPs. The final siRNA concentration was 0.125 mg/mL, and the particles were diluted in PBS to a specific concentration for each subsequent experiment. For consequential experiments, the LNPs were not dialyzed to remove ethanol.
LNP long-term stability at various pH and temperature values
The LNPs were formulated as originally described and then diluted in PBS that was either pH 7.4 (physiological pH), pH 3.04, or pH 8.97 with hydrochloric acid or sodium hydroxide, respectively. The final siRNA concentration of the LNP solutions was 0.125 mg/mL. The LNPs were then stored at either room temperature (25°C), in the refrigerator (2°C), or in the freezer (−20°C) for a span of 156 days. Stock solutions were not aliquoted, so each experiment involved them being brought to room temperature and then being returned to storage.
Nanoparticle characterization
The LNPs were diluted to an siRNA concentration of 0.5–1.25 μg/mL in PBS for the characterization studies. The siRNA entrapment efficiency was determined using Quant-iT Ribogreen assay (Invitrogen) following the manufacturer’s protocol. The siRNA entrapment efficiency is calculated using Equation 1:
|
|
where EsiRNA is the siRNA entrapment percentage, Ftotal is the total siRNA fluorescence, and Funencapsulated is the fluorescence of the siRNA outside of the nanoparticles. LNP particle size was measured using a Malvern Zetasizer Nano (Malvern Instruments, UK). Each LNP sample was measured three times, and size value was reported as the z-average hydrodynamic diameter.
Cell culture
HeLa cells were grown in DMEM supplemented with 100 mL/L of FBS, 10 IU/mL of penicillin, and 10 mg/mL streptomycin. The cells were incubated at 37°C in a 5% CO2 environment and subcultured by partial digestion with 0.25% trypsin and EDTA. Passages 10–50 were used for experiments.
Transfection of cells with LNPs in vitro
HeLa cells were stably modified to express firefly and Renilla luciferase and were seeded at a density of 15,000 cells per well in 96-well plates. Based on the timing used in previous experiments,3 LNPs were incubated with the cells for 24 hours at siLuc concentrations of 2–20 nM, depending on the experiment. Luciferase activity was assessed with a Dual-Glo Luciferase Assay Kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol. While firefly luciferase served as the target gene for knockdown, the Renilla luciferase activity served as a control.
Freeze–thaw studies
The LNPs were formulated as described previously and then diluted into PBS containing trehalose or sucrose for a final concentration of 0, 1, 5, 10, or 20% (w/v) sugar and 0.125 mg/mL siRNA. LNPs were then frozen overnight at −80°C and thawed the subsequent day.
Lyophilization of LNPs
Freshly formulated LNPs were stored at −80°C overnight or quickly frozen in liquid nitrogen. Freeze-drying was performed in a glass chamber for 12 hours using a Virtis Unitrap II freeze dryer. The lyophilized LNPs were stored in −80°C for no longer than a week. The LNPs were then reconstituted and vortexed in double distilled (DI) water with concentrations of ethanol from 0% to 30%. For the lyoprotectant studies, the formulated LNPs were diluted in PBS containing trehalose or sucrose at a final concentration of 1, 5, 10, or 20% (w/v) sugar and 0.125 mg/mL siRNA prior to lyophilizing as described earlier.
Results
Temperature and pH affected the long-term stability of LNPs
In the interest of making LNP storage as convenient as possible, we first evaluated stability under aqueous conditions. Both the pH of the storage solution and the storage temperature were investigated. The LNPs were formulated as previously described3 and diluted into PBS that had been adjusted to a pH of 3, 7.4, or 9. They were then stored at a temperature of either −20°C (standard freezer), 2°C (refrigerator), or 25°C (room temperature). For specific time points, the LNPs were brought to room temperature for measurements, and then the bulk solutions were returned to their designated temperatures for storage. Over the course of 156 days, the LNPs were examined for gene silencing efficacy, siRNA entrapment efficiency, and size. Gene silencing was assessed by transfecting Dual HeLa cells modified to stably express firefly and Renilla luciferase. LNPs contained siRNA against the firefly luciferase gene, and Renilla luciferase levels served as a built-in negative control.
As shown in Figure 1A, LNPs maintained potent luciferase gene silencing in Dual HeLa cells at a dose of 20 nM for ~58 days regardless of the storage temperature and pH. Control experiments showed that the pH-adjusted PBS solutions did not cause gene knockdown (Figure S1) and previous studies have shown that control LNPs containing off-targeting siRNA do not induce statistically significant changes in gene expression.3,5,7 At longer time points, the nanoparticles stored at room temperature lost efficacy. Interestingly, no major changes in siRNA entrapment, LNP size, or monodispersity were observed that could explain the loss in potency (Figure 1B–D). Entrapment of siRNA in the LNPs (Figure 1B) was measured using a Quant-iT Ribogreen™ Assay, which compares the amount of unencapsulated siRNA to the total siRNA of the formulation. A common entrapment pattern emerged, irrespective of storage condition. Entrapment levels decreased from days 0 to 14, increased from days 14 to 23, then steadily decreased for the remainder of the study. The increasing levels observed from days 14 to 23 may have been due to the degradation of unencapsulated siRNA during this time period. In contrast to room temperature conditions, LNPs stored in the freezer retained most of their efficacy over 156 days while experiencing an increase in z-average diameter and polydispersity index (PDI; Figure 1C and D). These results suggest that aggregation of the LNPs is most likely occurring when the LNPs are subject to freeze–thaw cycles. The pH of the PBS storage solution did not appear to affect LNP potency or properties at any of the temperatures tested. Overall, the LNPs remained stable when stored in a pH of 3, 7.4, or 9 at 2°C for a period of at least 156 days. Storing in unadjusted PBS (pH =7.4) would be the most practical option when a refrigerator is accessible.
Trehalose and sucrose stabilized LNPs through three freeze–thaw cycles
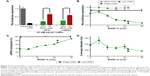
Considering that LNPs aggregated when subject to multiple freeze–thaw cycles, further experiments were performed to investigate a method of preventing aggregation. Trehalose and sucrose are two sugars that are widely used as cryoprotectants to improve the stability of proteins and nanoparticles at low temperatures.13,19,20 Incidentally, trehalose is found in nature within the tissues of the tardigrade (water bear) to aid in surviving extreme temperatures.21 Given the historical performance of these sugars for storage application, the LNPs were formulated and then diluted into a solution to a final concentration of 20% (w/v) trehalose or sucrose and subjected to three freeze–thaw cycles at −80°C. The LNPs were then tested for gene silencing efficacy, siRNA entrapment, size, and monodispersity. As shown in Figure 2, the LNPs with 0% sugar lost a significant amount of potency post-freeze–thaw. Fortunately, the addition of 20% trehalose or sucrose retained the gene silencing ability of freshly formulated LNPs through three freeze–thaw cycles. Of note, only the first freeze–thaw cycle appeared to cause damage to LNP efficacy when a cryoprotectant was not used (red bars in Figure 2); neither the second nor the third freeze–thaw significantly worsened gene silencing.
Figure 3A examines the amount of sugar necessary to retain LNP gene silencing ability. In these experiments, LNPs were formulated as previously described, diluted to five final concentrations of trehalose or sucrose between 0 and 20% (w/v), and used to dose HeLa cells with 2 nM siRNA. Gene silencing increased as a function of sugar concentration, with 20% sugar being necessary to retain efficacy. Fortunately, the siRNA entrapment was not affected by the presence of trehalose and sucrose before and after the freeze–thaw (Figure 3B). While freezing the LNPs without sugar resulted in large increases in nanoparticle size and PDI, the use of 20% sugar mitigated this increase (Figure 3C and D).
Lyophilized LNPs lost efficacy when reconstituted with DI water
To facilitate longer term storage and shipping, we investigated procedures for LNP lyophilization and reconstitution. Lyophilization, or freeze-drying, is extensively used in the pharmaceutical industry to improve the stability and increase the shelf life of many medications by removing the total water content from the drug formulation.12,15 In a dry powder form, LNPs could be conveniently shipped worldwide without the burden of maintaining cool or frozen temperature conditions. In addition, lyophilization may facilitate administration methods beyond nanoparticle injection. For example, freeze-dried nanoparticles could be loaded into a capsule for oral delivery applications, which could improve siRNA localization to a specific area in the intestines.
For lyophilization, LNPs in their original formulation medium were frozen overnight at −80°C and then placed on a freeze dryer for ~12 hours. Formulation medium contained 22% (v/v) ethanol and 78% aqueous buffer. Although buffer salts remained in the sample postlyophilization, the ethanol and water did not. Reconstitution in deionized (DI) water is preferable as the samples would not require dialysis for ethanol removal upon reconstitution. However, to compare the two techniques, LNPs were reconstituted with DI water both with and without 22% ethanol in the amount of volume lost. As demonstrated in Figure 4A, LNP potency was substantially reduced, from 80% to 35% gene silencing, when the sample was reconstituted with only DI water. In contrast, the LNPs reconstituted with the original 22% ethanol resulted in gene silencing of ~80%, which was similar to fresh LNP potency (~90% knockdown). Additionally, the method of freezing (−80°C or liquid nitrogen) before freeze-drying did not significantly affect the LNPs ability to alter gene expression. Lyophilized LNPs stored at −80°C over a period of 11 months continued to potently induce gene silencing in HeLa cells when reconstituted with 22% ethanol (Figure S2). This was likely caused by aggregation upon reconstitution.
To determine the minimum amount of ethanol needed to retain LNP efficacy, we investigated the effect of the ethanol concentration during reconstitution. As shown in Figure 4B, gene silencing improved with increasing ethanol content. Reconstituting the LNPs with 30% (v/v) ethanol, instead of the original 22% ethanol, fully restored the efficacy to ~90%. Silencing could not be directly attributed to the ethanol concentration, as the ethanol control solutions did not decrease firefly or Renilla luciferase expression when compared to the untreated cells. It is also worth noting that the lyophilized LNPs were reconstituted and diluted in PBS before delivery into HeLa cell medium, resulting in ethanol concentrations in the transfection medium of ≤0.03% (v/v).
LNP entrapment efficiency also improved with increasing ethanol upon reconstitution, which may explain the corresponding potency improvements (Figure 4C). Interestingly, reconstitution with 30% ethanol yielded a higher entrapment efficiency than fresh LNPs. As seen in Equation 1, higher entrapment values would result from a decrease in the amount of unencapsulated siRNA. This could possibly be due to the 30% ethanol allowing more of the siRNA to be encapsulated within the nanoparticles after reconstitution.
The size of the LNPs postlyophilization was also affected by the concentration of ethanol during reconstitution (Figure 4D). The LNPs reconstituted with 0 and 30% ethanol had z-average sizes of 370 and 170 nm, respectively. The addition of 30% ethanol resulted in a size comparable to the fresh LNPs. Similarly, the PDI of the LNPs also decreased with increasing ethanol volume percentage (Figure S3). Overall, the results indicate that reconstitution with ethanol greatly improved LNP stability. Unfortunately, the addition of ethanol to reconstitution solutions is often neither convenient nor practical, as dialysis into aqueous buffer would be required before use in animals or in the clinic.
The lyoprotectants trehalose and sucrose enabled LNP reconstitution without ethanol
Based on their stabilization properties under −80°C freezing conditions, trehalose and sucrose were utilized to improve the stability of lyophilized LNPs without the use of ethanol upon reconstitution. Ethanol can cause toxicity in living systems; as such, we would like to eliminate the need for ethanol when reconstituting and preparing the LNPs for administration.22 In these experiments, LNPs were formulated, diluted into sugar solutions to final concentrations of 0%–20% (w/v), frozen overnight at −80°C, and then lyophilized. After a dry powder was obtained, the LNPs were reconstituted with DI water in the amount of the original volume. The gene silencing, siRNA entrapment, size, and PDI were analyzed pre- and postlyophilization. Visually, the cake that formed after lyophilization collapsed for the LNPs that contained 0% sugar. The LNPs with sugar showed a stable freeze-dried cake with a surface area that increased with increasing sugar concentration. As demonstrated in Figure 5A, gene silencing increased as a function of sugar content, with the 20% (w/v) sucrose resulting in 86% gene silencing at a 2 nM siRNA dose. The lyophilized LNPs that contained 20% (w/v) trehalose also maintained high gene silencing of 80% compared to the fresh LNPs at 87%. Entrapment values were higher for the lyophilized LNPs that contained ≥5% (w/v) sugar (Figure 5B). In addition, the size and PDI of the LNPs decreased at the highest concentration of trehalose and sucrose (Figure 5C and D). These experiments indicated that the addition of the lyoprotectants trehalose and sucrose to the LNP solution preserved efficacy, even when reconstituted in pure DI water, without the presence of ethanol.
Discussion
Nucleic acid delivery systems, including RNA interference and mRNA systems, show great promise for the treatment of various diseases.23–25 However, many nanoparticle delivery vehicles experience instability during long-term storage, limiting clinical potential. It has been shown that a number of polymeric and liposomal delivery systems undergo particle fusion, hydrolysis, and drug leakage over time in aqueous solutions.10,26–31 The use of low temperatures for storage and/or nanoparticle lyophilization are the most widely used methods to overcome stability challenges. Previously, siRNA-loaded LNPs had not been examined for their long-term stability in aqueous media. Nonetheless, understanding and extending the stability of the LNP siRNA delivery system are important for logistics, minimizing costs and variability, and improving clinical potential.
At a range of temperatures, the LNPs maintained gene silencing efficacy for a period of 92 days in aqueous media. The LNPs stored at room temperature (25°C) lost the ability to silence genes after ~156 days. Interestingly, the loss in efficacy could not be explained by a decrease in entrapment percentage or aggregation of the LNPs. In a separate study from the McMillan group, siRNA-loaded PEGylated lipid nanoparticles, with a structure similar to our particle, were found to lose all efficacy after 1 month at 4°C and room temperature.29 In this case, there was also no increase in particle size over the 1-month time period. Alternatively, it is possible that the loss of efficacy in our LNPs could be caused by hydrolysis of the ester bond within the 306O13 lipidoid structure.1 Another possibility is that higher temperatures led to an increase in degradation of the siRNA within the aqueous solution.
Notably, altering the pH of the storage solution did not significantly affect LNP stability over time at different temperatures. This is interesting, as many drug delivery systems become unstable at low pH conditions. For example, Shu and Zhu32 reported that chitosan nanoparticles swell under acidic conditions, which resulted in undesired drug release. It is possible that, at a low pH, increased protonation of the amines within the lipidoid structure could have stabilized the electrostatic interaction between the amine groups and negatively charge siRNA backbone. The ability of our LNPs to silence genes after exposure to different pH solutions could be a significant advantage for particular applications, such as oral drug delivery. The LNPs would need to navigate the gastrointestinal (GI) tract, which varies in pH from 1 to 7, without becoming unstable.33
Storing the LNPs at −20°C resulted in an increase in z-average particle size as well as a significant amount of aggregation. During the freezing process, a phase separation occurs that results in the formation of an ice phase and the nanoparticle-concentrated solution phase.34,35 The separation of phases has been shown to lead to irreversible fusion of nanoparticles and subsequent aggregation.28 Additionally, ice crystal formation during freezing could have exerted mechanical stress on the LNPs, causing them to increase in size.28 The damage done to the LNPs, post freeze–thaw, was lessened with the addition of the cryoprotectants, trehalose, and sucrose, in a concentration-dependent manner. Date et al34 achieved similar stability retention, post-freeze–thaw, of rifampicin-loaded polymeric nanoparticles using 5%–20% (w/v) trehalose. Several studies have also demonstrated that frozen DNA lipoplex formulations maintained stability and transfection ability with the help of sugars.14,26,36 Data have suggested that the disaccharides interact directly with the polar groups of the lipids to prevent particle fusion during freezing and can also prevent ice formation by increasing the glass transition temperature of the solution.34,37 Overall, freezing the LNPs at −80°C could maintain their stability with the help of trehalose or sucrose; however, deep freezers are not always available, particularly during transport.
The process of freeze-drying has been employed since the beginning of recorded history as a way to preserve food.38 Freeze-drying or lyophilization removes the water content from a particle solution and allows facile transportation and storage at a range of temperatures. Lyophilization has been used to extend the shelf life of a broad array of delivery systems, including liposomes, polymeric nanoparticles, vaccines, and proteins usually with the help of lyoprotectants.10,12,15,39
In this study, LNPs experienced a significant decrease in gene silencing efficacy postlyophilization when reconstituted with DI water only. Interestingly, the addition of ethanol (5%–30% [v/v]) in the reconstitution solution restored gene silencing, siRNA entrapment, and lowered the z-average diameter in an ethanol concentration-dependent manner. We believe that ethanol is aiding in solubilization upon reconstitution of the lipid components in the LNPs, leading to higher gene silencing and less LNP aggregation. However, the inclusion of ethanol is undesirable, as it would necessitate dialysis before LNP administration in vivo.
As the freezing and drying steps in lyophilization exert many stresses on the LNPs, lyoprotectants are often used for improved stability. Sucrose and trehalose are lyoprotectants found in the formulations of commercially available lyophilized products, including AmBisome, LEP-ETU, Herceptin®, and Avastin® for increased long-term stability.40,41 These disaccharide lyoprotectants, added to the LNP solution, restored the gene silencing ability of lyophilized LNPs, which eliminated the need for ethanol during reconstitution. The 20% (w/v) sugar concentration provided the best particle stability in terms of gene silencing, siRNA entrapment, particle size, and monodispersity of the LNPs. Most likely, trehalose and sucrose maintain LNP stability through the water replacement model and/or the vitrification model.12,26 The water replacement model hypothesizes that the sugars interact with the polar head groups of the lipidoids, replacing the water in between the head groups. Upon freeze-drying, the spacing between the head groups is maintained, resulting in more stable LNPs. Alternatively, the vitrification model suggests that the addition of sugar preserves the LNP solution in a glassy matrix state, which prevents aggregation and ice crystal damage to the lipid bilayer.12
Conclusion
This study has added to our knowledge base regarding LNP stability at different pH conditions, temperatures, and under different physical states. For convenience in biological applications, we suggest storage of LNPs at physiological pH (7.4) in PBS, 2°C (refrigerator) for up to 160 days. If freezing is desired, the addition of 20% (w/v) sucrose or trehalose was found to be necessary to prevent nanoparticle aggregation and loss of efficacy. Ultimately, however, our data support the use of lyophilization for long-term storage needs. In this case, 20% (w/v) trehalose or sucrose must be added to the LNP to retain potency, assuming reconstitution in DI water. Alternatively, 30% ethanol can be added to the reconstitution solution without the use of lyoprotectant sugars; however, this approach is generally not recommended for most biological applications.
Acknowledgments
The authors would like to thank Christopher Knapp and Sevahn Vorperian for technical assistance. We also acknowledge the Center for Nucleic Acids Science and Technology as well as the DSF Charitable Foundation for funding.
Disclosure
KAW reports two patents related to the lipidoid materials described in this work: US Patents 9,439,968 and 9,227,917. The authors report no further conflicts of interest in this work.
References
Whitehead KA, Dorkin JR, Vegas AJ, et al. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat Commun. 2014;5:4277. | ||
Whitehead KA, Matthews J, Chang PH, et al. In vitro – in vivo translation of lipid nanoparticles for hepatocellular siRNA delivery. ACS Nano. 2012;6(8):6922–6929. | ||
Ball RL, Knapp CM, Whitehead KA. Lipidoid nanoparticles for siRNA delivery to the intestinal epithelium: in vitro investigations in a Caco-2 model. PLoS One. 2015;10(7):e0133154. | ||
Love KT, Mahon KP, Christopher G, et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci U S A. 2010;107(5):1864–1869. | ||
Kasiewicz LN, Whitehead KA. Silencing TNFα with lipidoid nanoparticles downregulates both TNFα and MCP-1 in an in vitro co-culture model of diabetic foot ulcers. Acta Biomater. 2015;32:120–128. | ||
Turnbull IC, Eltoukhy AA, Fish KM, et al. Myocardial delivery of lipidoid nanoparticle carrying modRNA induces rapid and transient expression. Mol Ther. 2015;24(1):66–75. | ||
Knapp CM, He J, Lister J, Whitehead KA. Lipidoid nanoparticle mediated silencing of Mcl-1 induces apoptosis in mantle cell lymphoma. Exp Biol Med (Maywood). 2016;241(9):1007–1013. | ||
Knapp CM, Guo P, Whitehead KA. Lipidoid tail structure strongly influences siRNA delivery activity. Cell Mol Bioeng. 2016;9(3):305–314. | ||
Aso Y, Yoshioka S. Effect of freezing rate on physical stability of lyophilized cationic liposomes. Chem Pharm Bull. 2005;53(3):301–304. | ||
Maitani Y, Aso Y, Yamada A, Yoshioka S. Effect of sugars on storage stability of lyophilized liposome/DNA complexes with high transfection efficiency. Int J Pharm. 2008;356(1–2):69–75. | ||
Torchilin V, Weissig V. Liposomes: A Practical Approach. OUP Oxford, New York; 2003. | ||
Chen C, Han D, Cai C, Tang X. An overview of liposome lyophilization and its future potential. J Control Release. 2010;142(3):299–311. | ||
Stark B, Pabst G, Prassl R. Long-term stability of sterically stabilized liposomes by freezing and freeze-drying: effects of cryoprotectants on structure. Eur J Pharm Sci. 2010;41(3–4):546–555. | ||
Del Pozo-Rodríguez A, Solinís MA, Gascón AR, Pedraz JL. Short- and long-term stability study of lyophilized solid lipid nanoparticles for gene therapy. Eur J Pharm Biopharm. 2009;71(2):181–189. | ||
Kasper JC, Winter G, Friess W. Recent advances and further challenges in lyophilization. Eur J Pharm Biopharm. 2013;85(2):162–169. | ||
Wang L, Hu X, Shen B, et al. Enhanced stability of liposomes against solidification stress during freeze-drying and spray-drying by coating with calcium alginate. J Drug Deliv Sci Technol. 2015;30:163–170. | ||
Yin F, Guo S, Gan Y, Zhang X. Preparation of redispersible liposomal dry powder using an ultrasonic spray freeze-drying technique for transdermal delivery of human epithelial growth factor. Int J Nanomedicine. 2014;9(1):1665–1675. | ||
Campardelli R, Trucillo P, Reverchon E. A supercritical fluid-based process for the production of fluorescein-loaded liposomes. Ind Eng Chem Res. 2016;55(18):5359–5365. | ||
Radmanovic N, Serno T, Joerg S, Germershaus O. Understanding the freezing of biopharmaceuticals: first principle modeling of the process and evaluation of its effect on product quality. J Pharm Sci. 2013;102(8):2495–2507. | ||
Fonte P, Soares S, Costa A, et al. Effect of cryoprotectants on the porosity and stability of insulin-loaded PLGA nanoparticles after freeze-drying. Biomatter. 2012;2(4):329–339. | ||
Hengherr S, Heyer AG, Köhler HR, Schill RO. Trehalose and anhydrobiosis in tardigrades – evidence for divergence in responses to dehydration. FEBS J. 2008;275(2):281–288. | ||
Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:63–74. | ||
Watanabe T, Umehara T, Yasui F, et al. Liver target delivery of small interfering RNA to the HCV gene by lactosylated cationic liposome. J Hepatol. 2007;47(6):744–750. | ||
Dahlman JE, Barnes C, Khan OF, et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol. 2014;9(8):648–655. | ||
Baba M, Itaka K, Kondo K, Yamasoba T, Kataoka K. Treatment of neurological disorders by introducing mRNA in vivo using polyplex nanomicelles. J Control Release. 2015;201:41–48. | ||
Anchordoquy TJ, Girouard LG, Carpenter JF, Kroll DJ. Stability of lipid/DNA complexes during agitation and freeze – thawing. J Pharm Sci. 1998;87(9):1046–1051. | ||
Felgner PL, Tsai YJ, Sukhu L, et al. Improved cationic lipid formulations for in vivo gene therapy. Ann N Y Acad Sci. 1995;772:126–139. | ||
Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev. 2006;58(15):1688–1713. | ||
Wu SY, Putral LN, Liang M, Chang HI, Davies NM, McMillan NA. Development of a novel method for formulating stable siRNA-loaded lipid particles for in vivo use. Pharm Res. 2009;26(3):512–522. | ||
Kundu AK, Chandra PK, Hazari S, et al. Stability of lyophilized siRNA nanosome formulations. Int J Pharm. 2012;423(2):525–534. | ||
Fonte P, Araújo F, Seabra V, Reis S, Van De Weert M, Sarmento B. Co-encapsulation of lyoprotectants improves the stability of protein-loaded PLGA nanoparticles upon lyophilization. Int J Pharm. 2015;496(2):850–862. | ||
Shu X, Zhu K. The influence of multivalent phosphate structure on the properties of ionically cross-linked chitosan films for controlled drug release. Eur J Pharm Biopharm. 2002;54(2):235–243. | ||
Van de Graaf KM. Anatomy and physiology of the gastrointestinal tract. Pediatr Infect Dis. 1986;5(1):S11–S15. | ||
Date PV, Samad A, Devarajan PV. Freeze thaw: a simple approach for prediction of optimal cryoprotectant for freeze drying. AAPS PharmSciTech. 2010;11(1):304–313. | ||
Kasper JC, Friess W. The freezing step in lyophilization: physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. Eur J Pharm Biopharm. 2011;78(2):248–263. | ||
Hinrichs WLJ, Mancenido FA, Sanders NN, et al. The choice of a suitable oligosaccharide to prevent aggregation of PEGylated nanoparticles during freeze thawing and freeze drying. Int J Pharm. 2006;311(1–2):237–244. | ||
Khatri N, Baradia D, Vhora I, Rathi M, Misra A. Development and characterization of siRNA lipoplexes: effect of different lipids, in vitro evaluation in cancerous cell lines and in vivo toxicity study. AAPS PharmSciTech. 2014;15(6):1630–1643. | ||
Hayashi H. Drying Technologies of Foods – Their History and Future. Drying Technology. 1989;7(2):315–369. | ||
Carpenter JF, Chang BS, Garzon-Rodriguez W, Randolph TW. Rational design of stable protein formulations. Pharm Biotechnol. 2002;13:109–133. | ||
Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomedicine. 2012;7:49–60. | ||
Ohtake S, Wang YJ. Trehalose: current use and future applications. J Pharm Sci. 2011;100(6):2020–2053. |
Supplementary materials
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.