Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 15
ABCA7 rs3764650 Polymorphism is Associated with Delayed Neurocognitive Recovery
Authors Yu L, Ji H, Zhou M, Guo Y , Liu J , Lei D , Han C , Ma T
Received 6 December 2021
Accepted for publication 16 March 2022
Published 30 March 2022 Volume 2022:15 Pages 301—309
DOI https://doi.org/10.2147/PGPM.S352810
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Lu Yu,1 Haiyan Ji,2 Minmin Zhou,1 Yaxin Guo,2 Junfeng Liu,2 Daoyun Lei,3 Chao Han,1 Tieliang Ma1
1Department of Anesthesiology, Affiliated Yixing Hospital of Jiangsu University, Yixing, Jiangsu, 214200, People’s Republic of China; 2Medical College of Jiangsu University, Zhenjiang, Jiangsu, 212013, People’s Republic of China; 3Department of Anesthesiology, Zhongda Hospital Southeast University, Nanjing, Jiangsu, 210029, People’s Republic of China
Correspondence: Chao Han, Email [email protected]
Background: Several studies have shown that ATP-binding cassette transporter A7 (ABCA7) gene variation is associated with cognitive impairment. This study was aimed to investigate the relationship between ABCA7 rs3764650 polymorphism and perioperative neurocognitive disorder (pNCD).
Methods: A total of 132 elderly patients aged 65 and over who underwent elective non-cardiac surgery were enrolled in the study, while 28 healthy volunteers matching age and sex were recruited as the control group. A battery of neuropsychological tests was conducted 1 day before, 7 days, and 3 months after surgeries. Delayed neurocognitive recovery (dNCR) and postoperative mild or major neurocognitive disorder (POCD) were determined using the Z value method. The venous blood sample of the surgical patients was taken before the operation. Genotyping of rs3764650 was performed using polymerase chain reaction amplification and restriction fragment length polymorphism analysis.
Results: The incidences of dNCR and POCD were 29.7% and 16.8% at 7 days and 3 months after surgery, respectively. The G allele frequency and GG frequency of dNCR patients were significantly higher than that of non-dNCR patients (43.3% vs 28.2%, P=0.035; 23.3% vs 4.2%, P=0.013, respectively) at 7 days following surgery. No significant differences in ABCA7 alleles between POCD and non-POCD patients were observed 3 months postoperatively.
Conclusion: ABCA7 rs3764650 gene polymorphism is associated with dNCR and GG genotype might be a predisposing factor for postoperative cognitive impairment in Chinese Han elderly populations.
Keywords: ATP-binding cassette transporter, rs3764650, polymorphism, postoperative neurocognitive disorders
Introduction
Recently, the trend of aging population has become increasingly apparent due to improvement in general living standards, health care, nutrition, and education. A growing number of elderly are requiring surgical interventions and procedures with the development of medical and health technologies. Concern has been growing over the last decade pertaining to postoperative neuropsychiatric complications, including postoperative delirium and postoperative cognitive dysfunction (POCD). POCD is a common neurological complication characterized by impaired memory and learning ability, loss of attention, and declined information processing speed.1 The incidence of POCD varies enormously from 10%-80%,2–4 depending on the cognitive performance tests, time of postoperative assessment, and the limitations of specificity and sensitivity of the current cognitive tests. Patients over the age of 65 years who underwent non-cardiac surgery had a 26% prevalence of POCD one weeks after surgery which decreased to 10% 3 months postoperatively.5 POCD is reversible in most patients, but a permanent cognitive impairment can sometimes ensue. Prolonged POCD increases the risk of dementia,6 which detrimentally affects the quality of life, and aggravates the burden on the family and society.
In 2018, new recommendations for the nomenclature of cognitive change associated with anesthesia and surgery facilitated the transition of the former research diagnosis POCD as a clinical diagnosis based on DSM-5 criteria.7 The concept of perioperative neurocognitive disorders (pNCD) was therefore proposed and included special nomenclatures depending on timing and magnitude (delayed neurocognitive recovery(dNCR), within the 30 days recovery period; postoperative mild or major neurocognitive disorder(POCD), from expected recovery 30 days to 12 months, respectively). The novel definition facilitates interdisciplinary communication and makes clinical diagnosis more standardized, although it does not clarify the specific neuropsychological tests. Despite extensive research on postoperative cognitive function, the pathogenesis of pNCD remains elusive and there is not any specific clinical predictor for its early detection.
ATP-binding cassette (ABC) transporters constitute a superfamily of highly conserved proteins involved in the membrane transport of a variety of substrates such as ions, amino acids, lipids, and sterols.8 Absolute ABC transporters contain a pair of conserved cytoplasmic domains termed ATP-binding cassettes(ABC)or nucleotide-binding domains(NBDs) as well as two transmembrane domains(TMDs) bundles each composed of six membrane-spanning helices. The NBDs hydrolyze ATP and drive conformational changes in the TMDs, thus allowing substrates to cross the lipid bilayer of the membrane and either be imported into or exported out of the cytoplasm.9 ATP-binding cassette transporter A7 (ABCA7) is a member of the subfamily of ABC transporters that predominantly function in distributing lipids and other lipophilic molecules across membranes.10 ABCA7, mainly expressed in the brain on microglia and neurons, is capable of fluxing phospholipids as well as enhancing phagocytosis of apoptotic cells,11 and ABCA7 deficiency affects brain lipid homeostasis, disturbs cognitive functions, and accelerates amyloid-β Aβproductionis.12 It has been reported that ABCA7 may play a role in the development of diverse neurodegenerative diseases, such as schizophrenia and Alzheimer’s disease (AD).13,14
The ABCA7 gene is located on chromosome 19p3.3, with 47 exons covering ~32 kb length.15 GWAS revealed several AD-related ABCA7 single-nucleotide polymorphisms(SNP), including rs3764650, rs3752246, and rs115550680.16 The ABCA7 SNP rs3764650 has been implicated in influencing ABCA7 expression levels in the brain and corresponds to 10%–20% increased risk of AD in Caucasians.17 This ABCA7 variant is associated with a later age of onset and shorter disease process, exacerbating cognitive decline in subjects diagnosed with mild cognitive impairment or AD.18 Furthermore, the risk allele for rs3764650 (G) is related to increased hippocampal atrophy,19 whereas aerobic fitness is linked to increased neurogenesis in the hippocampus.
Based on the above-mentioned findings, we hypothesized that rs3764650 polymorphism is associated with postoperative cognitive impairment, then the primary objective of the study was to explore the relationship between rs3764650 SNP and perioperative neurocognitive disorder(pNCD).
Materials and Methods
Study Design
This study was approved by the ethics committee of Affiliated Yixing Hospital of Jiangsu University (approval number IRB2019S036) and performed consistent with the principles of the Helsinki Declaration on Human Experimentation. All participants provided written informed consents.
From June 2018 to March 2019, consecutive patients scheduled for elective surgery under general anesthesia were invited to the trial. The inclusion criteria were the age of 65 and over, American Society of Anesthesiologists (ASA) classification of I-II, Han nationality, and non-cardiac surgery. The subjects meeting any of the exclusion criteria listed below were excluded: pre-existing neurological or clinically evident neurovascular disease, communication impairments, achromatopsia or hypochromatopsia, alcohol/drug abuse, preoperative mini-mental state examination (MMSE) scores less than 24, anxiety or depression (score >8 using Hospital Anxiety and Depression Scale (HADS) in a Chinese version), duration of surgery less than 2 hours, postoperative infection or severe complications, decline to participate. As repeated neuropsychological testing can produce practice effect and result in misinterpretation of outcomes,20 age- and gender-matched healthy volunteers from a nearby community were recruited as control group using the same exclusion criteria.
Anesthesia Protocols
Anesthesia protocols were standardized, and no anticholinergic agents were used as premedication. On arrival, all patients received standard monitoring, including electrocardiogram, blood pressure, oxygen saturation by pulse oximeter, end-tidal carbon dioxide, temperature, and bispectral index. Midazolam (0.04 mg/kg), Propofol (1.5–2 mg/kg), Fentanyl (4–6 ug/kg), and Cis-atracurium (0.2 mg/kg) were intravenously injected for anesthesia induction. Approximately 3 minutes after drug delivery, patients were intubated and ventilated to maintain a end-tidal carbon dioxide of 35 ± 5 mm Hg. Anesthesia was maintained either by intravenous infusion or by inhalation, keeping BIS value between 40 and 60. Upon completion of the surgery, neostigmine was used to reverse neuromuscular blockade. Subjects were transferred to the postoperative care unit after extubation. All aspects of clinical care were documented in each patient’s electronic medical record.
Neuropsychological Testing and Diagnosis of pNCD
All patients received a standard battery of neuropsychological tests 1 day before surgery, at 7 day, and 3 months after surgery, while the control volunteers underwent testing in a corresponding time interval. We used the test battery on basis of recommendations of ISPOCD group,21 which consisted of Visual Verbal Learning, Concept Shifting Task, Stroop Color Word Test, Memory Scanning Task, Letter-Digit Coding, and Reaction time testing with the Four Boxes Test. An independent experienced psychiatrist blinded to the trial conducted all neuropsychological tests.
In each test, a Z value was calculated through the difference between the postoperative score and the preoperative baseline score minus the mean change of the control group and then divided by the standard deviation of the change score of the control group. If the Z value exceeded 1.96, the test was considered as positive.
Peri-operative NCD was defined here according to 2018 recommendations for the nomenclature of cognitive change associated with anesthesia and surgery by the nomenclature consensus working group. The case was determined dNCR if two and more tests were positive 7 days after surgery, while POCD 3 months postoperatively.
Sequencing and Genotyping
Peripheral venous blood samples (5 mL) were collected before surgery and stored at −20 °C for genomic DNA and SNP analysis. Primers to detect rs3764650 loci polymorphism were designed and synthesized by Tianhao Company in Shanghai. They were then blasted in NCBI database to exclude any unspecific bindings. The sequences of primers are as follows (from 5’ to 3’): TCGTGTACCTGCAAGACCTG (forward); CATCTGGCACGACTGGTTC (reverse). Sequencing results were extracted from ABI3130 sequencing device and interpreted by Chroma2.3.1 software. Genotype of each blood sample was recognized by sequencing maps and peaks. Figure 1 shows how wild type, heterozygote, and mutant rs3764650 look like.
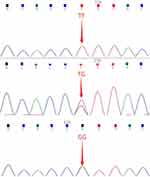 |
Figure 1 DNA sequencing map of ABCA7 rs3764650 wild type, heterozygotes, and mutant. |
Statistical Analysis
All the statistical analyses were performed with SPSS, version 25.0. Data with normal distribution were interpreted as mean±SEM (standard error). Continuous variables and categorical variables are described as means ± standard deviation and numbers or percentages appropriately. Categorical variables were analyzed by the chi-square (χ2)-test, and the continuous variables was analyzed by independent t test. The association between the genotypes and risk of pNCD was assessed by calculating values of odds ratios (ORs) and 95% confidence intervals (95% CIs). All analysis were two-sided and we considered P values <0.05 statistically significant.
Results
Study Population
A study flow diagram is shown in Figure 2. In total, 132 patients and 28 volunteers were initially included in the study. Of the 132 patients, 31 participants were excluded due to surgical duration less than 2 hours (8), postoperative death within 3 months (1), severe complications (11), reoperations (2), and loss to follow-up (9). 101 patients and 28 volunteers were finally analyzed. The demographic data between surgical patients and healthy volunteers were compatible (Table 1).
 |
Table 1 Baseline Characteristics of Surgical Patients and Volunteers |
 |
Figure 2 Flowchart showing the enrollment of participants. |
Cognitive Outcomes
Table 2 summarized the neuropsychological test results. According to the diagnostic criteria above, 30 patients (29.7%) were diagnosed as dNCR 7 days after surgeries, while 17 (18.2%) were diagnosed as POCD 3 months following surgeries. There is no significant difference in term of the demographic and clinical characteristics between pNCD and non-pNCD patients (Table 3).
 |
Table 2 Neuropsychological Test Results |
 |
Table 3 Comparison of Baseline and Clinical Characteristics in pNCD and Non-pNCD Patients |
Genotypes
The G allele frequency of dNCR patients was significantly higher than that in non-dNCR group (43.3% vs 28.2%, P=0.035). Additionally, the frequency of rs3764650 GG was higher in dNCR patients than in non-dNCR group (23.3% vs 4.2%, P=0.013), and recessive model of rs3775430 (GG vs AG+AA) also exhibited significant difference between the two groups (P=0.008). However, we did not observe the difference between POCD and No-POCD patients (Table 4).
 |
Table 4 Comparison of Genotype Frequency of Rs3764650 in pNCD and Non-pNCD Patients |
Discussion
Previous studies have investigated the relationship between gene polymorphisms and postoperative cognitive decline, including APOE-ε4, P-selectin, CRP, and BDNF,22–24 however, there was no reliable genetic indicators for pNCD susceptibility. In our study, we firstly explored the correlations between ABCA7 rs3764650 polymorphism and pNCD. We revealed that the frequency of G allele and GG genotype in dNCR patients was significantly higher than that of non-dNCR patients, and no significant difference at 3 months postoperatively was observed. The patients carrying a G allele and GG genotype at the rs3764650 locus have a higher risk for dNCR than an A allele and AA or AG carriers on postoperative 7 days. These results indicated that ABCA7 rs3764650 polymorphisms is associated with early postoperative cognitive impairment and GG genotype might be an independent risk factor for risk of dNCR in Chinese Han populations.
Several potential mechanisms of ABCA7 relevant to cognitive impairment have been proposed. Numerous studies have demonstrated that abnormal lipid metabolism are known to be closely related to cognitive impairment.25,26 As ABCA7 is 54% homologous in amino acid residue sequence to ABCA1 which regulates the generation of high-density lipoprotein (HDL) and the translocation of lipids,15,27 the major function of ABCA7 is likely to regulate the release of cellular lipids including cholesterol and phospholipids.28,29 In-vitro studies indicated that ABCA7 could promote the outflow of apolipoprotein-mediated phospholipid in HEK293 cells.11 In ABCA7 knock-out mice, serum cholesterol and HDL levels significantly decreased.28 ABCA7 contributes to lipid metabolism in the brain, which might affect diverse brain functions.30 Furthermore, aberrant lipid metabolism results in microvascular dysfunction, which is associated with worse cognitive performance.31
The accumulation and deposition of Aβ peptides in the brain is a central event of AD and also recognized as one of the potential mechanisms of pNCD pathogenesis.32 Clinical evidence showed that Aβ level significantly elevated both in peripheral blood and in cerebrospinal fluid of pNCD patients.33 In a recent study of lipidomic analysis in the brains of the control and ABCA7 knock-out mice, ABCA7 deficiency modified PE and SM lipid subspecies, which are also major components of lipid rafts. The modification of lipid raft function/components due to ABCA7 deficiency may facilitate the interaction between APP and BACE1, resulting in accelerating Aβ production in the brains.30 ABCA7 promotes degradation of amyloid precursor protein (APP) and inhibits secretion of Aβ by transporting low-density lipoprotein receptor-related proteins (LRP) to plasma membrane. Moreover, ABCA7 can potentiate intake of Aβ by macrophages via C1q complement pathway.34 Overexpression of ABCA7 could also promote cellular viability and decrease endoplasma reticulum (ER) stress, significantly alleviate the neurotoxicity of Aβ, and hence improve cognitive conditions of AD mice.35 Furthermore, in an in vitro Model of the Blood-Brain Barrier, a decrease in ABCA7 expression at the BBB provokes a reduction in ABCA1 and APOE secretion, leading to a decrease of cholesterol exchange across the BBB and Aβ peptide basolateral-to-apical transport.36 Hughes and colleagues37 confirmed a considerable association between ABCA7 rs3764650 polymorphism and Aβ amyloid plaque formation via PET imaging. The studies above all indicate the potential role of rs3764650 polymorphism in neurocognitive impairments via intracerebral aggregation of Aβ.
Substantial evidence suggests that cerebral atrophy serves as a predictor of pNCD in elderly patients.38 Large amounts of evidence have proven that rs3764650 polymorphism is associated with posterior cortical atrophy (PCA)39 and hippocampal atrophy,19 which further indicates that rs3764650 polymorphism might promote pathogenesis of pNCD via aggravating cerebral atrophy.
It is widely believed that risk factors for non-cardiac surgical patients developing POCD include advanced age, education, hypertension, aortic plaque, carotid artery stenosis, cerebrovascular disease, anesthesia time, length of surgery, blood oxygen saturation in brain surgery and systemic inflammation.40 However, we found no significant differences between NCD and no-NCD patients on these factors. This may be due to an insufficient sample size. Given the exploratory nature of the study, we did not calculate the sample size. So further validation study is required.
Another limitation of the study was the effects of the potential differences among different types of surgeries on pNCD. We ignored it due to the small sample size in each surgical subgroup. Moreover, we did not detect the concentration of ABCA7 in plasma or cerebrospinal fluid, which could partially reflect different expression level of ABCA7 due to rs3764650 polymorphism.
In conclusion, ABCA7 rs3764650 gene polymorphism is associated with early postoperative cognitive decline, and GG genotype might be a susceptibility factor for dNCR in Chinese Han populations.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the ethics committee of Affiliated Yixing Hospital of Jiangsu University with approval number IRB2019S036. The subject provided written consent. All methods were carried out in accordance with Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the General Program of Health Commission of Wuxi (grant number, MS201934), and Double hundred top talent project of Wuxi (grant number, HB2020109).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Hansen MV. Chronobiology, cognitive function and depressive symptoms in surgical patients. Dan Med J. 2014;61(9):B4914.
2. Coburn M, Fahlenkamp A, Zoremba N, et al. Postoperative cognitive dysfunction: incidence and prophylaxis. Anaesthesist. 2010;59(2):177–184.
3. Mason SE, Noel-Storr A, Ritchie CW. The impact of general and regional anesthesia on the incidence of post-operative cognitive dysfunction and post-operative delirium: a systematic review with meta-analysis. J Alzheimers Dis. 2010;22:67–79.
4. Miller D, Lewis SR, Pritchard MW, et al. Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non-cardiac surgery. Cochrane Database Syst Rev. 2018;8(8):CD012317.
5. Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857–861.
6. Needham MJ, Webb CE, Bryden DC. Postoperative cognitive dysfunction and dementia: what we need to know and do. Br J Anaesth. 2017;119(suppl_1):i115–i125.
7. Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth. 2018;121(5):1005–1012.
8. Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113.
9. Dean M, Allikmets R. Evolution of ATP-binding cassette transporter genes. Curr Opin Genet Dev. 1995;5(6):779–785.
10. Tanaka N, Abe-Dohmae S, Iwamoto N, et al. Helical apolipoproteins of high-density lipoprotein enhance phagocytosis by stabilizing ATP-binding cassette transporter A7. J Lipid Res. 2010;51(9):2591–2599.
11. Wang N, Lan D, Gerbod-Giannone M, et al. ATP-binding cassette transporter A7 (ABCA7) binds apolipoprotein A-I and mediates cellular phospholipid but not cholesterol efflux. J Biol Chem. 2003;278(44):42906–42912.
12. Sakae N, Liu CC, Shinohara M, et al. ABCA7 Deficiency Accelerates Amyloid-β Generation and Alzheimer’s Neuronal Pathology. J Neurosci. 2016;36(13):3848–3859.
13. Yamazaki K, Yoshino Y, Kawabe K, et al. ABCA7 Gene Expression and Genetic Association Study in Schizophrenia. Neuropsychiatr Dis Treat. 2020;16:441–446.
14. Aikawa T, Holm ML, Kanekiyo T. ABCA7 and Pathogenic Pathways of Alzheimer’s Disease. Brain Sci. 2018;8(2):27.
15. Kaminski WE, Orsó E, Diederich W, et al. Identification of a novel human sterol-sensitive ATP-binding cassette transporter (ABCA7). Biochem Biophys Res Commun. 2000;273(2):532–538.
16. Kim JH. Genetics of Alzheimer’s Disease. Dement Neurocogn Disord. 2018;17(4):131–136.
17. Vasquez JB, Fardo DW, Estus S. ABCA7 expression is associated with Alzheimer’s disease polymorphism and disease status. Neurosci Lett. 2013;556:58–62.
18. Carrasquillo MM, Crook JE, Pedraza O, et al. Late-onset Alzheimer’s risk variants in memory decline, incident mild cognitive impairment, and Alzheimer’s disease. Neurobiol Aging. 2015;36(1):60–67.
19. J M S, S J C, M M C, et al. Genetic risk factors for the posterior cortical atrophy variant of Alzheimer’s disease. Alzheimers Dement. 2016;12(8):862–871.
20. Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12–19.
21. Rasmussen LS, Larsen K, Houx P, et al. The assessment of postoperative cognitive function. Acta Anaesthesiol Scand. 2001;45(3):275–289.
22. Tardiff BE, Newman MF, Saunders AM, et al. Preliminary report of a genetic basis for cognitive decline after cardiac operations. The Neurologic Outcome Research Group of the Duke Heart Center. Ann Thorac Surg. 1997;64(3):715–720.
23. Mathew JP, Podgoreanu MV, Grocott HP, et al. Genetic variants in P-selectin and C-reactive protein influence susceptibility to cognitive decline after cardiac surgery. J Am Coll Cardiol. 2007;49(19):1934–1942.
24. Steinmetz J, Jespersgaard C, Dalhoff K, et al. Cytochrome P450 polymorphism and postoperative cognitive dysfunction. Minerva Anestesiol. 2012;78(3):303–309.
25. Agarwal M, Khan S. Plasma Lipids as Biomarkers for Alzheimer’s Disease: a Systematic Review. Cureus. 2020;12(12):e12008.
26. Lutski M, Weinstein G, Goldbourt U, et al. Plasma Lipids, Apolipoproteins, and Subsequent Cognitive Decline in Men with Coronary Heart Disease. J Alzheimers Dis. 2019;67(3):827–837.
27. Abe-Dohmae S, Ueda K, Yokoyama S. ABCA7, a molecule with unknown function. FEBS Lett. 2006;580(4):1178–1182.
28. Kim WS, Fitzgerald ML, Kang K, et al. Abca7 null mice retain normal macrophage phosphatidylcholine and cholesterol efflux activity despite alterations in adipose mass and serum cholesterol levels. J Biol Chem. 2005;280(5):3989–3995.
29. Abe-Dohmae S, Ikeda Y, Matsuo M, et al. Human ABCA7 supports apolipoprotein-mediated release of cellular cholesterol and phospholipid to generate high density lipoprotein. J Biol Chem. 2004;279(1):604–611.
30. Sakae N, Liu CC, Shinohara M, et al. ABCA7 Deficiency Accelerates Amyloid-beta Generation and Alzheimer’s Neuronal Pathology. J Neurosci. 2016;36(13):3848–3859.
31. Rensma SP, van Sloten TT, Houben AJHM, et al. Microvascular Dysfunction Is Associated With Worse Cognitive Performance: the Maastricht Study. Hypertension. 2020;75(1):237–245.
32. Lin X, Chen Y, Zhang P, et al. The potential mechanism of postoperative cognitive dysfunction in older people. Exp Gerontol. 2020;130:110791.
33. Wu Z, Zhang M, Zhang Z, et al. Ratio of beta-amyloid protein (Abeta) and Tau predicts the postoperative cognitive dysfunction on patients undergoing total hip/knee replacement surgery. Exp Ther Med. 2018;15(1):878–884.
34. Zlokovic BV, Deane R, Sagare AP, et al. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid β-peptide elimination from the brain. J Neurochem. 2010;115(5):1077–1089.
35. Mengqian L, Yuan Y, Bo H, et al. Study on Lentivirus-Mediated ABCA7 Improves Neurocognitive Function and Related Mechanisms in the C57BL/6 Mouse Model of Alzheimer’s Disease. J Mol Neurosci. 2017;61(4):489–497.
36. Lamartinière Y, Boucau M-C, Dehouck L, et al. ABCA7 Downregulation Modifies Cellular Cholesterol Homeostasis and Decreases Amyloid-β Peptide Efflux in an in vitro Model of the Blood-Brain Barrier. J Alzheimers Dis. 2018;64(4):1195–1211.
37. Hughes TM, Lopez OL, Evans RW, et al. Markers of cholesterol transport are associated with amyloid deposition in the brain. Neurobiol Aging. 2014;35(4):802–807.
38. Chen MH, Liao Y, Rong PF, et al. Hippocampal volume reduction in elderly patients at risk for postoperative cognitive dysfunction. J Anesth. 2013;27(4):487–492.
39. J M S, S J C, M M C, et al. Genetic risk factors for the posterior cortical atrophy variant of Alzheimer’s disease. Alzheimers Dement. 2016;12(8):862–871.
40. Wang W, Wang Y, Wu H, et al. Postoperative cognitive dysfunction: current developments in mechanism and prevention. Med Sci Monit. 2014;20:1908–1912.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
