Back to Journals » International Journal of General Medicine » Volume 9
Ability of procalcitonin to diagnose bacterial infection and bacteria types compared with blood culture findings
Authors Watanabe Y , Oikawa N, Hariu M, Fuke R, Seki M
Received 18 June 2016
Accepted for publication 12 August 2016
Published 30 September 2016 Volume 2016:9 Pages 325—331
DOI https://doi.org/10.2147/IJGM.S115277
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yuji Watanabe,1,2 Nozomi Oikawa,1,2 Maya Hariu,1,2 Ryota Fuke,1 Masafumi Seki1
1Division of Infectious Diseases and Infection Control, 2Laboratory for Clinical Microbiology, Tohoku Medical and Pharmaceutical University Hospital, Sendai City, Miyagi, Japan
Abstract: Procalcitonin (PCT) and C-reactive protein serve as biomarkers of infection in patients with sepsis/bacteremia. The present study assessed the clinical characteristics of 280 patients with suspected sepsis who were admitted to Tohoku Medical and Pharmaceutical University Hospital between January 2012 and December 2013. Among the patients, 133 and 147 were positive and negative for PCT, respectively. Patients who were PCT positive were older and more frequently male, had reduced levels of platelets and albumin, and increased levels of aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, creatinine, and C-reactive protein. Patients who were PCT positive had significantly higher blood culture positivity compared with those who were PCT negative, and the sensitivity and specificity of PCT for detecting positive blood cultures were 74.5% and 59.1%, respectively. Escherichia coli was detected in PCT-positive patients, whereas Staphylococcus epidermidis and Staphylococcus lugdunensis were frequently detected in PCT-negative patients. Levels of PCT were higher in the patients infected with gram-negative rods than those with gram-positive cocci. Furthermore, extended-spectrum β-lactamase (ESBL)-producing bacteria cases showed higher levels of PCT than those of non-ESBL cases. These results suggest that PCT may be a useful biomarker of sepsis, and it might serve as a strong tool to detect patients with severe gram-negative rod bacteremia including ESBL-producing bacteria cases early due to its relative high sensitivity.
Keywords: biomarker, sepsis, Escherichia coli, gram-negative rods, ESBL
Introduction
The syndrome of physiological, pathological, and biochemical abnormalities induced by infection called sepsis is a leading cause of mortality and critical illness worldwide.1–3 Initial definitions of sepsis were developed at a consensus conference in 1991 based on the prevalent view that it resulted from a host systemic inflammatory response syndrome (SIRS) to infection; however, such definitions have recently been modified.1,3
The inflammatory biomarkers C-reactive protein (CRP) and procalcitonin (PCT) have frequently been applied in Japan with respect to SIRS, and the detection of infectious bacteria in blood cultures is important for a correct diagnosis and treatment for sepsis.2,4 However, blood cultures require at least 1–2 days and are therefore impractical as a rapid test.5,6 Although CRP is a popular inflammatory marker, blood concentrations increase gradually and also nonspecifically in patients with inflammatory states such as collagen disorders and malignancies.2,4 Although levels of PCT do not increase with viral infections or autoimmune diseases, they increase more rapidly than those of CRP after the onset of bacterial infection and sepsis/bacteremia, and PCT has a long half-life in blood. Therefore, it should be a useful auxiliary test for bacterial infection.2,7,8
The present study measured PCT concentrations in blood samples from patients with suspected sepsis and/or bacteremia and compared laboratory findings between PCT-positive and -negative patients. In addition, we analyzed PCT levels due to isolated bacterial infection which might have been still an unclear issue
Patients and methods
Patients
We retrospectively reviewed the medical records of 280 patients with suspected bacteremia and sepsis in whom PCT had been assayed after admission to Tohoku Medical and Pharmaceutical University Hospital between January 2012 and December 2013. Age, sex, underlying disease, and laboratory data were evaluated. Patients aged <20 years were excluded from the study. If the same bacteria had been isolated from the same patient on several occasions within the period of one admission, we reviewed only the first episode of bacteremia. The serum PCT measurement and blood culture were performed at the same time point using the same blood samples. Ethical approval was obtained from the committee of ethics in Tohoku Medical and Pharmaceutical University (#2015-2-025) and patients signed written informed consent.
PCT assays
Blood concentrations of PCT were measured using the ECLusys 2010 electrochemiluminescence immunoassay (Roche, Basel, Switzerland). Blood PCT concentrations >0.5 µg/L were considered positive.2,7
Identification of bacteria
Blood samples were cultured in BacT/Alert bottles (Sysmex bioMérieux, Kobe, Japan). Positive samples were rapidly identified, and antimicrobial susceptibility was tested using the MicroScan WalkAway 96 plus system (Siemens, Munich, Germany).9,10 All bacterial isolates were identified by analyzing colony morphology and Gram staining.
Definition of bacteremia and sepsis
Bacteremia was defined as one or more positive cultures in blood samples from patients with clinical signs of infection such as fever, chills, and sweats with or without local signs and symptoms.5,10 Sepsis was suspected in accordance with the 2012 guidelines in which SIRS due to infection is classified as at least two of the following: temperature <36°C or >38°C, heart rate >90 beats/minute, respiratory rate >20 breaths/minute, PaO2 <4.3 kPa (32 mmHg), or white blood cell (WBC) count <4000 or >12,000/mm³.3
Statistical analysis
PCT-positive and -negative patients were compared using the Fisher’s exact test, Student’s paired t-test, and analysis of variance test (StatMate V; Atoms, Tokyo, Japan). P-values <0.05 were considered to indicate statistical significance.6,10
Results
Demographics of patients with suspected bacteremia
Table 1 shows the background and laboratory data for 133 PCT-positive and 147 PCT-negative patients with suspected bacteremia and/or sepsis. Age and sex basically differed between the two groups. The PCT-positive group was older and comprised more males than the PCT-negative group. Significantly fewer PCT-positive patients had autoimmune diseases including rheumatoid arthritis requiring treatment with immune modulators such as steroids. However, respiratory, heart, digestive, and neurological diseases or surgery were not significantly associated with PCT positivity.
Indicators of inflammation status, namely, WBC counts and CRP, were significantly increased in PCT-positive patients, compared with negative patients (Table 2).
In addition, significantly lower platelet counts suggested disseminated intravascular coagulation in the PCT-positive patients, although total bilirubin, aspartate aminotransferase, and alanine aminotransferase values did not differ between the groups. Renal function indicated by creatinine and blood urea nitrogen values was worse in PCT-positive patients than in PCT-negative patients. Serum albumin concentrations were also lower in the PCT-positive patients, suggesting a worse nutritional status than that of PCT-negative patients.
Bacteremia findings according to PCT assays and comparison with blood cultures
Table 3 shows the results of the PCT assays and cultures of 280 blood samples. Among these, bacteria were positive in 55 (19.6%) samples, among which 14 (25.5%) were positive by culture alone, whereas 92 (69.2%) of 133 were positive by PCT alone. Patients who were PCT positive showed significantly higher blood culture positivity compared with those who were PCT negative (14.6% vs 5.0%, respectively). The sensitivity and specificity of PCT to detect infection in blood cultures were 74.5% (41/55) and 59.1% (133/225), respectively.
  | Table 3 PCT and blood culture results Note: **Significant at P<0.01. Abbreviation: PCT, procalcitonin. |
Types of bacteria isolated from blood samples of PCT-positive and -negative patients
Detected pathogens from blood culture in PCT-positive and -negative patients are shown in Table 4. Staphylococcus epidermidis was the most prevalent type of bacteria, but significantly less predominant in the PCT-positive group along with Staphylococcus lugdunensis. The prevalence of Staphylococcus aureus, including methicillin-resistant S. aureus, was similar in both groups.
In contrast to these two gram-positive cocci and others, gram-negative rods of Escherichia coli were more prevalent in the PCT-positive patients than in the PCT-negative patients. All five species of Klebsiella pneumoniae, which are also gram-negative rods similar to E. coli, were isolated from PCT-positive patients. The prevalence of extended-spectrum β-lactamase (ESBL)-producing bacteria was not high and did not significantly differ between the two groups of patients
Levels of PCT and bacterial species
We analyzed correlations between PCT concentrations in the blood of patients from whom bacteria were isolated from blood cultures and the species of these bacteria.
First, PCT concentrations were significantly higher in blood culture-positive cases (n=55) than blood culture-negative cases (n-235) (6.0±28.4 vs 0.29±0.5, respectively, P=0.03). In addition, PCT concentrations were also significantly higher in patients infected with E. coli or K. pneumoniae than in those infected with S. epidermidis and gram-positive cocci other than S. aureus and S. epidermidis, but did not significantly differ between patients infected with S. aureus and any other patients (Figure 1).
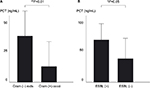
Overall, PCT concentrations were higher in patients harboring gram-negative rods than gram-positive cocci (Figure 2A). Furthermore, we also found that ESBL-producing bacteremia cases due to E. coli and Proteus mirabilis showed higher concentrations of PCT, compared with those cases due to non-ESBL-producing bacteria (Figure 2B).
Discussion
PCT has emerged as a promising marker for diagnosing bacterial infections because levels are higher in patients infected with bacteria than viruses and in those with nonspecific inflammatory diseases.2,7 Hence, PCT values might be used to support clinical decisions about the initiation and cessation of antibiotic therapy for bacterial infection.8
We analyzed PCT levels in the blood of 280 patients with suspected sepsis/bacteremia. We found that 133 of these patients who were PCT positive were older and mostly males. Values for WBC, lactate dehydrogenase, CRP, and albumin were increased, but platelets and renal function were decreased, compared with patients who were PCT negative, that is, PCT was increased when inflammatory and organ status was suggested to be worse, such as in disseminated intravascular coagulation, renal failure, malnutrition, and sepsis.
Sepsis has been defined as a SIRS caused by infection, and this definition has remained unchanged for over two decades.3 However, sepsis has recently been redefined according to sequential organ failure assessment scores as more complex status with organ dysfunction, similar to “severe sepsis” in the previous definition.1 Our findings matched the new definition because PCT positivity suggested not only SIRS but also organ failure.1
Furthermore, blood cultures were more frequently positive in patients who were PCT positive than PCT-negative patients, and the sensitivity and specificity of PCT for blood cultures in patients with suspected sepsis were relative high and low, respectively. These findings suggested that the old definition of sepsis based on SIRS criteria covers bacteremia and more. PCT values might be used to indicate patients with severe inflammatory status early including real bacteremia, and the low specificity of PCT might have been tolerant in the bedside to start the antibiotic therapy as soon as possible to save the patients.
Although the sensitivity of PCT was not quite high in detecting patients with confirmed bacteremia, PCT concentrations were significantly higher in patients who were infected with E. coli than in those infected with S. epidermidis and S. lugdunensis. Overall, PCT values were significant higher in patients infected with gram-negative rods than with gram-positive-cocci. These findings suggest that PCT could distinguish bacterial types.
Gram-negative rods, including E. coli and K. pneumoniae, produce endotoxins and frequently induce extreme inflammation and sepsis.3,7,11
Endotoxins are one of the strong inducers of PCT, and Dandona et al found significant PCT increase after endotoxin injection in normal subjects.12 Endotoxins are produced from gram-negative rods and these might be the reasons why PCT increases in gram-negative rods, but not in gram-positive cocci cases.
Levels of PCT are increased in patients infected with gram-negative bacteria, and our results confirmed this fact. Moreover, we found that ESBL-positive cases showed higher PCT concentrations than ESBL-negative cases. The frequency of infection with ESBL-producing E. coli and P. mirabilis that are resistant to third-generation cefem antibiotics is increasing worldwide including in Japan.13,14 If we could distinguish ESBL types and non-ESBL types by PCT concentrations, PCT might be very useful for detecting patients infected with such agents and facilitating rapid and appropriate antibiotic treatment, including carbapenem use. Concentrations of PCT were lower in patients with bacteremia caused by gram-positive cocci than gram-negative cocci. Gram-positive cocci, especially S. epidermidis, are frequent contaminants of blood cultures.15 Low PCT could exclude such contamination among patients with suspected sepsis and patients with high fever or extreme inflammation caused by conditions such as malignancies and collagen diseases.
A systematic review of individual patient data derived from a meta-analysis of 14 trials found no increased risk for mortality or treatment failure when PCT served as a guide to the initiation and duration of antibiotic therapy among patients with acute respiratory infections compared with controls, and such guidance significantly reduced antibiotic administration across various clinical settings and acute respiratory infection diagnoses.8 The guide to the initiation and duration of antibiotic therapy dependent on on the ability of PCT to distinguish bacterial species supported by the present study. Embedding PCT in clinical algorithms has the potential to improve antibiotic management for individual patients and has substantial clinical and public health implications in terms of reducing antibiotic exposure and the associated risk of acquiring resistance to antibiotics.8,16,17
In conclusion, we analyzed PCT concentrations and blood culture results in patients suspected with sepsis/bacteremia. Patients who were PCT positive had more severe inflammation and worse clinical status, including suspected organ failure, which matched the most recent definition of sepsis. The sensitivity of PCT for detecting bacteremia was relative high, although specificity was low in comparison to blood culture findings. However, PCT values were significantly higher among patients with bacteremia caused by gram-negative rods, including ESBL-producing types. PCT might help to detect bacteremia and severe organ failure patients early and lead to immediate antibiotic therapy.
Acknowledgement
This work was supported by Japanese Society for the Promotion of Science Grant-in-Aid for Scientific Research 26461158 (to MS)
Disclosure
The authors report no conflicts of interest in this work.
References
Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. | ||
Nishikawa H, Shirano M, Kasamatsu Y, et al. Comparative usefulness of inflammatory markers to indicate bacterial infection-analyzed according to blood culture results and related clinical factors. Diagn Microbiol Infect Dis. 2016;84(1):69–73 | ||
Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;42(2):580–637. | ||
Seki M, Watanabe A, Mikasa K, Kadota J, Kohno S. Revision of the severity rating and classification of hospital-acquired pneumonia in the Japanese Respiratory Society guidelines. Respirology. 2008;13(6):880–885. | ||
Yanagihara K, Kitagawa Y, Tomonaga M, et al. Evaluation of pathogen detection from clinical samples by real-time polymerase chain reaction using a sepsis pathogen DNA detection kit. Crit Care. 2010;14(4):R159. | ||
Seki M, Takahashi H, Yamamoto N, et al. Polymerase chain reaction-based active surveillance of MRSA in emergency department patients. Infect Drug Resist. 2015;8:113–118. | ||
Aikawa N, Fujishima S, Endo S, et al. Multicenter prospective study of procalcitonin as an indicator of sepsis. J Infect Chemother. 2005;11(3):152–159. | ||
Schuetz P, Briel M, Christ-Crain M, et al. Procalcitonin to guide initiation and duration of antibiotic treatment in acute respiratory infections: an individual patient data meta-analysis. Clin Infect Dis. 2012;55(5):551–562. | ||
Seki M, Gotoh K, Nakamura S, et al. Fatal sepsis caused by an unusual Klebsiella species that was misidentified by an automated identification system. J Med Microbiol. 2013;62(Pt 5):801–803. | ||
Isobe M, Uejima E, Seki M, et al. Methicillin-resistant Staphylococcus aureus bacteremia at a university hospital in Japan. .J Infect Chemother. 2012;18(6):841–847. | ||
Yamamoto N, Takegawa R, Seki M, et al. Pneumorachis associated with multiorgan infection due to Citrobacter koseri. Diagn Microbiol Infect Dis. 2013;77(4):370–372. | ||
Dandona P, Nix D, Wilson MF, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79(6):1605–1608. | ||
Yamaguchi K, Ishii Y, Tateda K, et al. Nationwide surveillance of parenteral antibiotics containing meropenem activities against clinically isolated strains in 2012. Jpn J Antibiot. 2014;67(2):73–107. | ||
Hawkey PM. Multidrug-resistant Gram-negative bacteria: a product of globalization. J Hosp Infect. 2015;89(4):241–247. | ||
Mirrett S, Weinstein MP, Reimer LG, Wilson ML, Reller LB. Relevance of the number of positive bottles in determining clinical significance of coagulase-negative staphylococci in blood cultures. J Clin Microbiol. 2001;39(9):3279–3281. | ||
Drozdov D, Schwarz S, Kutz A, et al. Procalcitonin and pyuria-based algorithm reduces antibiotic use in urinary tract infections: a randomized controlled trial. BMC Med. 2015;13:104. | ||
Lam SW, Bauer S, Duggal A. Procalcitonin-based algorithms to initiate or stop antibiotic therapy in critically ill patients: Is it time to rethink our strategy? Int J Antimicrob Agents. 2016;47(1):20–27. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.