Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Abnormal intrinsic functional activity in patients with cervical spondylotic myelopathy a resting state fMRI study
Received 25 March 2019
Accepted for publication 31 July 2019
Published 21 August 2019 Volume 2019:15 Pages 2371—2383
DOI https://doi.org/10.2147/NDT.S209952
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Cuili Kuang, Yunfei Zha
Radiological Department, Renmin Hospital of Wuhan University, Hubei, People’s Republic of China
Correspondence: Yunfei Zha
Radiological Department, Renmin Hospital of Wuhan University, No.99 ZhangZhiDong Road, Wuchang District, Wuhan, Hubei 430060, People’s Republic of China
Tel +86 135 5406 1833
Email [email protected]
Purpose: We employed resting-state fMRI analyses to reveal central functional reorganization in the brains of patients with cervical spondylotic myelopathy (CSM) and to provide complementary evidence of cortex reorganization in these patients.
Patients and methods: We obtained Fisher’s z transformation amplitude of low-frequency fluctuations (zALFF) and Fisher’s z transformation regional homogeneity (zReHo) measurements from 33 patients with CSM and 33 healthy controls (HC) and used the brain regions with significant alterations in the zALFF or zReHo values as seed regions. Then, we calculated Pearson’s correlation coefficients between the resting-state time courses of each seed and the time series of the rest of the brain. Lastly, we computed correlations between the altered zALFF, zReHo, and functional connectivity with Japanese Orthopaedic Association scores, Neck Disability Index score, and the duration of symptoms in patients with CSM.
Results: zALFF and zReHo values were increased in the left medial superior frontal gyrus (lSFGmed) and left supramarginal gyrus (lSMG) in patients with CSM compared with those in the HC group. Selecting lSFGmed as the seed, we observed increased functional connectivity between it and the left postcentral gyrus (lPoCG) and left rolandic operculum and decreased functional connectivity with the right medial superior frontal gyrus in patients with CSM. In addition, there was a significant increase in the functional connectivity between the lSMG (seed) and the left calcarine and lPoCG in patients with CSM. However, we did not find any significant correlation between the resting-state findings and the clinical performance of patients with CSM.
Conclusion: These observed intrinsic functional changes in the patients with CSM may be related to functional reorganization and reflect the innate cortical plasticity in patients with CSM. Notably, the increased connectivity between the lPoCG and the two seed ROIs indicates the adaptive changes in patients with CSM. These findings provide complementary evidence of cortex reorganization in CSM.
Keywords: ALFF, ReHo, seed-based functional connectivity, cervical spondylotic myelopathy
Introduction
Cervical spondylotic myelopathy (CSM) is the most common disorder of chronic spinal cord compression1 and is also regarded as a specific incomplete spinal cord injury (SCI).2,3 Studies on the local changes in the spinal column or cord of patients with CSM have reported the damage to nerve fibers within the lateral corticospinal tract as the primary cause of the main signs and symptoms observed in patients with CSM.4–6 In addition, several studies have reported a degree of functional recovery through cortical reorganization and plasticity7–9 and central nervous system alterations10–14 in patients with CSM. Recently, cerebral functional reorganization or plasticity secondary to neuronal damage in the spinal cord has been accepted as a vital phenomenon in patients with CSM.9,10,15 Previous studies7,9,16 on sensorymotor cortical plasticity have reported that there is a potential for dynamic reorganization of the brain after secondary injury during the progression of SCI.17 There are two major adaptive processes involved in cortical plasticity: 1) synaptic plasticity by modification of preexisting connections and 2) anatomical plasticity through sprouting of axons and dendrites to develop new circuitry.16,18 Nowadays, it is considered that the synchronous neural activity of the brain cortices is functionally connected and constitutes a functional network.19,20 Given the reported cortical reorganization in patients with complete and incomplete SCI, there is a need to determine whether alterations in intrinsic synchronous functional activity between neurons during resting-state exist, and this may provide information about the complementary functional activity in cortical reorganization. A recent resting-state functional MRI (rs-fMRI) study used the sensorimotor cortex as a priori region of interest (ROI) and found increased amplitude of low-frequency fluctuations (ALFF) in the priori cortex in patients with CSM.11 Similarly, another study chose the sensorimotor network as the ROI and observed local neural activity alterations within the priori regions in patients with CSM by measuring regional homogeneity (ReHo) at the resting state.12 Moreover, Zhou et al selected the sensorimotor network as the seed region and investigated seed-based functional connectivity strength changes at the voxel level.13 They observed altered thalamocortical functional connectivity in two distinct low-frequency bands in patients with CSM.14 However, these studies were all hypothesis-driven analyses of some brain region as the specific cortex. Recently, a rs-fMRI study based on data-driven analyses demonstrated that the ALFF/ReHo values in the occipital lobe were decreased and the functional connectivity between the visual cortex and posterior cingulate gyrus was increased in 27 patients with CSM as compared with those of 11 healthy controls (HCs).21
Resting-state fMRI is a powerful and commonly used technique for evaluating spontaneous neural activity.22,23 Specifically, ALFF and ReHo are two important data-driven algorithms for the local measurement of spontaneous neural activity. ALFF measures the amplitude of very low-frequency oscillations of the blood oxygen level-dependent (BOLD) signal at the single-voxel level,24 while ReHo evaluates the neural synchronization of a given voxel with its adjacent voxels (ie, local neural synchrony).25 The combination of the two data-driven analyses without a priori hypothesis may provide a more comprehensive pathophysiological evaluation of brain dysfunction in CSM than each method separately. Moreover, seed-based functional connectivity analysis is a classic approach that correlates the resting-state time courses of a selected ROI (ie, seed) to the time series of the rest of the brain.19,20 Exploring the relationship of the synchronous functional activity between adjacent or remote neurons may benefit the understanding of synaptic plasticity and anatomical plasticity in patients with CSM. Therefore, in addition to obtaining ALFF and ReHo values, we conducted seed-based functional connectivity analyses by selecting brain regions with significant ALFF and/or ReHo alterations as the seed regions to obtain more detailed information about the connectivity between these cortices. Subsequently, we assessed the relationship between clinical performance and the altered ALFF, ReHo, and functional connectivity in patients with CSM. Although a previous study21 discussed the alterations in resting-state functional activity in patients with CSM, it mainly focused on exploring the relationship of the resting-state-related alternations with visual disorders, and the number of patients with CSM and HCs were not well-matched. Therefore, we aimed to contribute to the current literature on cortical reorganization in patients with CSM by using larger and number-matched study groups in order to identify more elaborate central activity alterations linked to CSM.
Materials and methods
Participants
The present study was approved by the Institutional Review Board of Wuhan University and carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Thirty-three (17 females; mean age, 54.78±8.41 years [mean ± SD]; range, 42–67 years) patients with CSM were recruited from the Renmin Hospital of Wuhan University through convenience sampling, and 33 (18 females; mean age, 53.52±8.13 years, range, 40–63 years) HCs were enrolled through community health screenings or newspaper advertisements. There were no significant between-group differences in both age and gender. All participants were right-handed and gave written informed consent. Inclusion criterion was definitive CSM, which was based on clear evidence of cord compression on a cervical spine MRI, such as (1) cervical spondylosis, (2) an ossified posterior longitudinal ligament, or (3) demyelination with hyper-intensity of the cord on T2-weighted images.11 Meanwhile, two radiologists confirmed spinal cord compression when either the cord surface was clearly indented or the cord diameter had narrowed from compression. Seventeen patients with CSM had right-side lesions, 12 had left-side lesions, and four had bilateral lesions. Patients with more severe signs would be more likely to undergo decompression surgery; therefore, they were excluded from participation. The mean duration of symptoms from disease onset to the date of the MRI examination was 37.0±25.1 months (range, 3 months–8 years). The clinical severity of myelopathy was evaluated using the Japanese Orthopaedic Association (JOA) scores system26 and Neck Disability Index (NDI) questionnaires. The JOA score system evaluates the severity of myelopathy by assigning scores based on the degree of dysfunction, while the NDI measures the activities of daily living in patients with neck pain. Exclusion criteria included trauma- or infection-related cord compression or other neurological disorders, such as multiple sclerosis, or a history of trauma.11 Moreover, MRI data from subjects with a head motion >2.0 mm translation or >2.0º rotation in any direction were excluded.
MRI procedure
MRI images were acquired on a 3.0T MR scanner (GE Discovery MR 750) equipped with an eight-channel head coil. A single-shot gradient echo echo-planar imaging) sequence was used to acquire BOLD rs-fMRI data. The scanning parameters were as follows: repetition time (TR) =2 s, echo time (TE) =25 ms, flip angle =90º, slice thickness/gap =3.0/0.6 mm, number of slices =38, field of view (FOV) =24 cm×24 cm, readout bandwidth =250 kHz, and in-plane matrix =64×64. The duration of the resting-state scan was 490 s. During the scan, the subjects were asked to close their eyes and keep their mind blank but avoid falling asleep. For registration purposes, high-resolution anatomical images were acquired from each subject using a sagittal 3D T1-weighted BRAVO sequence with the following parameters: TR =7.2 ms, TE =2.7 ms, inversion time (TI) =450 ms, flip angle =12º, number of slices =160, slice thickness =1.0 mm, FOV =25.6 cm×25.6 cm, readout bandwidth =41.67 kHz, and in-plane matrix =256×256. Sagittal and axial conventional T1-weighted, T2-weighted, and T2-fluid-attenuated inversion recovery (T2-FLAIR) images were acquired from the brain and cervical spinal cord of each patient for diagnosis.
Data preprocessing
All rs-fMRI preprocessing steps were performed using the Data Processing & Analysis of Brain Imaging V2.327 running in MatLab 8.6.0 (Math Works, Natick, MA, USA). Preprocessing involved discarding the first 10 time points to allow the MR signal to reach a steady state and to allow the participants to get used to the scanner environment. Other preprocessing steps included spatial realignment to estimate and correct for subject head motion; slice-timing correction for different acquisition times of slices; and outlier detection, which involved artifact detection tool (ART)-based identification of outlier scans for scrubbing. The high-resolution T1-weight images were co-registered to the mean realigned fMRI images and segmented into gray matter, white matter, cerebrospinal fluid (CSF), and deformation field images. Next, the fMRI images were spatially normalized to the standard Montreal Neurological Institute (MNI) space and resampled to 3×3×3 mm.3 Temporal processing involved regressing out confounding factors (including 12 estimated head motion parameters, ART-based scrubbing parameters, and mean signals from white matter and CSF), linear trend removal, and band-pass filtration (0.01–0.08 Hz). Specifically, the mean signals from white matter and CSF were regressed out using the anatomical aCompCor strategy to reduce potentially spurious correlations among distant voxels.28 Two subjects from each group were excluded due to excessive head motion: one of the two patients with CSM had left spinal cord compression, and the other patient had bilateral spinal cord compression. Thus, 31 subjects from each group were included for further analysis.
ALFF and ReHo calculation
The time series were first smoothed with a Gaussian kernel of 6 mm full-width at half-maximum. The smoothed time series were transformed to frequency domains using the fast Fourier transform to obtain the power spectrum. Subsequently, we calculated the square root at each frequency of the power spectrum and obtained the averaged square root across the 0.01–0.08 Hz frequency range at each voxel. This averaged square root value was chosen as the ALFF value. Finally, this value was transformed using Fisher’s z transformation amplitude of low-frequency fluctuations (zALFF) and used for subsequent group-level analysis.
We obtained ReHo maps in a voxel-wise manner by calculating the Kendall correlation coefficient of a given voxel and its neighboring voxels (26 voxels) from the unsmoothed time series. Each ReHo map was also transformed using Fisher’s z transformation regional homogeneity (zReHo) and used for subsequent group-level analysis.
Seed-based functional connectivity calculation
We calculated the Pearson correlation coefficient between the resting-state time courses of the brain regions with significant alterations in the zALFF and/or zReHo (seed regions) and the time series of the rest of the brain. The Pearson correlation coefficient value represented seed-based functional connectivity. Similarly, each Pearson correlation coefficient was transformed using Fisher’s z transformation and used for subsequent group-level analysis.
Statistical analyses
Between-group analyses
Voxel-wise two-sample t-tests were performed to investigate the differences in the zALFF and zReHo values between patients with CSM and patients in the HC group. Age was included as a nuisance covariate. Type I error was controlled through the use of cluster-wise false discovery rate (FDR) correction (P<0.01), and the cluster forming threshold was P<0.001. Using the brain regions with significant alterations in the ALFF and ReHo analyses as the seed regions, we performed two-sample t-tests to investigate the between-group differences in each seed-based functional connectivity. Age was also included as a nuisance covariate. We controlled for type I errors through the use of cluster-wise FDR correction (P<0.01), and the cluster forming threshold was P<0.001.
Correlation analyses
For each region showing seed-based functional connectivity with a significant between-group difference, we computed the correlation between altered functional connectivity in patients with CSM with the JOA score, NDI score, and duration of symptoms. P<0.01 was set as the statistical significance threshold. In addition, for altered zALFF and zReHo with a significant between-group difference, the same correlation analyses with the three clinical measures (ie, the JOA score, NDI score, and duration of symptoms) were also conducted.
Results
Demographic and clinical data profiling
Table 1 and Figure 1 summarize the demographic and clinical data of patients with CSM and patients in the HC group. There were no significant differences in age (P=0.752) or gender (P=0.95) between the two groups. However, there were significant differences in the JOA scores, NDI scores, and duration of symptoms between the two groups (P<0.0001).
 |
Table 1 Demographic data and clinical measures for the cervical spondylotic myelopathy patients and healthy controls |
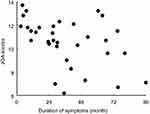 |
Figure 1 The scatter plot with duration of symptoms and JOA score of CSM patients. Abbreviation: JOA, Japanese Orthopaedic Association. |
ALFF and ReHo analyses
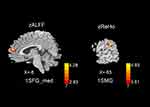
Figure 2 shows the differences in zALFF and zReHo values between the two groups. Table 2 lists the Brodmann’s Area (BA) regions, peak MNI coordinates, clusters size, and peak T-value of the differences in the zALFF and zReHo values between the two groups. Compared with patients in the HC group, patients with CSM had significantly increased zALFF in the left medial superior frontal gyrus (lSFGmed) (P<0.01, cluster-wise FDR corrected) and significantly increased zReHo in the left supramarginal gyrus (lSMG) (P<0.01, cluster-wise FDR corrected) (See Figure 2).
 |
Table 2 zALFF and zReHo differences between the two groups (P<0.01, cluster-wise FDR corrected) |
Seed-based functional connectivity analyses
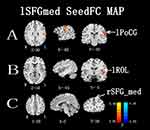
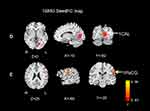
Figures 3 and 4 show the differences in functional connectivity between the two groups when lSFGmed and lSMG were used as the seed ROIs. We observed increased connectivity between the lSFGmed and the left postcentral gyrus (lPoCG) (Figure 3A) and the left rolandic operculum (lROL) (Figure 3B) in patients with CSM as compared to that in the HC group. In addition, connectivity was significantly decreased between the ISFGmed and the right SFGmed (rSFGmed) in the CSM group (Figure 3C). When the lSMG was selected as the seed ROI, we observed increased connectivity between the ISMG and the left calcarine (lCAL) and the IPoCG in patients with CSM (Figure 4D and E). Table 3 lists the BA regions, peak MNI coordinates, clusters size, and peak T-values of the functional connectivity differences between the two groups (P<0.01, cluster-wise FDR corrected).
 |
Table 3 Functional connectivity differences between the two groups (P<0.01, cluster-wise FDR corrected) |
Correlation analyses
Table 4 shows the results of correlation analysis of altered zALFF, zReHo, and functional connectivity with the clinical measures in the CSM group. There were no significant relationships between altered zALFF, zReHo, and functional connectivity with the JOA scores, NDI scores, or duration of symptoms.
 |
Table 4 Correlation between altered zALFF, zReHo, and functional connectivity with clinical measures in the CSM group |
Discussion
Through combining both ALFF and ReHo approaches to detect functional plasticity in patients with CSM, as well as investigating whole-brain intrinsic functional connectivity through seed-based functional connectivity analyses, we observed increased zALFF in the lSFGmed and increased zReHo in the lSMG in patients with CSM. Further, we observed increased functional connectivity between the ISFGmed (seed region) and the lPoCG and the lROL and decreased functional connectivity between the ISFGmed and the rSFGmed in patients with CSM. In addition, functional connectivity was increased between the lSMG (seed region) and the lCAL and lPoCG in patients with CSM as compared with that in the HC group. These findings on the resting-state-related spontaneous neural activity demonstrate the remarkable brain functional plasticity of patients with CSM. However, we did not find any significant correlations between these altered resting-state-related findings and the clinical performance of patients with CSM.
Increased zALFF and zReHo in patients with CSM
ALFF measures the intensity of spontaneous low-frequency oscillations during the resting state24 and reveals local cortical intrinsic dynamic functional activity.11 In the current study, we found that the zALFF was increased in the lSFGmed in patients with CSM. Studies have reported that the SFGmed holds a complete somatotopical representation of body movements through direct connections with the primary motor cortex and spinal cord.29–32 As a part of the prefrontal cortex, the SFGmed is also involved in multiple cognitive processes, such as execution of sequential movements, visuomotor association, etc.33 Recently, many studies have demonstrated cortical reorganization in patients with CSM after spinal cord compression.7,8,10–13,16,34 Specifically, a previous study demonstrated that the areas of cortical representation of the affected limb, such as the adjacent motor territories and the SFGmed cortex, were expanded in patients with CSM.7 Synaptic plasticity and anatomical plasticity are the two major adaptive processes of cortical reorganization in patients with CSM.16 The former involves modification of preexisting connections, while the latter involves the development of new circuity through sprouting of axons and dendrites. The interconnections of axons and dendrites maintain standard cortical representations under the inhibitory influence. However, there may be a disruption of the inhibitory influence in patients with CSM due to afferent or efferent fibers loss. These disinhibited connections between axons and dendrites in patients with CSM facilitate cortical reorganization.7 ALFF reflects the intrinsic local dynamic activity of neurons, and the observed increase in zALFF in the lSFGmed indicates the increased modulation of cortical activity occurring in patients with CSM. We suspect that this may be attributed to the disinhibitory influence and may be related to cortical plasticity.
ReHo reflects the resting-state neuronal synchronization of intraregional activities.25 Increased ReHo indicates that the functional activity of a neuron and its adjacent neurons are more temporally synchronous and reflects the perfect coordination of these neurons to achieve a specific function. Recently, studies14,35,36 have shown frequency-dependent changes in the ReHo in different neurologic conditions. According to a previous study, the rs-fMRI signal can be differentiated into four frequency bands: slow 2–5. Unlike the physiological fMRI signal in low frequencies (including slow-5 [0.01–0.027 Hz] and slow-4 [0.027–0.073 Hz]), the contributions of the signal in high frequencies (including slow 2–3 [>0.1 Hz]) to functional connectivity are minor.14,35 In this study, we analyzed changes in the ReHo in the frequency range from 0.01 Hz to 0.08 Hz that included the slow-5 and slow-4 but did not separate the two frequency bands. The selected frequency band of the study is in line with the physiological fMRI signal. In the present study, we obtained increased ReHo in the left SMG in patients with CSM. The SMG makes up the anterior part of the inferior parietal lobule (IPL)37 and consists of a somatosensory-related, higher-order associational cortex.38 Similarly, a recent study that utilized rs-fMRI12 reported increased ReHo in the right superior parietal lobule in patients with CSM. The SPL and IPL are parietal-integrated regions and are also known as the somatosensory association cortex.13 Studies have reported that the IPL is related to spatial perception and the interpretation of sensory information16 and operates as a sensorimotor interface rather than subserving only perceptual functions.39 Moreover, enhancement activation has been reported in the somatosensory association cortex following the postoperative recovery of function in patients with CSM.16,40 A longitudinal fMRI study demonstrated the highly plastic feature of cortical sensorimotor output maps with reorganization in response to changes in the peripheral and central nervous systems.8 The increased zReHo in the lSMG in patients with CSM may be attributed to functional integration and regulation of injury information from the primary sensory cortex.41 This may involve greater activity coherence in the sensory-related cortex to compensate for the decreased sensory loss. It has been reported that the clinical signs and symptoms of CSM are not directly consistent with the degree of spinal cord compression,10–13 and this inconsistency may be explained by the compensatory increase in the ReHo, due to sensory-motor function deficiency in the somatosensory association cortex in patients with CSM.
Altered seed-based functional connectivity in patients with CSM
The lSFGmed as the seed ROI
When the lSFGmed was selected as the seed ROI, we observed increased functional connectivity between the lSFGmed and lPoCG in patients with CSM. There have been many studies on the functional changes in the PoCG in patients with CSM. A rs-fMRI study reported the ALFF was increased in the PoCG in patients with CSM as compared with that of HC.11 Further, Bhaqavatula et al reported increased recruitment and activation of the PoCG in patients with CSM after decompression surgery,34 and Duggal et al reported cortical reorganization in patients with CSM that involved an increase in the degree of activation in both the precentral gyrus (PrCG) and PoCG.9 Studies have reported that neuronal plasticity allows neurons to compensate for injury and disease and adjust their activities in response to surrounding changes.34 For instance, patients who have paresis may compensate for the impairment of motor function by increased use of muscles unaffected by the disease or trauma,42 and the functional changes achieved are thought to take place as a result of altered connectivity or neurotransmission within the central nervous system.42 Previous studies43–45 have reported that the SFGmed is implicated in the control of sequential movement and visuomotor association. The PoCG, which belongs to the somatosensory cortex, receives somatosensory inputs from the thalamocortical systems and sends them to other parts of the somatosensory cortex.46 The increased functional connectivity between the two cortices potentially reflects the compensatory repair of the sensorimotor function in patients with CSM during the recovery stage. An SCI study reported that impaired movement in patients with SCI during the early postinjury stage may result in an overdependence on the associated sensorimotor areas, which play a compensatory role due to the impaired movement function.
In the present study, we also observed increased functional connectivity between the lSFGmed and lROL in patients with CSM. The insula/operculum is involved in interoception and interoceptive awareness and processes signals that are critical for self-awareness. A task-fMRI study observed that the bilateral ROL showed the highest selectivity for bodily self-consciousness based on cardio-visual manipulation and proposed that the ROL processes integrated exteroceptive-interoceptive signals that are necessary for interoceptive awareness and bodily self-consciousness.47 As mentioned above, the SFGmed holds a complete somatotopical representation of body movements through direct connections with the primary motor cortex and spinal cord.29–32 However, a previous magnetic resonance spectroscopy study has reported that there is a decrease in the N-acetylaspartate/creatine metabolite ratio in the primary motor cortex in patients with CSM and cortical levels of N-acetylaspartate/creatine is suggested as a meaningful biomarker in cervical myelopathy, indicative of neuronal damage or dysfunction.10 Therefore, the SFGmed cortex of patients with CSM may receive less or abnormal information from the primary motor cortex. The enhanced functional connectivity between the lSFGmed and lROL in patients with CSM in the current study may help explain the integration of the exteroceptive-interoceptive signals that are vital to processing bodily self-consciousness due to the deficient information input from the impaired primary motor cortex to resist the body unsteadiness of patients with CSM, which represents an adaptive strategy for functional compensation of patients with CSM.
We found a unique decrease in inter-hemispheric connectivity in the bilateral SFGmed in patients with CSM, which has not been reported before. We suspect that the decreased functional connectivity may be related to the ipsilateral influence on cortex reorganization in patients with CSM, which has been explored in several studies.11–13,16 This influence may allow for high-efficient and low-energy cortical reorganization in patients with CSM. However, further research is required to elucidate what the decreased inter-hemispheric connectivity implies.
The lSMG as the seed ROI
When the lSMG was selected as the seed ROI, we observed increased functional connectivity between the lSMG and lPoCG in patients with CSM. Studies have reported that the ipsilateral PoCG and PrCG seem to be extensively recruited in healthy subjects with increase in the motor task difficulty.16,48 Moreover, functional imaging studies have reported enhanced recruitment of the ipsilateral PoCG and PrCG in a wide variety of patients, such as those with stroke, peripheral denervation, and isolated myelitis.49–51 The increased functional connectivity between the lSMG and the lPoCG is consistent with the suggestion that the brain requires functional rearrangements with fascinating cross-modal plasticity when dealing with sensory loss in patients with CSM.13,52 Previous studies on CSM have explored sensory-motor cortical plasticity, which is the dynamic potential of the brain to reorganize following secondary injury in disease progression.7,9,16 Another explanation for the increased functional connectivity between the lSMG and lPoCG in the present study is an analogous cortex reorganization phenomenon explained in a previous SCI study.16 This previous SCI study reported that compensatory reorganization of somatosensory cortices may play a vital role in limiting the deterioration of motor function and contributing to sensorimotor recovery. Combined with the increased ReHo in the lSMG, the increased association between the lSMG and lPoCG may further compensate for sensorimotor function deficiency, which explains the inconsistency between the degree of spinal cord compression and the clinical signs and symptoms of patients with CSM.
In the current study, we also observed increased functional connectivity between the lSMG and the lCAL. Animal studies have reported that the IPL is involved in the “vestibular cortical circuit” in monkeys.53,54 Meanwhile, studies54–56 have also reported that the SMG is related to vestibular stimulation and involved in the increase in connectivity in patients with bilateral vestibular failure. The SMG contains multisensory neurons that receive visual and/or somatosensory input and also play a role in spatial attention during the control of eye movements.57 The calcarine, which is a sulcus of the medial surface of the occipital lobe, belongs to the primary visual cortex and is associated with visual information processing.58 Increased functional connectivity between the IPL and primary visual cortex has been reported and interpreted as a visual compensatory-dependent mechanism due to the dizziness in vestibular patients.55,56 Studies59,60 on the pathophysiological mechanism of cervical vertigo have indicated that there are many direct neurophysiological connections between the somatosensory, vestibular, and visual systems.61–63 In addition to the common symptoms, including neck pain and stiffness, motor and sensory deficits are also observed. A number of patients with CSM may also experience cervical vertigo. We suggest that the increased functional connectivity between the lSMG and lCAL may result from a similar visual compensatory-dependent mechanism as that seen in vestibular patients. Moreover, a similar resting-state study reported increased functional connectivity between the visual cortex and posterior cingulate lobe in patients with CSM.21 The spinal cord has been reported to have an innate ability to recover varying degrees of sensory, motor, and useful neurological function to accommodate the environmental changes. Thus, the increased association between the lSMG and lCAL, which potentially resulted from the visual compensatory-dependent mechanism, may be in line with the visual sensory recovery in patients with CSM.
Correlation between altered zALFF, zReHo, and functional connectivity with clinical measures of patients with CSM
Few fMRI studies on CSM10–13 have reported a significant correlation between functional alterations and JOA scores, NDI scores, or disease duration. Similarly, we did not find any significant correlations between altered zALFF, zReHo, or functional connectivity with these clinical indices. This indicates that the various changes in clinical performance in patients with CSM may be caused by the local insult to the spinal cord rather than intrinsic functional changes of the neurons. Two reasons may explain this phenomenon. First, the interplay between the ongoing destructive mechanisms and the innate reparative processes may eventually reach a balance that implies the innate adaptive or maladaptive plasticity of patients with CSM.12 Next, the JOA and NDI scores are obtained using the questionnaire method; therefore, subjective consciousness and lack of objectivity may influence the scores. Although the JOA system is recommended, it is associated with some clinical disadvantages, including sensitivity, effectiveness, and ignorance of its physical functions for the cervical spine (eg, range of motion of the neck, pain).12,26 Future task-fMRI studies or diffusion tensor imaging studies that could reveal the influence of microstructural white matter changes on cervical cord compression may help identify the elaborate correlation between neuroimage findings and clinical performances of patients with CSM.
Similar to previous fMRI studies, we observed increased zALFF in the lSFGmed and increased zReHo in the lIPL in patients with CSM as compared with subjects in the HC group, without concomitant changes in the lPoCG.11–13 It is worth noting that, unlike the previous hypothesis-driven studies,11–13 the present study was based on data-driven methods. A previous study11 that reported increased ALFF in the PoCG selected the sensorimotor cortex (SMC) as an a priori ROI. The researchers calculated the ALFF values within the SMC to obtain individual ALFF maps within a functional SMC mask and compared the group differences between 19 patients with CSM and 19 controls.11 However, we calculated and compared the ALFF difference within the whole brain between 31 patients with CSM and 31 HC. In addition, during the data preprocessing, we regressed out confounding factors, including 12 estimated head motion parameters, ART-based scrubbing parameters, and mean signals from white matter and CSF, which is different from the previous study. Thus, the diverse results may be due to the difference in research approaches, including data preprocessing and subject sizes, although seed-based functional connectivity analysis revealed a similar increase in the connections between the lPoCG and each of the chosen seed ROIs in the patients with CSM to those that were previously reported.7,8,10–13,16,34,40 The observed differences may reflect the adaptive changes in patients with CSM. Interestingly, there is an obvious pattern in our results. Except for the decreased functional connectivity between the rSFGmed and the lSFGmed, all of the other altered functional connectivity occurred in the left hemisphere. Previous studies have explored the ipsilateral characteristics of CSM.11–13,16 One of the main interpretations for this is the asymmetrical spinal cord compression or asymmetrical spinothalamic sensory loss.13 Other explanations may be the inconsistent maturity of inter-hemispheric regions or the reported ipsilateral recruitment of the PoCG and PrCG for the control of coordinated finger movements during the functional recovery of nonpermanently damaged corticospinal projections in patients with CSM.16
One of the limitations in this study is that we only recruited patients with CSM who had not undergone decompression surgery. Studies have demonstrated that the spinal cord has an innate ability to recover sensory motor function and is helpful in cortical plasticity.64 However, decompression surgery in patients with CSM may accelerate the cortical reorganization for recovery of useful neurological functions and optimization of adaptive strategies.16,34 Thus, the results of the study may have been improved by enrolling patients before and after decompression surgery. Another limitation is that the rs-fMRI analyses we implemented may have been affected by multiple factors, such as alertness/sleepiness, substance and medication intake, pain/discomfort, and head motion. Specifically, neck pain is one of the main symptoms of CSM; therefore, the validity of our results may have been affected by uncontrollable head movements caused by discomfort of neck pain during the image acquisition. These confounding factors should be taken into consideration in future studies. Lastly, In our study, Type I error was controlled through the use of cluster-wise FDR correction of P<0.01, which is conservative compared to P<0.001. We did not observe a significant correlation between the resting-state findings and clinical scores may be potentially associated with the generous threshold. Expanding the sample size and collecting more homogeneous patients with CSM may improve the reliability of the results. In addition, more advanced statistical methods may also contribute to the selection of the most suitable P-value.
Conclusion
In conclusion, we analyzed the intrinsic neuronal functional activity in the brain and found significant alterations in zALFF, zReHo, and functional connectivity in patients with CSM as compared with those of control participants. These resting-state intrinsic functional changes may be related to functional reorganization and may reflect the innate cortical plasticity in patients with CSM, which indicates the powerful adaptive nature of the brain. Thus, our findings provide complementary evidence regarding cortical reorganization in patients with CSM.
Abbreviations
CSM, Cervical spondylotic myelopathy; HC, Healthy Controls; SCI, spinal cord injury; ReHo, regional homogeneity; rs-fMRI, resting-state functional MRI; ALFF, amplitude of low-frequency fluctuations; lSFGmed, left medial superior frontal gyrus; lPoCG, left postcentral gyrus; FDR, false discovery rate; lCAL, left calcarine; lIPL, left inferior parietal lobule; rSFGmed, right medial superior frontal gyrus; PrCG, precentral gyrus; JOA, Japanese Orthopaedic Association; NDI, Neck Disability Index; MNI, Montreal Neurological Institute; BA, Brodmann’s Area; CSF, cerebrospinal fluid; rSPL, right superior parietal lobule; ROI, region of interest; BOLD, blood oxygen level-dependent; FWHM, full-width at half-maximum; T2WI, T2-weighted image; T1WI, T1-weighted image; FOV, field of view; TE, echo time; TR, repetition time; TI, inversion time; T2-FLAIR, T2-fluid-attenuated inversion recovery; ART, artifact detection tool; zALFF, Fisher’s z transformation amplitude of low-frequency fluctuations; zReHo, Fisher’s z transformation regional homogeneity; ROL, Rolandic operculum; DTI, diffusion tensor imaging.
Acknowledgments
We wish to express our gratitude to all participants for their time and effort. This work was supported by Renmin Hospital of Wuhan University. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nicholas JH Sharp and Aimon J Wheeler. Chapter 7- Cervical disc disease- Small Animal Spine Disorders (ed2).Elsevier. 2005: 93–120.
2. Yarbrough CK, Murphy RK, Ray WZ, et al. The natural history and clinical presentation of cervical spondylotic myelopathy. Adv Orthop. 2012;2012:480643. doi:10.1155/2012/480643
3. Nardone R, Holler Y, Brigo F, et al. Functional brain reorganization after spinal cord injury: systematic review of animal and human studies. Brain Res. 2013;1504:58–73. doi:10.1016/j.brainres.2012.12.034
4. Cui JL, Wen CY, Hu Y, et al. Entropy-based analysis for diffusion anisotropy mapping of healthy and myelopathic spinal cord. Neuroimage. 2011;54:2125–2131. doi:10.1016/j.neuroimage.2010.10.018
5. Konomi T, Fujiyoshi K, Hikishima K, et al. Conditions for quantitative evaluation of injured spinal cord by in vivo diffusion tensor imaging and tractography: preclinical longitudinal study in common marmosets. Neuroimage. 2012;63:1841–1853. doi:10.1016/j.neuroimage.2012.06.037
6. Wen CY, Cui JL, Liu HS, et al. Is diffusion anisotropy a biomarker for disease severity and surgical prognosis of cervical spondylotic myelopathy? Radiology. 2014;270:197–204. doi:10.1148/radiol.13121885
7. Holly LT, Dong Y, Albistegui-Dubois R, et al. Cortical reorganization in patients with cervical spondylotic myelopathy. J Neurosurg Spine. 2007;6:544–551. doi:10.3171/spi.2007.6.6.5
8. Jurkiewicz MT, Mikulis DJ, McIlroy WE, et al. Sensorimotor cortical plasticity during recovery following spinal cord injury: a longitudinal fMRI study. Neurorehabil Neural Repair. 2007;21:527–538. doi:10.1177/1545968307308498
9. Duggal N, Rabin D, Bartha R, et al. Brain reorganization in patients with spinal cord compression: a pre and post-surgical comparison using fMRI. Neurology. 2010;74:1048–1054. doi:10.1212/WNL.0b013e3181d6b0ea
10. Kowalczyk I, Duggal N, Bartha R. Proton magnetic resonance spectroscopy of the motor cortex in cervical myelopathy. Brain. 2012;135:461–468. doi:10.1093/brain/awr328
11. Zhou F, Gong H, Liu X, et al. Increased low-frequency oscillation amplitude of sensorimotor cortex associated with the severity of structural impairment in cervical myelopathy. PLoSOne. 2014;9:e104442. doi:10.1371/journal.pone.0104442
12. Tan Y, Zhou F, Wu L, et al. Alteration of regional homogeneity within the sensorimotor network after spinal cord decompression in cervical spondylotic myelopathy: a resting-state fMRI study. Biomed ResInt. v.2015; 2015;647958. doi:10.1155/2015/647958.
13. Zhou FQ, Tan YM, Wu L, et al. Intrinsic functional plasticity of the sensory-motor network in patients with cervical spondylotic myelopathy. Sci.Rep. 2015;5:9975. doi:10.1038/srep09975
14. Zhou F, Wu L, Liu X, et al. Characterizing thalamocortical disturbances in cervical spondylotic myelopathy: revealed by functional connectivity under two slow frequency bands. PLoS One. 2015;10:e0125913. doi:10.1371/journal.pone.0125913
15. Holly LT. Management of cervical spondylotic myelopathy with insights from metabolic imaging of the spinal cord and brain. Curr Opin Neurol. 2009;22:575–581. doi:10.1097/WCO.0b013e3283325ea7
16. Dong Y, Holly LT, Albistegui-Dubois R, et al. Compensatory cerebral adaptations before and evolving changes after surgical decompression in cervical spondylotic myelopathy: laboratory investigation. J Neurosurg Spine. 2008;9:538–551. doi:10.3171/SPI.2008.10.0831
17. Nishimura Y, Isa T. Compensatory changes at the cerebral cortical level after spinal cord injury. Neuroscientist. 2009;15:436–444. doi:10.1177/1073858408331375
18. Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–273. doi:10.1038/35067570
19. Biswal B, F Z Y, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi:10.1002/mrm.1910340409
20. Kelly C, Biswal B, Craddock RC, et al. Characterizing variation in the functional connectome: promise and pitfalls. Trends Cogn Sci. 2012;16:181–188. doi:10.1016/j.tics.2012.02.001
21. Chen Z, Wang Q, Liang M, et al. Visual cortex neural activity alteration in cervical spondylotic myelopathy patients: a resting-state fMRI study. Neuroradiology. 2018;60(9):921–932. doi:10.1007/s00234-018-2061-x
22. Lee MH, Smyser CD, Shimony JS. Resting-state fMRI: a review of methods and clinical applications. Am J Neuroradiol. 2013;34:1866–1872. doi:10.3174/ajnr.A3263
23. Mantini D, Perrucci MG, Del Gratta C, et al. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104:13170–13175. doi:10.1073/pnas.0700668104
24. Zang YF, He Y, Zhu CZ, et al. Altered base line brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi:10.1016/j.braindev.2006.11.009
25. Zang Y, Jiang T, Lu Y, et al. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi:10.1016/j.neuroimage.2003.12.030
26. Yonenobu K, Abumi K, Nagata K, et al. Interobserver and intra-observer reliability of the Japanese Orthopaedic Association scoring system for evaluation of cervical compression myelopathy. Spine. 2001;26:1890–1894. doi:10.1097/00007632-200109010-00014
27. Yan CG, Wang XD, Zuo XN, et al. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 2016;14:339–351. doi:10.1007/s12021-016-9299-4
28. Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi:10.1089/brain.2012.0073
29. Maier MA, Armand J, Kirkwood PA, et al. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb Cortex. 2002;12:281–296. doi:10.1093/cercor/12.3.281
30. Shallice T, Stuss DT, Picton TW, et al. Multiple effects of prefrontal lesions on task-switching. Front Hum Neurosci. 2007;1:2.
31. Krieghoff V, Brass M, Prinz W, et al. Dissociating what and when of intentional actions. Front Hum Neurosci. 2009;3:3. doi:10.3389/neuro.09.003.2009
32. Chouinard PA, Paus T. What have we learned from “perturbing” the human cortical motor system with transcranial magnetic stimulation? Front Hum Neurosci. 2010;4:173. doi:10.3389/fnhum.2010.00173
33. Zhang S, Jaime SI, Chiang-shan RL. Resting-state functional connectivity of the medial superior frontal cortex. Cereb Cortex. 2012;22:9–111. doi:10.1093/cercor/bhr088
34. Bhaqavatula ID, Shaukla D, Sadashiva N, et al. Functional cortical reorganization in case of cervical spondylotic myelophthy and changes associated with surgery. Neurosurg Focus. 2016;40(6):E2. doi:10.3171/2016.3.FOCUS1635
35. Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6:15–28. doi:10.1038/nrneurol.2009.198
36. Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi:10.1126/science.1099745
37. Mennemeier M. Inferior parietal lobule. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer; 2018:51–51. doi:10.1007/978-3-319-57111-9_1308.
38. Leichnetz GR. Supramarginal gyrus. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer; 2011:180–180. doi:10.1007/978-0-387-79948-3_369.
39. Mattingley JB, Husain M, Rorden C, Kennard C, Driver J. Motor role of human inferior parietal lobe revealed in unilateral neglect patients. Nature. 1998;392:179–182. doi:10.1038/32413
40. Tam S, Barry RL, Bartha R, et al. Changes in functional magnetic resonance imaging cortical activation after decompression of cervical spondylosis: case report. Neurosurgery. 2010;67:E863–E864. doi:10.1227/01.NEU.0000374848.86299.17
41. Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi:10.1073/pnas.0504136102
42. Puri B, Smith H, Cox I, et al. The human motor cortex after incomplete spinal cord injury: an investigation using proton magnetic resonance spectroscopy. J Neurol Neurosurg Psychiatry. 1998;65:748–754. doi:10.1136/jnnp.65.5.748
43. Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672.
44. Rushworth MF, Walton ME, Kennerley SW, et al. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi:10.1016/j.tics.2004.07.009
45. Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi:10.1038/nrn2478
46. Wang Z, Chen LM, Negyessy L, et al. The relationship of anatomical and functional connectivity to resting-state connectivity in primate somatosensory cortex. Neuron. 2013;78:1116–1126. doi:10.1016/j.neuron.2013.04.023
47. Blefari ML, Martuzzi R, Salomon R, et al. Bilateral Rolandic operculum processing underlying heartbeat awareness reflects changes in bodily self-consciousness. Eur J Neurosci. 2017;45(10):1300–1312. doi:10.1111/ejn.13567
48. Wexler BE, Fulbright RK, Lacadie CM, et al. An fMRI study of the human cortical motor system response to increasing functional demands. Magn Reson Imaging. 1997;15:385–396. doi:10.1016/S0730-725X(96)00232-9
49. Dong Y, Dobkin BH, Cen SY, et al. Motor cortex activation during treatment may predict therapeutic gains in paretic hand function after stroke. Stroke. 2006;37:1552–1555. doi:10.1161/01.STR.0000221281.69373.4e
50. Reddy H, Floyer A, Donaghy M, et al. Altered cortical activation with finger movement after peripheral denervation: comparison of active and passive tasks. Exp Brain Res. 2001;138:484–491. doi:10.1007/s002210100732
51. Rocca MA, Agosta F, Martinelli V, et al. The level of spinal cord involvement influences the pattern of movement-associated cortical recruitment in patients with isolated myelitis. Neuroimage. 2006;30:879–884. doi:10.1016/j.neuroimage.2005.10.013
52. Frasnelli J, Collignon O, Voss P, et al. Crossmodal plasticity in sensory loss. Prog Brain Res. 2011;191:233–249.
53. Guldin WO, Grüsser OJ. The anatomy of the vestibular cortices of primates. In: Collard M, Jeannerod M, Christen Y, editors. Le Cortex Vestibulaire. Paris: Ipsen, Editions IRVINN; 1996:17–26.
54. Helmchen C, Ye Z, Sprenger A, et al. Changes in resting-state fMRI in vestibular neuritis. Brain Struct Funct. 2013;219:1889–1900. doi:10.1007/s00429-013-0608-5
55. Della-Justina HM, Gamba HR, Lukasova K, et al. Interaction of brain areas of visual and vestibular simultaneous activity with fMRI. Exp Brain Res. 2014;233:237–252. doi:10.1007/s00221-014-4107-6
56. Gottlich M, Jandl NM, Wojak JF, et al. Altered resting-state functional connectivity in patients with chronic bilateral vestibular failure. Neuroimage Clin. 2014;4:488–499. doi:10.1016/j.nicl.2014.03.003
57. Dieterich M, Bense S, Lutz S, et al. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb Cortex. 2003;13:994–1007. doi:10.1093/cercor/13.9.994
58. Gilissen E, Zilles K. The calcarine sulcus as an estimate of the total volume of human striate cortex: a morphometric study of reliability and intersubject variability. J Himforsch. 1996;37:57–66.
59. Kristjansson E, Treleaven J. Sensorimotor function and dizziness in neck pain: implications for assessment and management. J Orthop Sports Phys Ther. 2009;39:364–377. doi:10.2519/jospt.2009.2834
60. Treleaven J, Jull G, Sterling M. Dizziness and unsteadiness following whiplash injury: characteristic features and relationship with cervical joint position error. J Rehabil Med. 2003;35:36–43.
61. Liu JX, Thornell LE, Pedrosa-Domellof F. Muscle spindles in the deep muscles of the human neck: a morphological and immunocytochemical study. J Histochem Cytochem. 2003;51:175–186. doi:10.1177/002215540305100206
62. Richmond FJ, Bakker DA. Anatomical organization and sensory receptor content of soft tissues surrounding upper cervical vertebrae in the cat. J Neurophysiol. 1982;48:49–61. doi:10.1152/jn.1982.48.1.49
63. Edney DP, Porter JD. Neck muscle afferent projections to the brainstem of the monkey: implications for the neural control of gaze. J Comp Neurol. 1986;250:389–398. doi:10.1002/cne.902500311
64. Fawcett JW, Curt A, Steeves JD, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. SpinalCord. 2007;45:190–205.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.