Back to Journals » Infection and Drug Resistance » Volume 12
Absence of cutaneous involvement in disseminated Talaromyces marneffei infection in an AIDS patient: a case report and literature review
Authors Pongpech N, Rotjanapan P
Received 6 March 2019
Accepted for publication 17 May 2019
Published 4 June 2019 Volume 2019:12 Pages 1493—1499
DOI https://doi.org/10.2147/IDR.S207819
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eric Nulens

Nisha Pongpech, Porpon Rotjanapan
Division of Infectious Diseases, Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Background: Talaromyces marneffei (T. marneffei) is an important opportunistic pathogen found in human immunodeficiency virus-positive individuals in Southeast Asia, Southern China, and Northeastern India. Patients with disseminated talaromycosis commonly develop multi-organ involvement including the skin. In this report, we describe the clinical presentation, investigation, management, and clinical outcome of an acquired immune deficiency syndrome (AIDS) patient with newly diagnosed disseminated talaromycosis without skin involvement.
Case presentation: A 27-year-old male with AIDS presented with acute onset of abdominal pain for 4 days and fever for 2 days. He had been diagnosed with AIDS, pneumocystis pneumonia, and presumptive smear-negative pulmonary tuberculosis 2 months previously. His initial CD4 count was 91 cells/mm3,. After a 3-week course of trimethoprim/sulfamethoxazole and anti-tuberculosis treatment, anti-retroviral therapy was initiated. Physical examination revealed left upper quadrant tenderness but no abnormal skin lesions. On this visit, his CD4 count rose to 272 cells/mm3, (19%). Computed tomography of the abdomen showed evidence of a small hypodense lesion with a thin enhancing rim at the spleen and extensive intra-abdominal lymphadenopathy. Empirical amphotericin B deoxycholate was administered in response to positive serum galactomannan, although this was switched to intravenous liposomal amphotericin B 1 week later because of acute kidney injury. Blood and bone marrow cultures for fungus grew T. marneffei on days 9 and 12, respectively. After 21 days of treatment, oral itraconazole replaced intravenous therapy. The patient was discharged home after 29 days in the hospital and continued to improve clinically at a follow-up visit as an outpatient.
Conclusion: Talaromycosis is a fairly common opportunistic infection among AIDS patients in Thailand, despite a rise in CD4 count which may reflect a change in immune status. To a lesser extent, a systemic disease without skin involvement can be expected in real clinical practice.
Keywords: talaromycosis, AIDS, disseminated fungal infection, cutaneous involvement
Background
Talaromyces marneffei (formerly Penicillium marneffei) is a thermally dimorphic fungus that was first isolated from a bamboo rat in 1956. The first disseminated talaromycosis in a human immunodeficiency virus (HIV)-infected patient was reported in 1988 in Chicago, USA.1 Talaromycosis has become an important cause of morbidity and mortality in HIV-infected patients who live in or travel to Southeast Asia, Southern China, and Northeastern India.2,3 However, this fungal infection increasingly occurs outside these epidemic areas as a result of increased migration and international travel.3 During the potent antiretroviral era, the incidence of talaromycosis in Thailand has declined significantly, but it remains the third most common cause of HIV-associated death, after tuberculosis and cryptococcosis.4,5 A seasonal pattern of talaromycosis with a mostly predominant peak is seen during the rainy season from May to November.6 Common presentations in HIV-infected patients are fever, lymphadenopathy, splenomegaly, and cutaneous lesions.7 We report a 27-year-old man with well-controlled acquired immune deficiency syndrome (AIDS) with disseminated talaromycosis without cutaneous involvement.
Case presentation
A 27-year-old Thai male with AIDS from Saraburi Province presented with acute abdominal pain that had lasted for 4 days. He originated from Mukdahan Province, in Northeastern Thailand, but had moved to Saraburi Province which is situated in the southern part of Northeastern Thailand, 200 km from Bangkok. He denied other travel histories outside these areas during the past year. He was diagnosed with AIDS 2 months before at another hospital, which recorded an initial CD4 count of 91 cells/mm3 (unknown percentage and HIV viral load) and no complete immune status evaluation was performed. At the time, he presented with respiratory distress which was later identified as pneumocystis pneumonia and presumptive smear-negative pulmonary tuberculosis. He was given a 3-week course of trimethoprim/sulfamethoxazole, and anti-tuberculosis treatment consisting of isoniazid, rifampicin, pyrazinamide, and ethambutol during hospitalization. Anti-retroviral therapy (ART) comprising tenofovir disoproxil fumarate, emtricitabine, and efavirenz was introduced 2 weeks later when he was an outpatient. The anti-tuberculosis regimen was adjusted to isoniazid, rifampicin, ethambutol, and levofloxacin after 4 days, and a chest radiograph demonstrated complete resolution of prior pulmonary opacities after 50 days of treatment. He reported 100% adherence to both anti-tuberculosis treatment and ART and no other new medications had been added to the regimen. The CD4 count 8 weeks before this admission was 91 cells/mm3 (unknown percentage).
At this admission, he presented with abdominal pain mainly in the left upper quadrant that he described as a dull ache, without radiation. The pain was not related to eating food, physical activity, or position. He reported no other associated gastrointestinal complaints such as diarrhea or nausea. Two days later, he developed a high-grade fever without chills, with the highest temperature of 38.9 °C. Abdominal pain persisted and partially responded to acetaminophen. In response to this, he attended the emergency department where a physical examination revealed a blood pressure of 120/75 mmHg, a heart rate of 110 beats/min, a temperature of 38.7 °C, and a respiration rate of 20 breaths/min. His abdomen was soft, normal bowel sounds were heard, but tenderness on palpation in the left upper quadrant was evident. The spleen could not be palpated. The remaining physical examination was unremarkable including a negative complete skin examination, with no cutaneous lesions on his face, trunk, or extremities, and a neurological examination within normal limits.
Initial laboratory investigations revealed hemoglobin levels of 9.8 g/dL, a white blood cell count of 14,000 cells/mm3, and a platelet count of 225,000 cells/mm3 on a complete blood count. Serum galactomannan (GM) levels by Platelia enzyme-linked immunosorbent assay (ELISA) (BioRad)® were 6.84. The CD4 count on admission was 272 cells/mm3 (19%). A fungal blood culture obtained on hospital day 1 grew white to tan-colored, velvety and flat colonies with red soluble pigment (Figure 1) on day 9. Direct microscopic examination of the colonies with lactophenol cotton blue staining demonstrated septate hyphae and smooth conidia aloft phialides directly borne on metulae (Figure 2). A bone marrow culture grew similar colonies to the blood specimen (Figure 3) on day 12 after the procedure. Contrast-enhanced computed tomography of the abdomen revealed a small hypodense lesion with a thin enhancing rim at the spleen and extensive intra-abdominal lymphadenopathy (Figure 4). At the time, the patient was not able to provide sputum sample for culture and due to the high-risk procedure, intra-abdominal lymph node biopsy for culture and histologic analysis was not performed.
 | Figure 1 Fungal blood culture demonstrating white to tan-colored, velvety and flat colonies with red soluble pigment. |
 | Figure 2 Lactophenol cotton blue staining from fungal blood culture demonstrating septate hyphae and smooth conidia aloft phialides which are borne to metulae. |
 | Figure 3 Bone marrow fungal culture demonstrating white to tan-colored, velvety and flat colonies with red soluble pigment. |
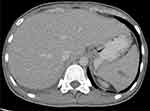 | Figure 4 Contrast-enhanced computed tomography of the whole abdomen demonstrating small hypodense lesion with a thin enhancing rim at the spleen and extensive intra-abdominal lymphadenopathy. |
Because of a concern of disseminated fungal infection on admission, empirical intravenous amphotericin B deoxycholate was given at a dose of 1.5 mg/kg/day for 7 days. This was switched to liposomal amphotericin B at 3.2 mg/kg/day in response to acute kidney injury for a total of 21 days. Before discharge, a loading dose of itraconazole was initiated at 600 mg/day for 3 days, then 400 mg/day for the maintenance phase. Of note, plasma itraconazole levels drawn on day 4 were 0.13 mcg/mL, with the highest value throughout the treatment course of 0.21 mcg/mL. The patient continued to improve clinically and was discharged from the hospital after 29 days. Repeat imaging was initially planned at 6 months but was deferred at the patient’s request. Serum GM levels 9 months after therapy had declined to 0.11, and he remained asymptomatic at the 12-month follow-up.
Discussion and conclusions
T. marneffei is a dimorphic fungus that can infect both immunocompromised and immunocompetent hosts. The yeast form with a centrally transverse septum is found at 37 °C whereas the hyphae form with a red diffusible pigment is found at room temperature (25 °C).8 Endemic areas of T. marneffei include Southeast Asia, Southern China, and Northeastern India.9
Previous studies suggested that inhalation of conidia was the primary route of infection that can later disseminate to the reticuloendothelial system, skin, and gastrointestinal organs. The bamboo rat is the only non-human host, and there is no evidence of direct transmission to humans.10 However, occupational exposure to plants and animals has been associated with human infection.3
Disseminated talaromycosis is the third most common opportunistic infection in the northern area of Thailand after tuberculosis and cryptococcosis. Talaromycosis has the highest incidence rate in the rainy season whereas other opportunistic infections show no seasonal predilection.6 The incubation period of the acute disease is approximately 1–3 weeks, while reactivation of the disease in immunocompromised hosts can occur many years after the initial exposure.3 T. marneffei mainly affects individuals with impaired cellular immunity, particularly those with low CD4 counts (ie, HIV-infected patients), and clinical manifestations may vary depending on organ system involvement. Moreover, the severity of the disease reflects the immune status of the individual. HIV-infected patients with talaromycosis commonly have a CD4 count below 100 cells/mm3 at diagnosis, but talaromycosis has also been reported in non-HIV infected patients, including those afflicted with lupus, diabetes mellitus, lymphoma, or in receipt of immunosuppressive drugs.7
Frequent presentations of talaromycosis in HIV-infected patients include fever (87.1%), followed by cutaneous lesions (40.5%), hepatomegaly (36.2%), lymphadenopathy (31.9%), and cough (27.6%).7 However, several studies have reported skin involvement in >80% of all patients.11–14 For example, a report from Manipur state, India in 2002 documented cutaneous lesions in 29 of 36 (81%) HIV-infected patients with disseminated talaromycosis.11 Similarly, a study from North Vietnam reported cutaneous lesions in 83% of patients.12 In HIV-uninfected individuals with talaromycosis, skin involvement remains the most common presentation (up to 78.6%), followed by fever according to Kawila et al7. Therefore, cutaneous involvement in disseminated talaromycosis is often used to aid diagnosis.
Umbilicated lesions are the most common form of cutaneous lesion found in this patient group, and these are usually distributed over the face and neck areas.7 A study from King Chulalongkorn Memorial Hospital, Bangkok during 2001–2010 reported umbilicated skin lesions in 78.5% of talaromycosis cases,13 whereas a study from Ramathibodi Hospital, Bangkok during 2005–2010 reported such lesions in 46% of all cases.14 Despite the anticipated skin manifestation among individuals with talaromycosis, regardless of the HIV serostatus, there is no definite pathophysiologic mechanism to explain why this is such a common finding, and no distinguished associated factors among infected individuals with and without skin involvement have been identified.
In the present case, dull abdominal pain and fever indicated an inflammatory process of the solid organ system rather than the hollow viscus. And based on the location of the pain in the left upper quadrant, the inflammation of the spleen was suspected. The etiology of the inflammation with relatively rapid progression made indolent infection such as tuberculosis less likely. Therefore, a concern of splenic abscess secondary to either bacterial or endemic fungal infections was raised. In this patient, fever was not an initial presentation which suggested a lower likelihood of hematogenous bacterial infection as the culprit but rather fungal infection. His clinical presentation did not suggest talaromycosis at first diagnosis despite the fact that the clinical course began in the rainy season. Instead, histoplasmosis, cryptococcosis, and other non-infectious etiologies including lymphoma were suspected. A diagnosis of talaromycosis can be recognized by clinicians when the patient presents with classic cutaneous lesions but is often missed when no skin lesions are documented in individuals suspected to have systemic infections. In 1997, Kuntipong et al reported six patients from Chiangrai Province, in Northern Thailand, who experienced hepatic talaromycosis without skin lesions and presented with high-grade fever, acute abdominal pain, and elevated alkaline phosphatase levels. T. marneffii was isolated from the blood of all six patients. Abundant septate hyphae were identified from the hepatic histopathology of two patients. Only two patients completed conventional amphotericin B treatment followed by itraconazole; they recovered fully but the remaining patients died before the treatment was started because of delayed diagnosis in three patients and leaving the hospital against medical advice by one.15
In 2006, a 26-year-old male with HIV infection who resided in Guangxi Province, China presented with a 2-month history of high-grade fever and cough without skin lesion. His chest radiograph was normal, and pulmonary tuberculosis was provisionally diagnosed. He was treated with anti-tuberculosis drugs for 1 month without clinical improvement. He was then referred to West China Hospital where bronchoscopy revealed granulomatous nodules at the bronchial orifices to the left lower basilar segment. His blood, bone marrow, and bronchoscopic biopsy cultures were positive for T. marneffei. A combination of amphotericin B deoxycholate and itraconazole treatment was instituted with an excellent clinical response.16
Talaromycosis can present either as a part of a new infection or as part of immune reconstitution inflammatory syndrome (IRIS). IRIS can occur during immune recovery after the initiation of ART that leads to an increased CD4 count and a decline of the HIV viral load. The emergence of a pre-existing undiagnosed infection is known as “unmasking IRIS”, and is less common than newly acquired infection.17 To date, only four cases have been reported worldwide, of which three presented as paradoxical IRIS.18 In 2007, Gupta et al reported a 35-year-old treatment naïve HIV-infected male from Manipur, India who presented with fever, weight loss, and heaviness in the abdomen for 2 months. His CD4 count was 4 cells/mm3 (unknown percentage), and chest computed tomography revealed a small round opacity in the basal segment of the left lung. He was given ART (stavudine, lamivudine, and nevirapine) and empirical anti-tuberculosis drugs despite a negative sputum acid-fast bacilli smear. One month later, he returned to the hospital with marked lymphadenopathy and hepatosplenomegaly. No abnormal cutaneous lesions were appreciated. T. marneffei was isolated from axillary lymph node aspirates and blood cultures. The final diagnosis was IRIS from T. marneffei. After a 2-week course of the amphotericin B deoxycholate, he continued with itraconazole 400 mg/day for 10 weeks and thereafter maintenance therapy of 200 mg/day. His condition dramatically improved.19
The clinical syndrome of our patient developed 2 months after the initiation of ART, making unmasking IRIS the most plausible diagnosis although this could not be verified. Other reasons to support unmasking IRIS include the lack of recent travel history to endemic areas and no notable exposure to soil, plants, or reservoir animals.
Cytological and histological examinations of talaromycosis clinical samples may reveal either intracellular or extracellular yeasts following periodic acid–Schiff or silver methenamine staining. The detection of non-budding yeast cells with a central transverse septum would give a presumptive diagnosis while confirmation by microbiological culture is considered the gold standard.11 The specimen that gives the highest yield in fungal culture is bone marrow (100%) followed by skin biopsy (90%) and blood culture (76%). The time to positivity of the blood culture varies from 1.5–7 days with a median time of 4 days. The morphology of the colony is granular with a greenish-yellowish or grayish color and the production of diffusible red pigment.2 Septate hyphae were also identified on Gram staining of the colonies in our patient.
Serology and direct antigen detection were not performed in this case because of their lack of availability at the national level. GM is a carbohydrate molecule composed of a backbone of mannose residues with side chains of β 1–5 linked galactofuranosyl residues. It is found in the cell wall of mold-like fungi, especially Aspergillus spp. and Talaromyces spp., but it is also found in other species such as Fusarium spp., Alternaria spp., and Histoplasma spp..20,21 In 1992, Stynen discovered a monoclonal antibody (EB-A2) which specifically targeted galactofuranosyl residues, and later resulted in the development of a diagnostic ELISA.22,23 The GM assay is generally used to detect Aspergillus spp., but serum GM is used as a surrogate marker of talaromycosis in some institutes.24
Thailand National Guidelines on HIV/AIDS Treatment and Prevention 2017 recommends the administration of amphotericin B deoxycholate 0.7–1 mg/kg/day for 7–14 days, followed by itraconazole 200 mg three times daily for 3 days and then 200 mg twice daily for 10–12 weeks. No routine plasma itraconazole level monitoring is recommended in clinical practice. The once-daily 200 mg itraconazole regimen is recommended as secondary prophylaxis among patients who sustain low CD4 counts until the value has reached >100 cells/mm3 for at least 6 months.25
Voriconazole is a broad-spectrum antifungal agent that has activity against yeast and mold forms of T. marneffei. Voriconazole was reported to have superior activity compared with amphotericin B and itraconazole according to an in vitro study, but in vivo data are limited.26 In 2017, Ouyang and colleagues studied 17 disseminated talaromycosis cases with and without a diagnosis of HIV infection who had been treated with voriconazole. Voriconazole had been administered at a loading dosage of 6 mg/kg every 12 h on the first day followed by 4 mg/kg every 12 h for at least 3 days, then 200 mg twice daily thereafter. The total duration of treatment was solely determined by the investigators dependent on clinical responses. Patients were monitored from treatment initiation until 16 weeks of treatment. Three of 17 patients prematurely discontinued treatment because of financial concerns and other unidentified reasons, 10 had a complete recovery, three had partial recovery, and one was a clinical failure at week 16. No adverse effects were recorded during the study period. This study concluded that voriconazole is an effective alternative and more convenient than the standard itraconazole regimen.27 However, voriconazole is considered a high-cost drug in lower-middle income countries and according to the national guidelines and reimbursement program, voriconazole has not yet been approved for the treatment of invasive fungal infection other than aspergillosis. And to comply with the national policy on a reimbursement program, when conventional amphotericin B is contraindicated, liposomal amphotericin B can be used. Therefore, voriconazole was not the option in this patient.
In conclusion, despite the rarity of disseminated talaromycosis among well-controlled AIDS patients without skin involvement, a history of residing in or visiting endemic areas should lead to a consideration of talaromycosis in the differential diagnosis. Indeed, high awareness of this condition remains key to early diagnosis and treatment success. Notably, unmasking IRIS is not the major presentation of talaromycosis compared with newly acquired infection.
Abbreviation list
AIDS, Acquired Immune Deficiency Syndrome; ART, Anti-retroviral therapy; BP, Blood pressure; ELISA, enzyme-linked immunosorbent assay; GM, Galactomannan: HIV, Human Immunodeficiency Virus; IRIS, Immune Reconstitution Inflammatory Syndrome.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Series Editor of this journal.
Ethics approval and consent to participate
Ethical approval was granted by the Research Ethics Committee of the Faculty of Medicine Ramathibodi Hospital, Mahidol University (approval no. MURA2018/345).
Acknowledgments
The authors would like to thank the Faculty of Medicine at Ramathibodi Hospital, Mahidol University for their permission to conduct this study and Subencha Pinsai, MD for assistance in clinical management.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Piehl MR, Kaplan RL, Haber MH. Disseminated penicilliosis in a patient with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1988;112:1262–1264.
2. Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirisanthana T. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344:110–113.
3. Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/AIDS. Lancet Infect Dis. 2017;17:e334–e343. doi:10.1016/S1473-3099(17)30303-1
4. Wong KH, Lee SS, Chan KC, et al. Redefining AIDS: case exemplified by Penicillium marneffei infection in HIV-infected people in Hong Kong. Int J STD AIDS. 1998;9:555–556.
5. Supparatpinyo K, Chiewchanvit S, Hirunsri P, Uthammachai C, Nelson KE, Sirisanthana T. Penicillium marneffei infection in patients infected with human immunodeficiency virus. Clin Infect Dis. 1992;14:871–874. doi:10.1093/clinids/14.4.871
6. Le T, Wolbers M, Chi NH, et al. Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Vietnam. Clin Infect Dis. 2011;52:945–952. doi:10.1093/cid/cir028
7. Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis. 2013;13:464. doi:10.1186/1471-2334-13-464
8. Antinori S, Gianelli E, Bonaccorso C, et al. Disseminated Penicillium marneffei infection in an HIV-positive Italian patient and a review of cases reported outside endemic regions. J Travel Med. 2006;13:181–188. doi:10.1111/j.1708-8305.2006.00039.x
9. Wong SY, Wong KF. Penicillium marneffei infection in AIDS. Patholog Res Int. 2011;2011:10.
10. Cao C, Liang L, Wang W, et al. Common reservoirs for Penicillium marneffei infection in humans and rodents, China. Emerg Infect Dis. 2011;17:209–214. doi:10.3201/eid1702.100718
11. Ranjana KH, Priyokumar K, Singh TJ, et al. Disseminated Penicillium marneffei infection among HIV-infected patients in Manipur state, India. J Infect. 2002;45:268–271.
12. Larsson M, Nguyen LH, Wertheim HF, et al. Clinical characteristics and outcome of Penicillium marneffei infection among HIV-infected patients in northern Vietnam. AIDS Res Ther. 2012;9:24. doi:10.1186/1742-6405-9-24
13. Uppathamnarakorn P, Jindamporn A, Suankratay C Penicilliosis, cryptococcosis, and histoplasmosis: a comparative study between epidemiology, clinical features, microbiology, treatment, and outcome.
14. Chantharit P, Watcharananan S, Sunkanuparph S Comparison of clinical characteristics and survival among patients with cryptococcosis, histoplasmosis, and penicillosis in Ramathibodi Hospital, 2005–2010.
15. Kantipong, Panich V, Pongsurachet V, Watt G. Hepatic penicilliosis in patients without skin lesions. Clin Infect Dis. 1998;26:1215–1217. doi:10.1086/520282
16. Zhiyong Z, Mei K, Yanbin L. Disseminated Penicillium marneffei infection with fungemia and endobronchial disease in an AIDS patient in China. Med Princ Pract. 2006;15:235–237. doi:10.1159/000092189
17. Elston JWT, Thaker H. Immune reconstitution inflammatory syndrome. Int J STD AIDS. 2009;20:221–224. doi:10.1258/ijsa.2008.008449
18. Sudjaritruk T, Sirisanthana T, Sirisanthana V. Immune reconstitution inflammatory syndrome from Penicillium marneffei in an HIV-infected child: a case report and review of literature. BMC Infect Dis. 2012;12:28. doi:10.1186/1471-2334-12-166
19. Gupta S, Mathur P, Maskey D, et al. Immune restoration syndrome with disseminated Penicillium marneffei and cytomegalovirus co-infections in an AIDS patient. AIDS Res Ther. 2007;4:21. doi:10.1186/1742-6405-4-21
20. Swanink CM, Meis JF, Rijs AJ, Donnelly JP, Verweij PE. Specificity of a sandwich enzyme-linked immunosorbent assay for detecting Aspergillus galactomannan. J Clin Microbiol. 1997;35:257–260.
21. Wong KF. Marrow penicilliosis: a readily missed diagnosis. Am J Clin Pathol. 2010;134:214–218. doi:10.1309/AJCPWVBQCW13DJLO
22. Huang YT, Hung CC, Hsueh PR. Aspergillus galactomannan antigenemia in penicilliosis marneffei. Aids. 2007;21:1990–1991. doi:10.1097/QAD.0b013e3282eeb413
23. Stynen D, Sarfati J, Goris A, et al. Rat monoclonal antibodies against Aspergillus galactomannan. Infection and Immunity. 1992;60:2237–2245.
24. Mennink-Kersten MA, Donnelly JP, Verweij PE. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect Dis. 2004;4:349–357. doi:10.1016/S1473-3099(04)01045-X
25.
26. Liu D, Liang L, Chen J. In vitro antifungal drug susceptibilities of Penicillium marneffei from China. J Infect Chemother. 2013;19:776–778. doi:10.1007/s10156-012-0511-7
27. Ouyang Y, Cai S, Liang H, et al. Administration of voriconazole in disseminated Talaromyces (Penicillium) marneffei infection: a retrospective study. Mycopathologia. 2017;182:569–575. doi:10.1007/s11046-016-0107-3
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
