Back to Journals » International Medical Case Reports Journal » Volume 15
Abdominal Tuberculosis Mimicking Ovarian Cancer: A Case Report and Review of the Literature
Authors Rinaldi I , Muthalib A, Gosal D, Wijayadi T, Sutedja B, Setiawan T, Gunawan A, Susanto N, Magdalena L, Handjari DR, Kurniawan F , Rifani A, Winston K
Received 17 November 2021
Accepted for publication 25 February 2022
Published 11 April 2022 Volume 2022:15 Pages 169—185
DOI https://doi.org/10.2147/IMCRJ.S348434
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ronald Prineas
Ikhwan Rinaldi,1,2 Abdul Muthalib,1,2 Djaja Gosal,2 Teguh Wijayadi,2 Barlian Sutedja,3 Tjondro Setiawan,4 Andika Gunawan,5 Nelly Susanto,4 Lingga Magdalena,4 Diah Rini Handjari,6,7 Fetisari Kurniawan,6,7 Aisyah Rifani,8 Kevin Winston8
1Divison of Hematology and Medical Oncology, Department of Internal Medicine, Cipto Mangunkusumo National General Hospital, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia; 2Department of Internal Medicine, Gading Pluit Hospital, Jakarta, Indonesia; 3Departement of General Surgery, Gading Pluit Hospital, Jakarta, Indonesia; 4Departement of Radiology, Gading Pluit Hospital, Jakarta, Indonesia; 5Departement of Nuclear Medicine, Gading Pluit Hospital, Jakarta, Indonesia; 6Department of Anatomical Pathology, Cipto Mangunkusumo National General Hospital, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia; 7Departement of Pathology, Gading Pluit Hospital, Jakarta, Indonesia; 8Departement of Internal Medicine, Cipto Mangunkusumo National General Hospital, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Correspondence: Ikhwan Rinaldi, Division of Hematology and Medical Oncology, Department of Internal Medicine, Cipto Mangunkusumo National General Hospital, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia, Tel +62 811-177-997, Email [email protected]
Introduction: Tuberculosis (TB) is a disease with high morbidity and mortality resulting from infection by Mycobacterium tuberculosis. TB can disseminate to any organ system of the body resulting in extrapulmonary tuberculosis. Interestingly, CA-125, which is a biomarker for some cancer, also rises in benign diseases such as pulmonary and extrapulmonary tuberculosis which may complicate diagnosis. In this case report, we present an abdominal tuberculosis patient that was initially presented as ovarian cancer.
Case Report: A 30-year-old woman admitted to the emergency department with chief complaint of fatigue and shortness of breath since 3 months ago. She had lost around 20 kg weight in the past 5 months. She was previously suspected with ovarian cancer because of the characteristic features of malignancy, high levels of CA-125, and positive PET scan. She was later diagnosed with abdominal TB. Subsequently, the patient was given anti-TB drugs, and the patient showed clinical improvement.
Conclusion: In the case of an elevated CA-125, clinicians should consider extrapulmonary TB as a differential diagnosis of ovarian cancer, especially in countries with high burden of tuberculosis.
Keywords: extrapulmonary tuberculosis, CA-125, ovarian cancer, malignancy, case report
Introduction
Tuberculosis (TB) is an infectious disease with high morbidity and mortality caused by Mycobacterium tuberculosis. Indonesia is a country which has been designated as a “high burden country” for tuberculosis infection.1 A study conducted in year 2014–2015 showed that Indonesia had approximately 1 million cases of TB.2 Furthermore, TB is the fourth highest cause of disability-adjusted life years (DALYs) in Indonesia.3 Globally, WHO estimates around 1.3 million deaths each year from TB infection.4 Thus, TB is a global public health threat, especially in developing countries.
TB is transmitted through inhalations of droplets carrying TB bacterium.5 Patients who have active pulmonary TB infection usually have symptoms such as chronic cough with hemoptysis, fever, and weight loss.6 While pulmonary TB infection represents the dominant clinical phenotype of TB infection, around 15–20% of TB infection occurs extrapulmonary or concomitantly with pulmonary TB due to hematogenous spread or direct spread via adjacent lymph nodes.7,8 The location of extrapulmonary TB (EPTB) is heterogeneous, which can range from central nervous system involvement to genitourinary system involvement.7,8
Diagnosing EPTB such as abdominal tuberculosis can be very difficult due to the lack of specific symptoms. Furthermore, EPTB can mimic other diseases. For example, abdominal tuberculosis can mimic ovarian cancer because it also shows ascites and pelvic mass.
One of the cancer biomarkers for ovarian carcinoma is CA-125. Interestingly, CA-125 has been reported to rise in patients with pulmonary and extrapulmonary tuberculosis such as peritoneal tuberculosis.9 A study conducted by Ichiki et al showed that about 44.4% of the patients with active pulmonary TB had elevated serum CA-125.10 Without clinical suspicion of tuberculosis infection, elevation of serum CA-125 may lead to oncological diagnosis.
In this case report, we present a patient referred to our cancer center suspected of having ovarian cancer based on clinical symptoms, elevated CA-125 levels, and PET-scan results. Initial examinations did not lead to abdominal tuberculosis diagnosis. After further examinations, the definitive diagnosis was changed to abdominal tuberculosis. This is the first case report of abdominal TB mimicking ovarian cancer in Indonesia. Other unique feature of this case is the concomitant presence of lymph node tuberculosis and bone tuberculosis. We believe this case report demonstrates difficulties in diagnosing abdominal tuberculosis. This case report also shows that clinical improvements of abdominal tuberculosis may not occur in the first month after Tuberculosis treatment started.
Case Report
A 30-year-old woman presented to the emergency department on 10 September 2020 with chief complaint of worsening shortness of breath for the past 2 months.
Patient also had dry cough and fatigue. She had lost around 20 kg weight in the past 5 months. She denied any symptoms of fever, angina, changes in bowels habit, and tremor. The patient denied any contact with positive COVID-19 patient, history of diabetes, heart failure, kidney disease, and liver disease. The patient was tested negative for COVID-19 PCR on 29 Augustus 2020. No similar symptoms were observed in her family.
She previously visited another hospital because she had right pleural effusion and then suspected of abdominal cancer. Pleural puncture was conducted 1 week before for cytology examination at the previous hospital but the procedure failed to gain adequate sample. The patient also denied family history of malignancies.
Physical examination on 10 September 2020 showed normal vital signs with blood pressure 132/90 mmHg, respiratory rate 23x per minute, heart rate 119 beats per minute, and temperature of 36.3 Celsius. Additionally, oxygen saturation was 93%. There was no altered mental status. Lung auscultation revealed decreased vesicular sound on the right pulmonary area. Rales and wheezing were not present during auscultation. Cardiac auscultation revealed distant heart sound. Abdominal examination showed presence of minimal ascites. No peripheral edema was observed. The rest of the physical examinations were normal.
Complete blood count and other laboratory examinations were performed on 10 September 2020 (Table 1). The patient’s lab revealed that she had anemia with 8.1 g/dL of hemoglobin, slightly elevated Aspartate transaminase (AST), normal alanine transaminase (ALT), and reduced albumin level. SARS‐CoV‐2 PCR swab test was negative and other laboratory tests were normal. The cancer biomarker results which was available 2 days later showed elevated CA-125 (121.07 U/mL). Due to the presence of severe anemia, the patient received packed red cell transfusion of 600 mL.
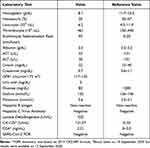 |
Table 1 Laboratory Test Result (September 10th, 2020) |
Her initial chest radiograph (CXR) on 10 September 2020 showed bilateral pleural effusion, mild right lung infiltrate/pneumonia, and cardiomegaly due to pericardial effusion (Figure 1).
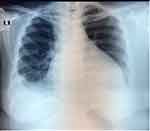 |
Figure 1 Initial chest x ray on 10 September 2020 which showed pleural effusion and pericardial effusion. |
Her non-contrast chest CT scan taken on the same day also showed bilateral pleural effusion and pericardial effusion (Figure 2). Lymph node enlargement on anterior mediastinum, paratracheal, tracheobronchial, and right hilum were observed. The lymph node enlargement was initially suspected due to metastasis. Further information obtained from the chest CT scan was the presence of lytic lesion on sternal bone, head of right humerus, and thoracal vertebrae. Finally, the CT scan indicated presence of pneumonia on left lower lung. Due to the presence of non-COVID-19 pneumonia, the patient was then treated with Cefixime 200 mg 2x1, Ceftriaxone 2g injection, Omeprazole 20 mg 2x1, and supportive therapies.
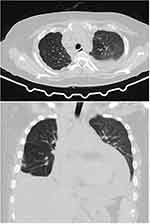 |
Figure 2 Thorax CT scan showed pleural and pericardial effusion. |
On 11 September 2020, a cardiac echocardiography was taken showing normal ventricles volumes, systolic function, diastolic function, and valves, tricuspid annular plane systolic excursion (TAPSE) of 2 cm, ejection fraction (EF) of 75%, inferior vena cava collapsibility of more than 50%, and pericardial effusion of 3.8 cm on plax view.
Arterial blood gas analysis was performed on 11 September 2020 (Table 2). Based on the results, the patient had acute uncompensated respiratory alkalosis with high likelihood caused by pneumonia.
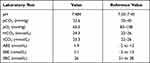 |
Table 2 Arterial Blood Gas Result (September 11th, 2020) |
On 12 September 2020, further laboratory examinations were conducted. Results showed bleeding time 2.5 seconds; clotting time 4 seconds; prothrombin time 12.2 seconds (control 10.6 seconds); partial thromboplastin time 33.9s seconds (control 35.2 seconds); and CA 15–3 level 14.7 U/mL.
Positron emission tomography (PET) scan was conducted on 14 September 2020 (Figure 3). The PET scan was conducted 50 minutes after injection of F-18 Fluorodeoxyglucose (FDG). The PET scan showed non-metabolic (non FDG avid) thickening on the region of bilateral adnexa. The radiologist suspected primary malignancy originating from thickening of bilateral adnexa which could be in the form of ovarian cancer. Scan also revealed multiple osteolytic hypermetabolic metastases located in bone marrow of right humerus head, sternal bone region, several bilateral ribs, pelvic bone, and most of cervical/sacral vertebra bone. There were also multiple hypermetabolic metastases at lymph nodes of cervical, right mediastinal, and right axilla. Cardiomegaly was observed with hypermetabolic pericardial effusion which suggested pericarditis or pericardial metastasis. Hypermetabolic pleural effusion with suspicion of pleural metastasis was also visible. Finally, there was presence of non-metabolic bleakness at the area of upper omentum with suspicion of metastatic seeding in the form of carcinomatosis omental and there was presence of non-metabolic asymmetrical thickening of ascending colon suggestive of inflammation or cancer mass.
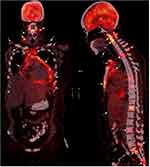 |
Figure 3 PET scan on 14 September 2020 showing positive intake on bones, pericardial effusion, and lymph nodes. |
CXR on 15 September 2020 showed no improvement in pericardial effusion. However, pigtail catheter was implemented. Similarly, there was no improvement in pleural effusion. The lung infiltrate was no longer present. In concordance to the resolution of lung infiltrate on CXR, the patient’s dyspnea was improved. The cefixime antibiotic was still continued based on pneumonia guideline.
On 16 September 2020, obstetrician gynecologist was consulted for evaluation of possible ovarian cancer based on the elevated CA-125 and results from PET-scan. The obstetrician gynecologist reported that there were no signs of abnormalities such as cancer or cysts on gynecological organs based on the USG. These findings were in contrast with the PET-scan.
On 17 September 2020, the patient had improved hemoglobin level to 10.6 mg/dL. Cyfra 21–1, a biomarker for lung cancer was tested with result of 0.68 (reference <3.3).
Analysis of pleural and pericardial effusion were completed by pathologist on 18 September 2020. A total of 450 mL of xerohemorrhagic fluid was taken from pleural aspiration. Cytologic examination of pleural fluid showed lymphocytes, leukocytes, and mesothelial cells with no presence of malignant cells. Pericardial effusion aspiration showed color of xerohemorrhagic with a total of 200 mL aspirated. Samples of pericardial fluid were sent for cytologic examination which showed presence of leukocytes and no presence of malignant cells.
On 18 September 2020, lymph nodes biopsy was taken with results came on 21 September 2020. Based on the pathological anatomy examination, the biopsy showed tubercles surrounded by lymphocytes. The tubercles contained epithelioid cells, datia Langhans (giant multinucleated) cells, and necrosis. No malignant cells were observed. Based on these findings, the pathological anatomy report concluded the diagnosis as tuberculous lymphadenitis. A meeting with attending physicians at that time concluded that the patient’s symptoms did not match pathology report and thus further differential diagnosis should be considered.
Due to improvement of the patient’s dyspnea, on 19 September 2020 the patient was discharged and scheduled for outpatient visit for further examinations.
On 30 September 2020, another pleurocentesis was conducted. A total of 400 mL of xerohemorrhagic fluid was aspirated from the pleura. Microscopic examination showed no presence of malignant cells. The pleural fluid was then examined for ADA (Adenosine Deaminase) and the result was 31.6 (Tuberculosis if ADA > 40), which indicated that the cause of pleural effusion was other than TB. However, due to lack of specific etiologies found other than possible ovarian cancer, it was decided that the patient should receive anti-tuberculosis drug starting on 1 October 2020 with monitoring of symptoms.
On 2 October 2020, another CXR was taken. When compared with CXR on 15 September 2020, no change in volume of pleural effusion and pericardial effusion was observed. Patient was given bondronate 6 mg through normal saline infusion for 90 minutes.
Colonoscopy on 6 October 2020 revealed no abnormal findings other than Peyer's patches hyperplasia at the terminal ileum. A total of 3 biopsies of the Peyer's patches was taken and examined for pathology. Microscopic examination by the pathologist on 8 October 2020 showed mild to moderate infiltration of lamina propria by inflammatory cells, indicating chronic ileitis. No malignant cells were found during microscopic examination. Laboratory examinations on 31 October 2020 are shown on Table 3.
 |
Table 3 Laboratory Test Result (31 October, 2020) |
Despite receiving tuberculosis drugs, initially the patient had worsening ascites and increasing level of CA-125. Another consultation was made to obstetrician gynecologist on 10 November 2020. Based on examinations and ultrasound by the obstetrician gynecologist, it was concluded that both left and right ovarium was normal with no signs of malignancy. Transvaginal ultrasonography conducted by the obstetrician gynecologist showed normal size uterus retroflexion, normal size of both ovarium, and ascites on pouch of Douglas. The obstetrician gynecologist also recommended diagnostic laparoscopy and biopsy to differentiate between peritoneal cancer and peritoneal tuberculosis. Furthermore, the obstetrician gynecologist revealed that the patient told her that the patient’s IGRA test was positive previously on 5 September 2020. This IGRA test was not previously told to other physicians.
On 24 November 2020 the patient came to the outpatient clinic due to bloating and abdominal enlargement for the last 7 days. The patient was then suggested to be hospitalized. Abdominal paracentesis was then conducted. Result of paracentesis showed that neither malignant cell or tuberculosis cells were detected. A chest radiograph and non-contrast chest CT scan were also performed. Her CXR showed pleural effusion higher than last month and pericardial effusion with same size compared to 2 October 2020 (Figure 4). No lung infiltrates and metastatic nodes were observed on CXR. The non-contrast chest CT scan revealed slightly elevated bilateral pleural effusion, decreased pericardial effusion, and bone metastasis compared with 1 month before.
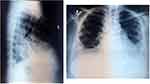 |
Figure 4 CXR showed more severe pleural effusion compared to previous month. |
On 25 November 2020, pleural puncture was conducted and 1.320 mL of yellow fluid was taken. No malignant cells were observed. Ascites fluid had ADA of < 5. Analysis of CA-125 laboratory test on 26 November 2020 showed the highest level which was 769.18 units per mL.
Diagnostic laparoscopy was conducted on 28 November 2020. The liver appeared very large and the surface is full of white patches (Figure 5). Omentum looked thickened. Biopsies were taken from liver and omentum to be analyzed by pathologist.
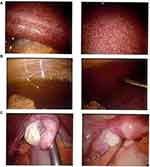 |
Figure 5 Omentum and liver were seen on diagnostic laparoscopy. (A) Liver enlargement with white patches on its surface. (B) Thickened omentum. (C) Normal ovarium and fallopian tube. |
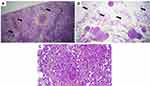
Biopsies results were obtained on 30 November 2020 (Figure 6). Liver biopsy revealed fatty infiltration of liver and granuloma containing inflammatory cells. Omentum biopsy revealed granuloma containing epithelioid cells and Langhans-type cells. These results suggested tuberculosis infection of omentum and liver.
Patient reported improvement after approximately two months of anti-TB treatment. Furthermore, CA-125 level decreased to 244.57 units per mL (Table 4). No side effects of tuberculosis drugs such as jaundice, liver enzymes elevation, peripheral neuropathy, and vision changes were found during the intensive phase of tuberculosis treatment regiment. CA-125 level decreased further into 13.81 units per mL after approximately 3 months of antituberculosis treatment, and 4.45 units per mL after approximately 9 months of treatment. (Table 5) (Figure 7).
 |
Table 4 Laboratory Test Result (22 December 2020) |
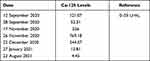 |
Table 5 CA-125 Serial Examination Before, During, and After Treatment |
 |
Figure 7 Serial CA-125 examination. |
The patient was still alive and well on August 24th 2021. She did not feel any clinical symptoms and felt much better compared to last year. She underwent thoracic CT-scan and whole abdomen CT-scan on the same day.
Thoracic CT scan revealed lessened pleural effusion when compared with previous CT-scan with minimal right pleural effusion observed. There was fibrosis on lower region of both lungs. Lymph nodes of axillary region, anterior region, and paratracheal region were no longer enlarged. Pericardial effusion was no longer observed but there was thickening of pericardium. Lytic sclerotic lesions were observed on right humerus head, several thoracal vertebrae body, several lumbar vertebrae body, several ribs, and sternal bone.
Whole abdominal CT scan showed lytic sclerotic lesions on whole lumbar vertebral body and several sacral vertebrae (Figure 8). CT scan also revealed lumbar spondyloarthrosis with mild narrowing of intervertebral disc of lumbar vertebra 4–5 and bulging disk on lumbar vertebra 3–4 and lumbar vertebra 4–5. No enlargement of paraaortic and pelvic lymph nodes was observed. Thickening of colon was no longer observed. Liver showed regular surface, normal left lobe size, slightly enlarged right lobe size, and homogeneous parenchymal density. After contrast injection, no pathological lesions were observed. Liver vascularization was within normal range. Biliary system was normal. Gynecological organs also showed no pathologies other than Nabothian cyst on cervix and follicular cyst on both ovarium after contrast injection (Figure 9).
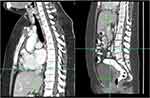 |
Figure 8 CT-Scan with contrast after TB treatment showed lytic sclerotic bone lesions on vertebrae. |
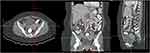 |
Figure 9 Abdominal CT-scan showed presence of follicular cyst. |
Discussion
EPTB is defined as tuberculosis infection located other than pulmonary tissue. According to an epidemiology study conducted in China, skeletal TB and pleural TB constitute the most common form of EPTB, with prevalence of 41.1% and 26.0% respectively.11 However, the study did not specify the prevalence of abdominal or peritoneal TB. Recent study by Kang et al in 2020 from China showed that prevalence of peritoneal tuberculosis was approximately 4.79%.12 Unfortunately, up to this date, there has been no study which describe the prevalence of abdominal tuberculosis or peritoneal tuberculosis in Indonesia, even though Indonesia is one of the highest country with tuberculosis burden. Indeed, research of EPTB is difficult to be conducted due to low disease prevalence of EPTB, heterogeneous symptoms, and difficulty in diagnosis.
Diagnosing EPTB poses a unique challenge for clinicians worldwide. There is often lack of specific symptoms and history of tuberculosis contacts. Symptoms also varies depending on the site of EPTB. For example, the symptoms of abdominal tuberculosis consisted of abdominal swelling, abdominal pain, abdominal mass, weight loss, fever, and ascites.13–15 Meanwhile, central nervous system tuberculosis usually involves fever, headache, altered mental status, and neurological deficits.16
We believe this case report demonstrates difficulties in diagnosing abdominal tuberculosis. Another important factor that may complicate diagnosis is that this patient came to a tertiary cancer center. This is the first case report in Indonesia that described a patient with TB presenting with symptoms suggesting ovarian cancer. Furthermore, this case report also showed that PET-scan may cause false positive from enhanced cellular metabolism, whether the cells are malignant or not. Thus, further examinations such as biopsies are needed to make proper diagnosis.
The patient in this case report initially came with worsening dyspnea, dry cough, and fatigue. Physical examination also showed mild ascites initially. Further workups revealed elevated CA-125, pleural effusion, pericardial effusion, and signs of ovarian cancer from PET-scan. Indeed, all of these led to suspicion of ovarian cancer. After treating the patient’s non-COVID pneumonia, the symptoms of dyspnea improved but there was no resolution in pleural effusion, pericardial effusion, and ascites which showed there were additional etiologies that should be explored in this patient.
Diagnosis of Extrapulmonary Tuberculosis
A study by Tandirogang et al in Indonesia showed that only 4.3% of patients with EPTB had contact with tuberculosis patients.17 Our case report also support this as the patient did not have any contacts with tuberculosis patients. Furthermore, the patient’s family also had no symptoms suggestive of tuberculosis infection.
Repeated analysis of pleural fluid and pericardial fluid in this patient failed to observe the presence of malignant cells. Meanwhile, biopsies on lymph nodes showed tuberculosis lymphadenitis based on pathology examination. However, this was contrary to PET-Scan result which indicated ovarian malignancy with lymph nodes, bone, pericardial and pleural metastasis. Furthermore, symptoms were not suitable to lymph nodes tuberculosis. Thus, at that time, we considered biopsy results from lymph nodes to be incidental finding. Further cancer workups were conducted after PET-scan as there was suspicion of malignancy due to non-specific symptoms, elevated CA-125, and PET-scan result itself. However, further examinations failed to find malignancy and obstetrician gynecologist ruled out abnormalities in reproductive system based on ultrasound. Of interest is that the pleural puncture examination based on ADA indicated non-tuberculosis disease. As a result, based on PET-scan and lymph nodes biopsy, we considered the possibility of ovarian cancer with concomitant tuberculosis infection. Hence, tuberculosis treatment was started on 1 October 2020.
The patient experience worsened ascites and abdominal enlargement on first month of treatment, which led to possibility of ovarian cancer. We further consulted obstetrician-gynecologist, but no abnormalities of reproductive organs were found. However, this time, another obstetrician-gynecologist revealed that the patient failed to mention her positive IGRA test, thus abdominal tuberculosis was considered as differential diagnosis.
Extrapulmonary TB was confirmed after diagnostic laparoscopy and biopsy were conducted. The most effective method for diagnosing extrapulmonary TB in this case was tissue biopsy through laparoscopy. This is in accordance with previous literatures which stated that the gold standard for determining the diagnosis of abdominal TB was laparoscopy with biopsy.13,18–20 In addition, it should be noted that infection and inflammation can also cause positive PET scan.21
In EPTB, it is challenging to obtain material from infected tissue and often, there is a small number of bacteria along with extensive tissue damage.7 Therefore, conventional smear microscopy acid-fast bacilli (AFB) smear and culture showed low sensitivity resulting in relatively high rate of false negatives.7,22,23 For examples, the sensitivity for microscopy is around 0%-40%.7 Cultures which is a gold standard for tuberculosis diagnosis yielded wide variances in sensitivity for extrapulmonary tuberculosis; ie peritoneal biopsy (38–92%), liver biopsy (33–50%), and pericardial fluid (25–77%).22 Another issue from cultures is long duration needed to obtain culture results and the need for specific facilities for culture, which may not be available in certain areas.24
According to Lee et al in a literature review article, EPTB should depend on histopathology examination from biopsy in-order to confirm diagnosis.7 Authors further added for peritoneal tuberculosis, biopsy from laparoscopic examination resulted in diagnostic yield of 85%-95%.26
IGRA is a method to detect tuberculosis from level of interferon-γ released by Th1 cells. A study conducted in Indonesia demonstrated that IGRA yielded sensitivity of 87.71% and specificity of 63% for EPTB.23 However, the study consisted of mainly lymph node tuberculosis (39 patients) and there were only 2 confirmed peritoneal tuberculosis patients.23 Hence, sensitivity and specificity for peritoneal tuberculosis or other type of EPTB may be different.
GeneXpert has poor sensitivity for detection of abdominal tuberculosis and peritoneal tuberculosis.27–30 A study by Ahmad et al on 21 patients with abdominal tuberculosis using sample of ascites fluid and biopsies showed sensitivity of 28.57% for GeneXpert.27 Another study conducted by Kumar et al on 37 intestinal tuberculosis showed sensitivity of 8.1%.28 Similarly, a study showed that GeneXpert was only positive in 26.1% cases of abdominal tuberculosis using samples from biopsies.30 On the other hand, a study by Tortoli et al using 94 cavitary fluids that included 60 peritoneal fluids showed a sensitivity of 50%.29 Finally, forest plot from a Cochrane review that included 20 studies showed that pooled sensitivity of GeneXpert for peritoneal fluid was 50%.31 Based from available data, GeneXpert is not recommended for abdominal TB diagnosis. All in all, the best diagnostic approach for peritoneal tuberculosis is biopsies from laparoscopic examination, although the disadvantage is that this approach is invasive and may cause some side effects for the patient.
Extrapulmonary Tuberculosis and CA-125
Recently, CA-125, a common tumor marker for ovarian cancer diagnosis and follow-up, has been implicated to be associated with tuberculosis infection.32–34 A small retrospective study by Fortun et al showed that CA-125 was increased in pulmonary tuberculosis patients when compared with EPTB patients.35 According to the study, mean value of Ca-125 value was 104.9 IU/mL and based from the analysis of the study, the optimal cut-off value was 32.5 IU/mL that corresponded with sensitivity and specificity of 68.6% and 77.8% respectively.35 Meanwhile, Kim et al conducted a study on 100 patients with active pulmonary tuberculosis.32 Out of these patients, the authors observed that 38 out of 100 patients had elevated CA-125. It is interesting that they found female gender was associated with elevation of CA-125 compared to males.32 However, the study included a significant proportion of young females that may cause higher CA-125 levels due to premenopausal status. Additionally, a recent systematic review and meta-analysis concluded that CA-125 has a potential role for pulmonary tuberculosis diagnosis but further studies should be conducted.36
The role of CA-125 in extrapulmonary tuberculosis is still relatively uncertain due to lack of large studies. Chen et al compared 23 peritoneal tuberculosis patients and 47 primary peritoneal cancer on clinical characteristics.25 Results of the study showed that both peritoneal tuberculosis and primary peritoneal cancer patients have elevated CA-125 levels. However, the elevation was statistically higher on primary peritoneal cancer patients.25 The study did not observe age difference between the two diseases. This is in contrast with a study by Yin et al that found patients with peritoneal tuberculosis were younger than peritoneal mesothelioma cancer patients.37 Another findings from the study is that peritoneal tuberculosis patients had higher proportions of pleural effusions, mesenteries, and abdominal visceral involvement.37
As CA-125 is found in epithelial cells of endometrium, lung tissues, and mesothelial cells, there are other causes of CA-125 other than ovarian cancer.38–40 This includes pulmonary cancer, breast cancer, pancreatic cancer, and gastrointestinal cancer.36 Other conditions may include pregnancy, pelvic inflammatory disease, uterine myoma, endometriosis, liver cirrhosis, and heart failure.40 Patients with benign or malignant pleural effusions can also have elevated CA-125 levels.41,42
In this patient, there were no signs of other cancers. The patient also did not have pregnancy at that point and did not show abnormalities in reproductive tissues from obstetrician gynecologist examination. This patient also did not have signs of liver cirrhosis from biopsy, which is gold standard for liver cirrhosis diagnosis. However, the patient had fatty liver. Clinical heart failure was also negative due to normal ventricles volumes, normal systolic function, normal diastolic function, normal valves, TAPSE of 2 cm, and normal EF of 75%. Thus, this left tuberculosis as differential diagnosis. However, on August 2021, CT-scan revealed that the patient had follicular cyst on both ovaria. It is possible that the follicular cyst may cause ascites, pleural effusion, and pericardial effusion. However, there have not been any literatures describing this association. This patient also did not fulfill Meigs’ syndrome triad of ascites, pleural effusion, and benign ovarian tumor. Most literatures described fibroma in Meigs’ syndrome not follicular cyst.43
Skeletal Tuberculosis
Historical evidence of skeletal tuberculosis comes from tuberculosis infections on bones of Egyptian mummies. Hematological seeding is a mechanism, in which tuberculosis spread to the bones. A common site for skeletal TB is vertebral bodies due to rich vascular supply.44,45 The sites of infection often involve more than one vertebra, usually on the thoracic and lumbar vertebrae.
Many cases of skeletal TB have infection sites concomitant with vertebral bodies.44–46 For example, venous drainage from vertebra often results in infection sites on the ribs. TB can also be found on sternum, cranium, and long bones.47
Diagnosing skeletal TB can be difficult due to its insidious onset and back pain symptom which are often neglected by both the patient and clinician. Bone scans show elevated metabolism in skeletal TB, but this can also occur in other bacterial infection or metastases. A study by Sinan et al showed that majority of spinal tuberculosis lesions were fragmentary and lytic.48 Biopsy shows caseating granuloma, but processing bone sample is difficult.44
In our case, we first thought that the lesions on the bones of our patient indicated metastases. However, as explained above, further workups on cancer diagnosis did not reveal primary cancer locations. The bone lesions of our patient had predilection of skeletal TB such as vertebrae lesions with ribs, sternum, and long bones. Furthermore, there was mild narrowing of intervertebral disc of lumbar vertebra 4–5 and bulging disk which indicated tuberculosis infection. After TB treatment, some of the lesions become lytic sclerotic which may indicate healing process of the bone. Unfortunately, we did not conduct bone biopsy as the gold standard for skeletal TB is histopathological examination.
Tuberculosis Treatment
Anti-tuberculosis regimen consists of two phases: intensive phase and continuation phase. The intensive phase consisted of isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (EMB) administration for 2 months. Meanwhile, the continuation phase consisted of 4 months of isoniazid and rifampin administration, which is widely used as regimen for pulmonary tuberculosis. This regimen has consistently been shown to reduce mortality and progression of the disease.49–51
The need for 6 months in treatment duration is due to Mycobacterium tuberculosis trait of slow-growing bacteria and in-order to prevent development of antibiotic resistance. This long treatment duration can become an issue from poor compliance. This was tackled by development of directly observed therapy (DOT) to increase patient’s compliance.52,53 In the future, it is expected that new regimen with shorter duration can be developed. Furthermore, new drugs that offer better efficacy with lower side effects are urgently needed.
Another issue from the long duration of TB treatment is the need for side effects monitoring. There are several side effects that should be monitored such as impaired hearing from streptomycin, optic neuritis from ethambutol, and liver enzymes elevation from rifampin, isoniazid, and pyrazinamide.
Usually, the tuberculosis regimen is given daily or intermittently. Difference of efficacy and toxicity between the dosing regimens remains unclear. A retrospective cohort study in Indonesia showed that there was no differences in sputum conversion profile and treatment success between daily and intermittent regiment.51 A meta-analysis conducted by Kasozi et al concluded that treatment success was similar between daily and intermittent regiment.54 On the contrary, the relapse rates were higher in intermittent regiment but the default rates were lower in intermittent regiment. Nevertheless, the guideline for Tuberculosis by WHO in 2017 no longer recommended intermittent dosing due to increased risk of relapse.
The treatment for extrapulmonary TB is the same as the pulmonary TB, however, there are differences in the treatment durations depending on the location of the extrapulmonary tuberculosis. According to a guideline from Infectious Diseases Society of America, tuberculosis treatment of 6–9 months are effective for most extrapulmonary tuberculosis.55 Treatment for peritoneal, lymph node, and genitourinary TB is taken for 6 months in duration. Meanwhile, if TB is located in the central nervous system, the duration of treatment is taken for 9–12 months.55 An important consideration that should be taken when treating extrapulmonary tuberculosis is that intermittent dosing is not recommended due to lack of clinical trials.55
Corticosteroid is generally not recommended in extrapulmonary tuberculosis with the exception of tuberculous meningitis.55 For abdominal tuberculosis, there are currently lack of good quality studies to determine the evidence.56
Mycobacterium Tuberculosis like all microorganisms has the capability for evolution. Thus, the bacterium may acquire drug resistance especially in patients with poor adherence. Identification of drug resistant Tuberculosis is important and this has been helped by molecular genetic testing such as GeneXpert and real time PCR.57 Multidrug-resistant TB (MDR TB) is defined as resistance of at least isoniazid and rifampin.58 On the other hand, extensively drug-resistant TB (XDR TB) is defined as resistance to either both isoniazid and rifampin together with at least one fluoroquinolone drug and at least one injectable drugs.58
According to WHO guideline, patients with rifampicin-susceptible and isoniazid-resistant tuberculosis should be treated with rifampicin, ethambutol, pyrazinamide and levofloxacin combination for at least 6 months.59
Conclusion
In the case of an elevated CA-125 in a woman, the clinicians should consider EPTB as a differential diagnosis of ovarian cancer. Further exploration, especially diagnostic laparoscopy with biopsy, is needed for suspected ovarian cancer and EPTB.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. No institutional approval was required to publish the case report.
Acknowledgement
All authors would like to thank Brenda Cristie Edina, Jeremy Rafael Tandaju and Indy Larasati Wardhana for their help in English language editing and galley proof editing. We also thank Brenda Cristie Edina for her input in manuscript evaluation.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Aggarwal AN. Quality of life with tuberculosis. J Clin Tuberc Mycobact Dis. 2019;17:100121. doi:10.1016/j.jctube.2019.100121
2. Surya A, Setyaningsih B, Suryani Nasution H, et al. Quality tuberculosis care in Indonesia: using patient pathway analysis to optimize public–private collaboration. J Infect Dis. 2021;216:S724–32. doi:10.1093/infdis/jix379
3. Mboi N, Murty Surbakti I, Trihandini I, et al. On the road to universal health care in Indonesia, 1990–2016: a systematic analysis for the Global Burden of Disease Study. Lancet Lond Engl. 2021;392:581–591. doi:10.1016/S0140-6736(18)30595-6
4. Global tuberculosis report 2021 [internet]. [cited November 05, 2021]. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021.
5. Turner RD, Chiu C, Churchyard GJ, et al. Tuberculosis infectiousness and host susceptibility. J Infect Dis. 2017;216:S636–43. doi:10.1093/infdis/jix361
6. Zumla A, Raviglione M, Hafner R, Fordham von Reyn C. Tuberculosis. N Engl J Med. 2013;368:745–755. doi:10.1056/NEJMra1200894
7. Lee JY. Diagnosis and treatment of extrapulmonary tuberculosis. Tuberc Respir Dis. 2015;78(2):47–55. doi:10.4046/trd.2015.78.2.47
8. Rodriguez-Takeuchi SY, Renjifo ME, Medina FJ. Extrapulmonary Tuberculosis: pathophysiology and Imaging Findings. Radiogr Rev Publ Radiol Soc N Am Inc. 2019;39:2023–2037. doi:10.1148/rg.2019190109
9. Maheshwari A, Gupta S, Rai S, et al. Clinical and laboratory characteristics of patients with peritoneal tuberculosis mimicking advanced ovarian cancer. South Asian J Cancer. 2021;10:102–106. doi:10.1055/s-0041-1736030
10. Ichiki H, Shishido M, Nishitani K, et al. [Evaluation of CEA, SLX and CA125 in active pulmonary tuberculosis]. Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31:1522–1527. Japanese.
11. Pang Y, An J, Shu W, et al. Epidemiology of extrapulmonary tuberculosis among inpatients, China, 2008–2017. Emerg Infect Dis. 2019;25(3):457–464. doi:10.3201/eid2503.180572
12. Kang W, Yu J, Du J, et al. The epidemiology of extrapulmonary tuberculosis in China: a large-scale multi-center observational study. PLoS One. 2020;15(8):e0237753. doi:10.1371/journal.pone.0237753
13. Wu DC, Averbukh LD, Wu GY. Diagnostic and therapeutic strategies for peritoneal tuberculosis: a review. J Clin Transl Hepatol. 2019;7:140–148. doi:10.14218/JCTH.2018.00062
14. Suntur BM, Kuşçu F. Pooled analysis of 163 published tuberculous peritonitis cases from Turkey. Turk J Med Sci. 2018;48(2):311–317. doi:10.3906/sag-1701-32
15. Shi X-C, Zhang L-F, Zhang Y-Q, Liu X-Q, Fei G-J. Clinical and laboratory diagnosis of intestinal tuberculosis. Chin Med J. 2016;129(11):1330–1333. doi:10.4103/0366-6999.182840
16. Rock RB, Olin M, Baker CA, Molitor TW, Peterson PK. Central nervous system tuberculosis: pathogenesis and clinical aspects. Clin Microbiol Rev. 2008;21(2):243–261. doi:10.1128/CMR.00042-07
17. Tandirogang N, Mappalotteng WU, Raharjo EN, Paramitai S, Bulan DE, Yasir Y. The spatial analysis of extrapulmonary tuberculosis spreading and its interactions with pulmonary tuberculosis in Samarinda, East Kalimantan, Indonesia. Infect Dis Rep. 2020;12:8727. doi:10.4081/idr.2020.8727
18. Guirat A, Affes N, Rejab H, Trigui H, Ben Amar M, Mzali R. [Role of laparoscopy in the diagnosis of peritoneal tuberculosis in endemic areas]. Med Sante Trop. 2015;25:87–91. French. doi:10.1684/mst.2014.0420
19. Muroni M, Rouet A, Brocheriou I, Houry S. Abdominal tuberculosis: utility of laparoscopy in the correct diagnosis. J Gastrointest Surg off J Soc Surg Aliment Tract. 2015;19(5):981–983. doi:10.1007/s11605-015-2753-z
20. Dervisoglu E, Sayan M, Sengul E, Yilmaz A. Rapid diagnosis of Mycobacterium tuberculous peritonitis with real-time PCR in a peritoneal dialysis patient. APMIS. 2006;114(9):656–658. doi:10.1111/j.1600-0463.2006.apm_456.x
21. Harkirat S, Anana S, Indrajit L, Dash A. Pictorial essay: PET/CT in tuberculosis. Indian J Radiol Imaging. 2008;18(2):141–147. doi:10.4103/0971-3026.40299
22. Public Health agency of Canada. Canadian tuberculosis standards 7th edition: 2014 [internet]; 2014 [cited November 7, 2021]. Available from: https://www.canada.ca/en/public-health/services/infectious-diseases/canadian-tuberculosis-standards-7th-edition.html.
23. Rumende CM, Hadi EJ, Tanjung G, Saputri IN, Sasongko R. The benefit of interferon-gamma release assay for diagnosis of extrapulmonary tuberculosis. Acta Medica Indonesia. 2018;50:138.
24. Pfyffer GE, Wittwer F. Incubation time of mycobacterial cultures: how long is long enough to issue a final negative report to the clinician? J Clin Microbiol. 2012;50(12):4188–4189. doi:10.1128/JCM.02283-12
25. Chen I-H, Torng P-L, Lee C-Y, Lee K-H, Hsu H-C, Cheng W-F. Diagnosis of peritoneal tuberculosis from primary peritoneal cancer. Int J Environ Res Public Health. 2021;18:10407.
26. Chow KM, Chow VC-Y, Szeto CC. Indication for peritoneal biopsy in tuberculous peritonitis. Am J Surg. 2003;185:567–573. doi:10.1016/S0002-9610(03)00079-5
27. Ahmad R, Changeez M, Khan JS, et al. Diagnostic accuracy of peritoneal fluid genexpert in the diagnosis of intestinal tuberculosis, keeping histopathology as the gold standard. Cureus. 2021;10:e3451. doi:10.7759/cureus.3451
28. Kumar S, Bopanna S, Kedia S, et al. Evaluation of Xpert MTB/RIF assay performance in the diagnosis of abdominal tuberculosis. Intest Res. 2017;15(2):187–194. doi:10.5217/ir.2017.15.2.187
29. Tortoli E, Russo C, Piersimoni C, et al. Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. Eur Respir J. 2012;40(2):442–447. doi:10.1183/09031936.00176311
30. Udgirkar S, Jain S, Pawar S, Chandnani S, Contractor Q, Rathi P. Clinical profile, drug resistance pattern and treatment outcomes of abdominal tuberculosis patients in Western India. Arq Gastroenterol. 2019;56(2):178–183. doi:10.1590/s0004-2803.201900000-35
31. Kohli M, Schiller I, Dendukuri N, et al. Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018;8:CD012768. doi:10.1002/14651858.CD012768.pub2
32. Kim ES, Park KU, Song J, et al. The clinical significance of CA-125 in pulmonary tuberculosis. Tuberc Edinb Scotl. 2013;93:222–226. doi:10.1016/j.tube.2012.10.014
33. Huang W-C, Tseng C-W, Chang K-M, Hsu J-Y, Chen J-H, Shen G-H. Usefulness of tumor marker CA-125 serum levels for the follow-up of therapeutic responses in tuberculosis patients with and without serositis. Jpn J Infect Dis. 2011;64:367–372.
34. Ozsahin SL, Turgut B, Nur N, Dogan OT, Erselcan T, Berk S. Validity of the CA125 level in the differential diagnosis of pulmonary tuberculosis. Jpn J Infect Dis. 2008;61:68–69.
35. Fortún J, Martín-Dávila P, Méndez R, et al. Ca-125: a useful marker to distinguish pulmonary tuberculosis from other pulmonary infections. Open Respir Med J. 2009;3(1):123–127. doi:10.2174/1874306400903010123
36. Zhao P, Yu Q, Zhang A, He F, Xu S, Serum CL. CA-125 for the diagnosis of pulmonary tuberculosis: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:1091. doi:10.1186/s12879-021-06772-7
37. Yin W, Zheng G, Chen Y, et al. CT differentiation of malignant peritoneal mesothelioma and tuberculous peritonitis. Radiol Med. 2016;121(4):253–260. doi:10.1007/s11547-015-0609-y
38. Kabawat SE, Bast RC, Bhan AK, Welch WR, Knapp RC, Colvin RB. Tissue distribution of a coelomic-epithelium-related antigen recognized by the monoclonal antibody OC125. Int J Gynecol Pathol off J Int Soc Gynecol Pathol. 1983;2:275–285. doi:10.1097/00004347-198303000-00005
39. Nouwen EJ, Pollet DE, Eerdekens MW, Hendrix PG, Briers TW, De Broe ME. Immunohistochemical localization of placental alkaline phosphatase, carcinoembryonic antigen, and cancer antigen 125 in normal and neoplastic human lung. Cancer Res. 1986;46:866–876.
40. Miralles C, Orea M, España P, et al. Cancer antigen 125 associated with multiple benign and malignant pathologies. Ann Surg Oncol. 2003;10:150–154. doi:10.1245/ASO.2003.05.015
41. How SH, Liam CK, Jamalludin AR, Chin SP, Zal AB. Serum cancer antigen 125 in patients with pleural effusions. Med J Malaysia. 2006;61:558–563.
42. Hussain S, Grayez J, Grigorian A, Green J. Massive pleural effusion and marked increase of CA-125. Postgrad Med J. 2004;80:300–301. doi:10.1136/pgmj.2003.012377
43. Saha S, Robertson M. Meigs’ and Pseudo‐Meigs’ syndrome. Australas J Ultrasound Med. 2012;15:29–31. doi:10.1002/j.2205-0140.2012.tb00140.x
44. Garg RK, Somvanshi DS. Spinal tuberculosis: a review. J Spinal Cord Med. 2011;34(5):440–454. doi:10.1179/2045772311Y.0000000023
45. Rajasekaran S, Soundararajan DCR, Shetty AP, Kanna RM. Spinal tuberculosis: current concepts. Glob Spine J. 2018;8:96S–108S. doi:10.1177/2192568218769053
46. Ferrer MF, Torres LG, Ramírez OA, Zarzuelo MR, Del Prado González N. Tuberculosis of the spine. A systematic review of case series. Int Orthop. 2012;36(2):221–231. doi:10.1007/s00264-011-1414-4
47. Pigrau-Serrallach C, Rodríguez-Pardo D. Bone and joint tuberculosis. Eur Spine J. 2013;22:556. doi:10.1007/s00586-012-2331-y
48. Sinan T, Al-Khawari H, Ismail M, Ben-Nakhi A, Sheikh M. Spinal tuberculosis: CT and MRI features. Ann Saudi Med. 2004;24(6):437–441. doi:10.5144/0256-4947.2004.437
49. Service, Hong Kong Chest, and British Medical Research Council. Controlled trial of 6-month and 8-month regimens in the treatment of pulmonary tuberculosis: the results up to 24 months. Tubercle. 1979;60:201–210. doi:10.1016/0041-3879(79)90001-1
50. Hong Kong Chest Service/British Medical Research Council. Controlled trial of 4 three-times-weekly regimens and a daily regimen all given for 6 months for pulmonary tuberculosis. Second report: the results up to 24 months. Tubercle. 1982;63:89–98. doi:10.1016/S0041-3879(82)80044-5
51. Siane A, Ascobat P, Instiaty I, Agustin H. Comparative effectiveness of tuberculosis treatment daily versus intermittent regimen in Indonesian TB-DM patients: real world patient database study. Acta Medica Indones. 2020;52:25–30.
52. Tola HH, Tol A, Shojaeizadeh D, Garmaroudi G. Tuberculosis treatment non-adherence and lost to follow up among TB patients with or without HIV in developing countries: a systematic review. Iran J Public Health. 2015;44:1–11.
53. Shao Y, Yang D, Xu W, et al. Epidemiology of anti-tuberculosis drug resistance in a Chinese population: current situation and challenges ahead. BMC Public Health. 2011;11(1):110. doi:10.1186/1471-2458-11-110
54. Kasozi S, Clark J, Doi SA. Intermittent versus daily pulmonary tuberculosis treatment regimens: a meta-analysis. Clin Med Res. 2015;13:117–138. doi:10.3121/cmr.2015.1272
55. Nahid P, Dorman SE, Alipanah N, et al. Official American thoracic society/centers for disease control and prevention/infectious diseases society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63:e147–95. doi:10.1093/cid/ciw376
56. Soni H, Bellam BL, Rao RK, et al. Use of steroids for abdominal tuberculosis: a systematic review and meta-analysis. Infection. 2019;47(3):387–394. doi:10.1007/s15010-018-1235-0
57. Seki M, Kim C-K, Hayakawa S, Mitarai S. Recent advances in tuberculosis diagnostics in resource-limited settings. Eur J Clin Microbiol Infect Dis. 2018;37(8):1405–1410. doi:10.1007/s10096-018-3258-y
58. Jang JG, Chung JH. Diagnosis and treatment of multidrug-resistant tuberculosis. Yeungnam Univ J Med. 2020;37:277–285. doi:10.12701/yujm.2020.00626
59. World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment [internet]; 2019 [cited January 4, 2022]. Available from: https://apps.who.int/iris/handle/10665/311389.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.