Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
A WeChat-Based Rehabilitation Platform for Children and Adolescents with Congenital Heart Disease to Promote Cardiac FITness (HeartFIT): Protocol for a Mixed-Methods Strategy from Evidence-Based Design to Pilot Study
Authors Li Y, Zhou Y , Chen M, Fu MR, Luo B , Yu P, Zheng H, Liu F
Received 17 November 2021
Accepted for publication 19 April 2022
Published 29 April 2022 Volume 2022:15 Pages 907—920
DOI https://doi.org/10.2147/JMDH.S349519
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yuan Li,1– 3,* Yaxin Zhou,4,5,* Miao Chen,6 Mei R Fu,7 Biru Luo,1– 3 Pengming Yu,4,5,8 Hong Zheng,1,9 Fangfei Liu1,9
1Nursing Department, West China Second University Hospital, Sichuan University, Chengdu, People’s Republic of China; 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, People’s Republic of China; 3West China School of Nursing, Sichuan University, Chengdu, People’s Republic of China; 4Rehabilitation Medicine Center, Sichuan University, Chengdu, People’s Republic of China; 5West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 6Department of Cardiovascular Surgery, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 7Rutgers University, School of Nursing, Camden, NJ, USA; 8Key Laboratory of Rehabilitation Medicine in Sichuan Province, Chengdu, People’s Republic of China; 9Department of Pediatric Cardiology, West China Second University Hospital, Sichuan University, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Biru Luo, Nursing Department, West China Second University Hospital, Sichuan University, No. 20, Section 3, South Renmin Road, Chengdu, Sichuan, 610041, People’s Republic of China, Tel +86-1 818 060 9180, Email [email protected] Miao Chen, Department of Cardiovascular Surgery, West China Hospital, Sichuan University, No. 37, Guoxue Alley, Wuhou District, Chengdu, Sichuan, 610041, People’s Republic of China, Tel +86-1 898 060 6107, Email [email protected]
Abstract: Progress in medical and surgical care has tremendously improved the survival rates of children with congenital heart disease (CHD). However, reduced aerobic capacity and health-related issues remain a threaten to quality survival and prevention of related complications among children and adolescents with CHD. This research program aims to develop and evaluate a WeChat-based health platform (HeartFIT) to facilitate cardiac rehabilitation and promote physical fitness for this rapidly expanding young population. The study protocol describes the use of an iterative process of using a mixed-methods strategy to develop, refine, and pilot test the proposed HeartFIT platform. A sequential problem-solving process comprising four iterative phases with ongoing end-user input will be implemented. In phase 1, relevant literature was systematically reviewed (completed) and then child-parent dyads will be interviewed to understand the broad context and the requirements and considerations of the target population toward the WeChat-based rehabilitation platform. In phase 2, key features and priority functionalities for the platform will be ideated and refined, and a digital interactive prototype will be created. In phase 3, heuristic evaluation and three rounds of end-user testing will be conducted to ensure further refinement and usability of the prototype. In phase 4, a prospective pilot study will be performed to investigate the feasibility, acceptability, and preliminary efficacy of the developed platform over a 12-week intervention period. If HeartFIT intervention is feasible, acceptable, and demonstrates promising efficacy, an adequately powered randomized controlled trial (future work) will be deployed to test the real-world effectiveness of the intervention.
Keywords: congenital heart disease, physical activity, exercise therapy, cardiorespiratory fitness, digital health intervention, user-centered design
Introduction
Congenital heart disease (CHD) is the most common birth defect, with a global prevalence of nearly 1.8 cases per 100 live births.1 China is among the countries with the highest burden of CHD.1 Over the past decades, advances in prenatal diagnosis as well as medical and surgical care have led to a dramatic reduction in mortality and enabled more pediatric patients with CHD to survive into adulthood.2 This changing landscape of CHD makes it imperative to ensure long-term quality of life in this population.2
Physical activities and structured exercises provide immediate and long-lasting health benefits and serve as cornerstones for cardiovascular fitness in patients with CHD.3,4 However, we are confronted with a “global epidemic” of childhood inactivity as less than 1 in 10 youth meet the exercise target of at least 60 minutes of moderate‐to‐vigorous physical activity daily.5 Children with CHD might be even less active than their healthy peers due to parental overprotection, diminished predilection, and misconception on pros and cons of activity engagement.6 The additive health risks resulting from physical inactivity may predispose these patients to a broad spectrum of subsequent disorders from cardiometabolic morbidities to psychosocial challenges. Furthermore, cardiorespiratory fitness is impaired in children with CHD and deteriorates faster than healthy controls.7 Impaired cardiorespiratory function is a known risk not only for cardiovascular morbidity and mortality but also for all-cause mortality.8,9 Maximal oxygen consumption is considered as the gold standard measure of cardiorespiratory fitness that is determined by the product of the cardiac output and the arteriovenous oxygen difference.10 Regular physical activity is highly rewarding in stimulating both the cardiac output and oxygen extraction through complex physiological mechanisms (eg, invigorating myocardial contractility, ventricular compliance, musculoskeletal adaptations, mitochondrial functions, etc.).11 Therefore, efforts to optimize physical activity levels and enhance cardiorespiratory fitness in early life are essential to ensure that beneficial effects on health and quality of life for this large and rapidly expanding population.
Cardiac rehabilitation featuring developmentally appropriate, individually tailored physical activity prescriptions is recognized as a safe, cost-effective, and promising strategy to encourage health-promotion behaviors in children and adolescents with CHD, and to help them thrive physically, emotionally, and socially.12,13 Yet, suboptimal patient adherence and limited service availability coupled with high costs remain the major obstacles to allow the traditional center-based rehabilitation programs to be widely implemented and achieve the full program potential.14
Technology innovation has improved the accessibility, affordability, and connectivity of health care. As the most popular social application and a de facto part of daily lives of most Chinese people (1.26 billion active users), WeChat is far more than a mobile application for instant messaging, Official Accounts offered via WeChat can be used as a platform to push feeds to subscribers, interact with subscribers and provide them with multi-purpose services.15 WeChat has been recognized as an effective venue to develop and deliver digital health program in management of chronic disease in China.16 In addition, children and adolescents who are growing up in the digital era typically are skilled in using digital devices for knowledge acquisition and information exchange. Thus, the use of WeChat to develop and deliver a digital rehabilitation program for children and adolescents with CHD to promote cardiac fitness (HeartFIT) with multilevel engagement of the targeted users and experts represent an innovative approach to implement customized and attractive rehabilitation interventions to eliminate geographical and logistical barriers.
This study protocol describes the use of an iterative process of using a mixed-methods strategy to develop, refine, and pilot test the proposed WeChat-based HeartFIT rehabilitation platform. This research program has the potential to improve physical health and psychosocial wellbeing of children and adolescents with CHD.
Methods and Analysis
Objectives
User Involvement Streams proposed by Shah et al17 comprised of four iterative study phases will be followed to ensure that end users’ needs and preferences are fully considered and properly translated into technical solutions.18,19 Specific objectives aligning with each study phase include: Phase 1. background analysis to understand the context and preferences of end users, Phase 2. conceptualization and prototyping to create an initial prototype platform, Phase 3. usability evaluation to inform iterative development of the platform, and Phase 4. pilot randomized controlled trial (RCT) to assess the feasibility, acceptability, and preliminary efficacy of the HeartFIT intervention. A schematic of the workflow is shown in Figure 1.
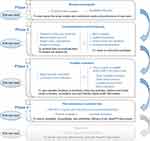 |
Figure 1 Schematic overview of the study workflow. |
Phase 1 Background Analysis
The background analysis consists of a scoping review of related literature and a qualitative study with target users to achieve context and user understanding.
Phase 1A Scoping Review
We have completed the scoping review. Findings from the review enabled us to learn about the broad context and allowed our development of the HeartFIT rehabilitation platform building upon existing knowledge and expertise.
We conducted a systematic literature search of the MEDLINE, Embase, Cochrane Library, and CINAHL databases from inception to August 31, 2021 to identify evidence describing and examining digital health development, implementation, and evaluation in cardiac rehabilitation for pediatric patients with CHD and their families. After rigorous study selection, four studies depicting various digital health tools were ultimately included in this review.20–23 For the purposes of this paper, only key conceptual information including the main features, development, usability, and feasibility of the digital health tools, as well as the facilitators and barriers to digital health adoption in clinical practice gathered from the scoping review is presented here (Table 1). Complete search syntax (Supplementary File 1), study selection criteria (Supplementary File 2) and synthesized data (Supplementary Files 3, 4, and 5) can be found in the online Supplementary Materials.
 |
Table 1 Overview of Clinically Studied Digital Health Tools |
The digital health tools20–23 were all web-based apps or websites with particular features like delivering health information and exercise suggestions, generating action plans, motivation/encouragement, behaviors tracking, self-monitoring, physical activity counselling, and rewarding systems to promote physically active lifestyle for children and adolescents with CHD. However, none of the included studies presented clear details on the design process or behavioral theory foundations of their tools; and only two of the studies mentioned stakeholder involvement during the tool development.20,21 Another major gap highlighted by the review is that none of the studies performed usability testing for their specific tools, which might explain why end users did not find the web-based app to be a useful tool in the “Smart Heart” study.23,24 The review results suggest that research into the use of digital health tools to promote cardiac fitness for children and adolescents with CHD is still in its infancy and that there is a need to develop an evidence-driven, user-friendly digital tool to facilitate cardiac rehabilitation for this population.
Phase 1B Qualitative Interviews
After background analysis of the context, the next step will be to conduct qualitative semi-structured interviews to understand the end-user child-parent dyads’ expectations, requirements and preferences of using a WeChat-based pediatric cardiac rehabilitation platform.
We will use a purposive sampling method18,19 to recruit child-parent dyad participants from pediatric cardiology clinics of West China Hospital and West China Second University Hospital. Inclusion criteria are as follows: (1) age between 8 and 18 years, (2) repair of congenital heart defects at least 6 months ago, (3) familiarity with smartphones and mobile applications, and (4) informed parent consent and child assent. Exclusion criteria include patients who have any contraindications for exercise, or known genetic or chromosomal disorders, or intellectual and developmental disabilities.
The one-to-one interviews will be conducted by a well-trained researcher in a quiet and separate room and be guided by a topic guide, involving a combination of open-ended questions25 on habitual physical activity patterns, perceptions and experiences of pediatric cardiac rehabilitation, interest in a WeChat-based platform for fitness promotion, and requirements and preferences regarding features, contents, and personalization of the proposed tool. Data collection will continue until data saturation is reached. All interviews will be audio-recorded and transcribed verbatim. To ensure the credibility of data analysis, we will use a modified iterative descriptive data analysis method to examine data, compare codes, challenge interpretations, and develop themes inductively.26
Phase 2 Conceptualization and Prototyping
The goal of this phase is to conceptualize the idea of the digital platform, to define key features and priority functionalities, and to create an initial interactive prototype.
The individual and family self-management theory27 will be utilized to steer the design process with the aim to enhance the long-term engagement and effectiveness of the proposed tool. The CALO-RE taxonomy, a 40-item list of behavior change techniques purported to help people change their physical activity behaviors28 will be considered to inform the tool design. In addition, personalized communication strategies29 will be used to improve communication to individual users and enhance persuasiveness for behavioral change.
With an in-depth understanding of the broad context and user expectations, we will cluster and codify relevant findings from the scoping review and qualitative interviews as discrete features to be incorporated into the HeartFIT platform as well as ideas generated from a brainstorming session by the research team. Design considerations including users’ requirements and preferences, theoretical constructs, behavior change techniques and tailoring methods will be listed and mapped to each of the discrete features to help define the platform functionalities and how they need to be designed. We will then disseminate the ideated features along with their corresponding design considerations to an expert panel including healthcare professionals and end users with digital health familiarity to seek further opinions. The ideated features will be refined after the expert panel consultations and thereon a final list of platform features and functionalities will be determined.
On the basis of design conceptualization, an interactive prototype will be built, tested and continuously refined in collaboration with three software partners. Representatives of target users will be frequently called upon to give unstructured feedback throughout the prototyping process.
Phase 3 Usability Evaluation
Usability is an outcome of use that implies the extent to which a product can be used by specified users to achieve specified goals with effectiveness, efficiency, and satisfaction in a specified context of use.30 Usability testing is critical for the success of a novel digital health intervention. The HeartFIT prototype will go through usability testing to gain valuable feedback in creating a feasible, acceptable and user-friendly intervention before embarking on a small-scale implementation.
Study Design
We will employ the iterative convergent mixed methods design which provides a clear framework for integrating quantitative and qualitative data to assess usability.31 Two types of usability testing on HeartFIT will take place: heuristic evaluation and user testing.
Participants
The heuristic evaluation will involve collaborative efforts of both the development and research teams, who are formally prepared in human-computer interaction and user experience logic.32,33 For end-user testing, current evidence suggests that 85% of usability issues could be identified with the first 5 participants and recommends 3 iterative studies with 5 users each to maximize usability.33–35 Thereby, we plan to recruit 5 child-parent dyads representative of the future end users to take each round of the usability testing.
Procedure
Heuristic evaluation is a usability inspection method in which the technology is evaluated against Nielsen’s 10 principles for interaction design.18,32,33 Any deviations from the classic principles will be referred to as a heuristic violation and the overall severity of the identified violations will be rated into five categories: (0) no problem, (1) cosmetic problem only, (2) minor problem, (3) major problem, and (4) usability catastrophe. Heuristic evaluators will also provide additional comments regarding the user interface. The initial HeartFIT platform will be iteratively refined based on feedback from heuristic evaluation.
Three iterative rounds of usability testing with 5 end users each will be conducted in a quiet room. The study participants will first complete a brief questionnaire covering demographics, disease condition, and technology use and interest. Then, they will be given a demonstration of the HeartFIT and be required to complete several tasks with the interactive platform independently. Each participant will be asked to think aloud their thoughts while completing the predetermined tasks designed to explicate HeartFIT features and also to freely explore the platform.18,35 The “think aloud” method will generate data on participants’ ongoing thought processes as they complete a set of training tasks.35 Thinking aloud questions such as “Tell me what you are thinking,” “What are you looking at?”, or “What’s on your mind?” will be used to elicit additional information about the mHealth technology as they move through the user interface.31,33,35 We will record device screen display, task duration and participant’s utterances with a screen recording application. Field notes will be documented to describe nonverbal reactions, the frequency, nature, and location of issues that participants encounter during the usability tasks. In addition, participants will be asked to complete the mHealth app usability questionnaire (MAUQ-Patient Version), which is designed specifically to evaluate the usability of interactive mHealth apps, with higher scores indicating better usability.36 At the end of each testing round, a short interview will be performed to address the users’ understanding of particular features and contents provided by the platform, their overall impressions of HeartFIT, and their intention to use HeartFIT. The progression of feedback from the iterative usability testing with end users will be utilized to iteratively improve the prototype.
Data Analysis
Demographic and quantitative data will be analyzed using descriptive statistics. Audio recordings of the think-aloud protocols will be verbatim transcribed and summarized thematically. Qualitative data from heuristic evaluation and end-user interviews will also be summarized thematically.
Phase 4 Pilot Randomized Controlled Clinical Trial
The HeartFIT study is among the first efforts to innovatively develop and systematically evaluate a WeChat-based rehabilitation intervention for children and adolescents with CHD. We will follow the latest Medical Research Council (MRC) guidance for developing and evaluating complex interventions37 to undertake a process evaluation in parallel with the pilot trial to gain insight into the feasibility of the intervention. The main significance of the pilot study is to assess preliminary efficacy of the intervention to help children and adolescents with CHD enhance cardiac fitness and adopt a physically active lifestyle, as well as its potential to improve psychosocial wellbeing of the pediatric patients and to provide evidence of feasibility, acceptability, and appropriateness of implementing the HeartFIT intervention in a home-based setting.
Study Design
This study will use a prospective, 12-week, two-arm, open-label, pilot RCT design. The intervention length of 12 weeks is chosen since health habit formation and physiological adaptations to exercise occur in this period of time.38,39 Outcomes of the pilot RCT will be quantitatively measured and qualitatively evaluated using concurrent semi-structured interviews. The pilot testing phase of the research program has been prospectively registered with the Chinese Clinical Trial Registry (Trial registration number ChiCTR2100050259) and will be reported in accordance with the SPIRIT and TIDieR guidelines.40,41
Participants
Subject recruitment will be carried out at two participating medical centers located in southwest China. In addition to the eligibility criteria described in phase 1B, participants will be further required to demonstrate reductions in peak exercise capacity during the cardiopulmonary exercise test (CPET), with a maximum oxygen uptake (VO2max) < 80% of predicted VO2max and/or a ventilatory anaerobic threshold (VAT) < 55% of predicted VO2max, to ensure adequate margin for improvement.42
We will identify potential child-parent dyads for enrolment via on-site screening of patients at outpatient visits, customized searching of the electronic medical record, and referrals from physicians and other healthcare providers. Potential eligible patients who are interest in participation in the trial will be invited to have an either face-to-face or telephone meeting in which the researchers will explain the study in detail and allow time for questions. Potential patients will attend an incremental CPET to ensure final eligibility. Child-parent dyads who meet the eligibility criteria will be invited to sign informed consent forms and complete baseline outcome assessment. The anticipated time schedule of participant enrolment, interventions and assessments is depicted in Table 2.
 |
Table 2 Schedule of Enrolment, Interventions, and Assessments* |
Randomization
Participants will be randomized in a 1:1 ratio to either the HeartFIT intervention group or the control group with variable block sizes of 2, 4 or 6, stratified by enrolling site. The allocation sequence will be generated by an independent researcher using SAS software, version 9.4 (SAS Institute, Cary, North Carolina) and the randomization assignment will be concealed until participants finalizing the initial assessment. All participants and researchers involved will be aware of the group affiliation but endpoint evaluation will be done by blinded medical staff.
Interventions
Participants allocated to the control group will continue their routine follow-up visits at outpatient clinics, with no access to the HeartFIT intervention during the study period. Child-parent dyads will receive basic health education regarding physical activity as necessary following the CPET test. They will be asked to build connection with the researcher via WeChat, and record and upload exercise logs biweekly using a standardized form. Upon completion of the study, participants in the control group will be given the opportunity to receive the same intervention.
Those randomized to the intervention group will receive the HeartFIT rehabilitation intervention performed by a multi-disciplinary team. To individually tailor the intervention, we will first conduct a comprehensive rehabilitation assessment guided by the individual and family self-management theory27 to learn about context and process factors that could potentially affect the intervention implementation. Health education in relation to the benefits of pediatric cardiac rehabilitation will be delivered and followed by a motivational interviewing to discuss options for enhancing activity levels over the succeeding 12 weeks and elicit participant motivation for actually changing and sustaining positive health behaviors. Each child-parent dyad will be granted a secure user account to login the HeartFIT platform and be demonstrated how to use the tool using teach-back method. An instruction video illustrating each function module of the platform will also be contained in the platform itself to help familiarize participants with the system whenever they need. In case of confusion or uncertainty, users will be directed to the research coordinator on the back end for further support. The HeartFIT platform will convey exercise prescriptions for home-based rehabilitation to promote cardiac fitness. The exercise prescriptions will follow expert recommendations for physical activity in pediatric patients with CHD3,43 and be created on the basis of individuals’ rehabilitation assessment. Specifically, the individualized exercise prescriptions will consist of four 45-minute sessions per week and every session will include aerobic, strength, and flexibility training. To maximize program engagement, participants are allowed to choose their preferred exercise activities from a variety of activities. The exercise prescriptions training intensity will be set at the heart rate corresponding to the ventilatory threshold of each subject assessed at the baseline CPET test and be reviewed by an experienced physical therapist specialized in pediatric cardiac rehabilitation. The exercise plans were also devised to allow participants the choice of a variety of activities. Participants will be required to upload their exercise performance to the digital platform to facilitate the identification and verification of adherence to the prescribed exercise plan. Other essential functions and features will be determined and incorporated into the platform according to study results derived from the conceptual design process. In addition, we will underline the importance of safety before exercises commence and throughout the intervention period and child-parent dyads will be followed up by phone every other week to check potential problems or adverse effects during rehabilitation. Follow-ups will also ensure participants’ proper use of the system, modify the exercise prescriptions as appropriate, and encourage maintenance of healthy behaviors.
Outcome Measures
Data collection will begin at the baseline visit following informed consent and progress throughout the study period and into the postintervention visit. The timeline of outcome assessment is summarized in Table 2.
Primary outcomes are changes in cardiorespiratory fitness values at 12 weeks measured by individual maximal CPET test with continuous breathing gas analysis on a bicycle ergometer, and is performed under medical supervision. The pre- and post-intervention assessments will be carried out by the same physiotherapists who are blinded to the intervention arm assignment. Secondary outcomes for the pilot trial will focus on potential effectiveness of the intervention to enhance behavioral change, physical health, and psychosocial functioning (Table 2). A triaxial accelerometer (Actigraph wGT3X-BT, Pensacola, FL, USA) will be used to objectively track daily physical activity and sedentary behaviors in free-living conditions. Medical staff (physiotherapists and dieticians) who are not involved in the study program will perform the pre- and post-intervention assessments of patients’ physical health in relation to body composition (InBody 770, Cerritos, CA, USA) and grip strength (Hand Dynamometer EH101, Zhongshan, China). All patient-reported psychosocial outcomes will be collected and managed electronically using REDCap system, including self-reported physical activity level (the physical activity questionnaire for older children and adolescents, PAQ-C/PAQ-A), health-related quality of life (Pediatric Quality of Life InventoryTM, PedsQLTM), self-efficacy for physical activity (the children’s self-perceptions of adequacy in and predilection for physical activity, CSAPPA), emotional wellbeing (the revised child anxiety and depression scale, RCADS), and parental activity support (the activity support scale for multiple groups, ACTS-MG).
Feasibility of implementing the HeartFIT intervention program will be assessed using 3 metrics (Table 2): (1) recruitment rate calculated as the number of actual consenting participants divided by the number of eligible patients approached; (2) retention rate demonstrated by the proportion of participants with valid postintervention outcome data; and (3) treatment adherence assessed by frequency, intensity, time, and type of registered exercise sessions. Participants dropping out midway will be contacted, where possible, to learn about their reasons for attrition and experience with the intervention. We will also assess acceptability and satisfaction of the intervention using both qualitative and quantitative metrics. Specifically, participants in the treatment group will be interviewed at the postintervention visit to capture their lived experience with the HeartFIT intervention in terms of its acceptability and perceived satisfaction. A modified Treatment Evaluation Inventory44 including 9 items will be adopted to quantitatively evaluate the treatment acceptability.
Adverse events are defined as any unexpected or uncomfortable signs or symptoms or any accidental injuries that could be possibly connected to the prescribed exercises over the intervention course. We will disclose all occurrences of adverse events in our final paper and report to the Ethics Committee as required.
Sample Size
Because of the pilot nature of the proposed study, we did not perform a formal sample size calculation. Nevertheless, to ensure the reliability of the standard deviation estimate from the pilot study to power a future trial with 90% power where the expected effect size is small (0.1 ≤ δ < 0.3),20 the optimal pilot sample size of 25 per arm is recommended.45 As such, we will target 68 participants to account for a 25% attrition20 and ensure at least 25 subjects for each group at follow-up.
Data Management
Each participant will be assigned a unique study ID and all personally identifiable information will be carefully de-identified prior to using the data. Study data will be maintained on a password-protected computer and backed up to a secure external hard drive, with access restricted to authorized researchers and staff. Automatic plausibility controls will be set to detect any inconsistencies or inaccuracies during data entry.
Data Analysis
We will first conduct a process evaluation to assess fidelity and quality of implementation of the HeartFIT intervention and gather participants’ attitude toward the program. The quantitative metrics of study feasibility and treatment acceptability will be reported using descriptive statistics. Qualitative process data acquired from interviews will be summarized using a content analysis method.
We will evaluate the preliminary program efficacy at 12 weeks through separate analysis of covariance (ANCOVA) models for each secondary outcome, with the post-intervention value as the dependent variable, group assignment (intervention or control) as the independent variable, and the baseline value of the specific outcome as the covariate. The outcome evaluation will primarily be based on the conservative intention-to-treat (ITT) analysis, and per protocol (PP) analysis will be adopted as a secondary analysis. In addition, multiple imputation methods will be undertaken in both the ITT and the PP analyses, when appropriate, to handle missing data as a form of sensitivity analysis. Although the pilot study may not be powered to detect significant differences in efficacy, this will help reveal the preliminary trends and trend direction of the intervention effectiveness. A two-tailed P < 0.05 will be considered significant for all statistical analyses.
Ethics Approval
This study received ethical approval from the Ethics Committee of West China Second University Hospital (No. 2021-063). The ethical principles of written informed consent, privacy and confidentiality, voluntary participation and data anonymization will be maintained throughout the research process. All study procedures will comply with the standards set by the Declaration of Helsinki.
Discussion
The ultimate goal of this ongoing program of research is to promote physical fitness for children and adolescents with CHD. As the essential step, this proposed study aims to iteratively develop and systematically evaluate an evidence-driven and user-centered WeChat-based platform to facilitate cardiac rehabilitation.
Over the past decade, there is a growing trend in the design of digital health systems that are aimed to promote physical fitness for individuals with chronic conditions.46 Overall, the efficacy of digital health interventions can be considerably enhanced through a more systematic approach to designing, developing, and reporting of the interventions.46 However, the majority of studies targets adult population; and even fewer studies focused on children and adolescents.46,47 The phase 1A scoping review was undertaken to only find four previous studies presenting different digital health tools intended to promote physical activity or facilitate exercise training for children and adolescents with CHD.20–23 However, none of the studies systematically considered the integration of behavior change techniques in the tool design or clearly presented their development process, nor did they proceed with usability testing for their specific tools. To our knowledge, this research program will be the first known example of applying a user-centered design approach and a sequentially phased problem-solving process to maximize the usability, quality, and behavior change potentials of a digital rehabilitation platform for children and adolescents with CHD, making it a novel contribution to pediatric cardiac rehabilitation.
The sequentially phased problem-solving process used in iteratively developing and evaluating the HeartFIT platform represent a rigorous approach to ensure it is easy to use, efficient and satisfying to operate. The thorough background analysis will enable us to learn about the broad context and understand needs and preferences of end users. The conceptualization and prototyping will translate the ideated features and functionalities into a functional prototype. Then, the HeartFIT prototype will undergo iterative usability testing to achieve an acceptable level of usability. Last, a pilot RCT will be launched to examine the feasibility and preliminary efficacy of implementing the HeartFIT intervention in a home-based setting among children and adolescents with CHD. The results of this study can contribute to a better understanding of the acceptability, appropriateness and early effectiveness of the HeartFIT intervention and, if positive, the proposed HeartFIT intervention could be scaled up with full-scale trial and be potentially integrated into the users’ daily lives to help children and adolescents with CHD adopt a physically active lifestyle and also be adapted and tailored to children and adolescents with other chronic cardiac conditions.
The convenience of the WeChat-based rehabilitation platform, which could help to overcome many traditional obstacles such as traveling and scheduling, and improve the delivery and effectiveness of healthcare service is likely to be particularly appealing. But undoubtedly, barriers to clinical research will ensue during the program’s implementation stage. However, we have designed the study to minimize such barriers. Firstly, recruiting child-parent dyad participants who are interested and willing to devote their time to this study is a potential challenge. Nevertheless, the remote and interactive features of HeartFIT that delivers personalized rehabilitation program may draw children and parents to the study. Secondly, selecting representative end-users of the targeted population is also a challenge.48 Yet, we have designed specific inclusion and exclusion criteria to ensure the selection of representative end-users in our usability testing. This will help to refine the prototype based on the end users’ needs and preference and increase successful potential in the subsequent pilot testing and dissemination of HeartFIT in this population in future. Thirdly, in the pilot study phase, potential challenges may occur for participants to strictly adhere to the prescribed exercise plan for a 12-week period, especially for those who have to transition from a sedentary lifestyle to a physically active lifestyle. However, the personalized program may help to overcome such barriers. Finally, the pilot testing may not be able to detect statistical significance of the study outcomes (eg, the physical fitness or psychosocial wellbeing), but we do expect positive behavior changes (eg, walking longer distance or less sedentary time) that eventually will lead to positive patient outcomes.
Acknowledgments
We would like to thank Prof. Kaiyu Zhou for her ongoing contribution to the study design and conduct. We would also like to thank nurses, physicians, and physical therapists at the Department of Cardiovascular Surgery of West China Hospital and Department of Pediatric Cardiology of West China Second University Hospital for their support of the research program.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Clinical Research Fund of West China Second University Hospital (Grant No. KL078) and the Provincial Key Research and Development Program of Sichuan (Grant No. 2018SZ0194). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zimmerman MS, Smith AGC, Sable CA, et al. Global, regional, and national burden of congenital heart disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc Heal. 2020;4:185–200. doi:10.1016/S2352-4642(19)30402-X
2. Bouma BJ, Mulder BJM. Changing landscape of congenital heart disease. Circ Res. 2017;120:908–922. doi:10.1161/CIRCRESAHA.116.309302
3. Takken T, Giardini A, Reybrouck T, et al. Recommendations for physical activity, recreation sport, and exercise training in paediatric patients with congenital heart disease: a report from the Exercise, Basic & Translational Research Section of the European Association of Cardiovascular Prevention and Rehabilitation, the European Congenital Heart and Lung Exercise Group, and the Association for European Paediatric Cardiology. Eur J Prev Cardiol. 2012;19:1034–1065. doi:10.1177/1741826711420000
4. Longmuir PE, Brothers JA, De Ferranti SD, et al. Promotion of physical activity for children and adults with congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2013;127:2147–2159. doi:10.1161/CIR.0b013e318293688f
5. Guthold R, Stevens GA, Riley LM, et al. Global trends in insufficient physical activity among adolescents: a pooled analysis of 298 population-based surveys with 1.6 million participants. Lancet Child Adolesc Heal. 2020;4:23–35. doi:10.1016/S2352-4642(19)30323-2
6. Caterini JE, Campisi E, Cifra B. Physical activity promotion in pediatric congenital heart disease: are we running late? Can J Cardiol. 2020;36:1406–1416. doi:10.1016/j.cjca.2020.07.003
7. Amedro P, Gavotto A, Guillaumont S, et al. Cardiopulmonary fitness in children with congenital heart diseases versus healthy children. Heart. 2018;104:1026–1036. doi:10.1136/heartjnl-2017-312339
8. Gupta S, Rohatgi A, Ayers CR, et al. Cardiorespiratory fitness and classification of risk of cardiovascular disease mortality. Circulation. 2011;123:1377–1383. doi:10.1161/CIRCULATIONAHA.110.003236
9. Imboden MT, Harber MP, Whaley MH, et al. Cardiorespiratory fitness and mortality in healthy men and women. J Am Coll Cardiol. 2018;72:2283–2292. doi:10.1016/j.jacc.2018.08.2166
10. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins; 2013.
11. Patel PN, Zwibel H. Physiology, Exercise. StatPearls; 2019.
12. Curran T, Gauthier N, Duty SM, et al. Identifying elements for a comprehensive paediatric cardiac rehabilitation programme. Cardiol Young. 2020;30:1473–1481. doi:10.1017/S1047951120002346
13. Tikkanen AU, Oyaga AR, Riano OA, et al. Paediatric cardiac rehabilitation in congenital heart disease: a systematic review. Cardiol Young. 2012;22:241–250. doi:10.1017/S1047951111002010
14. Thomas RJ, Beatty AL, Beckie TM, et al. Home-based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019;140:e69–e89. doi:10.1161/CIR.0000000000000663
15. Tencent. WeChat official accounts platform; 2022. Available from: https://mp.weixin.qq.com/?lang=en_US&token=.
16. Chen X, Zhou X, Li H, et al. The value of WeChat application in chronic diseases management in China. Comput Methods Programs Biomed. 2020;196:105710. doi:10.1016/j.cmpb.2020.105710
17. Shah SG, Robinson I, Alshawi S. Developing medical device technologies from users’ perspectives: a theoretical framework for involving users in the development process. Int J Technol Assess Health Care. 2009;25:514–521. doi:10.1017/S0266462309990328
18. Van Cleave JH, Fu MR, Bennett AV, et al. The development, usability, and reliability of the Electronic Patient Visit Assessment (ePVA) for head and neck cancer. Mhealth. 2019;5:21. doi:10.21037/mhealth.2019.06.05
19. Fu MR, Axelrod D, Guth AA, et al. mHealth self-care interventions: managing symptoms following breast cancer treatment. Mhealth. 2016;2:28. doi:10.21037/mhealth.2016.07.03
20. Klausen SH, Andersen LL, Søndergaard L, et al. Effects of eHealth physical activity encouragement in adolescents with complex congenital heart disease: the PReVaiL randomized clinical trial. Int J Cardiol. 2016;221:1100–1106. doi:10.1016/j.ijcard.2016.07.092
21. Lemire O, Yaraskavitch J, Lougheed J, et al. Impacting child health outcomes in congenital heart disease: cluster randomized controlled trial protocol of in-clinic physical activity counselling. Contemp Clin Trials. 2020;91:105994. doi:10.1016/j.cct.2020.105994
22. Meyer M, Brudy L, Fuertes-Moure A, et al. E-health exercise intervention for pediatric patients with congenital heart disease: a randomized controlled trial. J Pediatr. 2021;233:163–168. doi:10.1016/j.jpeds.2021.01.058
23. Rombeek M, De Jesus S, Altamirano-Diaz L, et al. The use of smartphones to influence lifestyle changes in overweight and obese youth with congenital heart disease: a single-arm study. Pilot Feasibility Stud. 2017;3:59. doi:10.1186/s40814-017-0207-y
24. Rombeek M, De Jesus S, Prapavessis H, et al. Improving remote lifestyle intervention studies in children: participant and caregiver feedback of the smart heart study. Patient Educ Couns. 2020;103:1326–1334. doi:10.1016/j.pec.2020.02.016
25. DiCicco-Bloom B, Crabtree BF. The qualitative research interview. Med Educ. 2006;40:314–321. doi:10.1111/j.1365-2929.2006.02418.x
26. Magny-Normilus C, Whittemore R, Wexler DJ, et al. Barriers to type 2 diabetes management among older adult Haitian immigrants. Sci Diabetes Self Manag Care. 2021;47:382–390. doi:10.1177/26350106211040435
27. Polly R, Sawin K. The individual and family self-management theory: background and perspectives on context, process, and outcomes. Nurs Outlook. 2009;57:217–225. doi:10.1016/j.outlook.2008.10.004
28. Michie S, Ashford S, Sniehotta FF, et al. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Heal. 2011;26:1479–1498. doi:10.1080/08870446.2010.540664
29. Monteiro-Guerra F, Rivera-Romero O, Fernandez-Luque L, et al. Personalization in real-time physical activity coaching using mobile applications: a scoping review. IEEE J Biomed Heal Informatics. 2020;24:1738–1751. doi:10.1109/JBHI.2019.2947243
30. International Organization for Standardization. Ergonomics of human-system interaction-part 11: usability: definitions and concepts; 2018. Available from: https://www.iso.org/standard/63500.html.
31. Alwashmi MF, Hawboldt J, Davis E, et al. The iterative convergent design for mobile health usability testing: mixed-methods approach. JMIR Mhealth Uhealth. 2019;7:e11656. doi:10.2196/11656
32. Nielsen J. Usability inspection methods. In:
33. Fu MR, McTernan ML, Qiu JM, et al. The effects of Kinect-enhanced lymphatic exercise intervention on lymphatic pain, swelling, and lymph fluid level. Integr Cancer Ther. 2021;20:1–14. doi:10.1177/15347354211026757
34. Nielsen Norman Group. Why you only need to test with 5 users?; 2000. Available from: https://www.nngroup.com/articles/why-you-only-need-to-test-with-5-users/.
35. Fu MR, Axelrod D, Guth AA, et al. Usability and feasibility of health IT interventions to enhance self-care for lymphedema symptom management in breast cancer survivors. Internet Interv. 2016;5:56–64. doi:10.1016/j.invent.2016.08.001
36. Zhou L, Bao J, Setiawan IMA, et al. The mHealth app usability questionnaire (MAUQ): development and validation study. JMIR Mhealth Uhealth. 2019;7:e11500. doi:10.2196/11500
37. Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: following considerable development in the field since 2006, MRC and NIHR have jointly commissioned an update of this guidance to be published in 2019; 2019. Available from: https://mrc.ukri.org/documents/pdf/complex-interventions-guidance/.
38. Gardner B, Lally P, Wardle J. Making health habitual: the psychology of “habit-formation” and general practice. Br J Gen Pract. 2012;62:664–666. doi:10.3399/bjgp12X659466
39. Jacques M, Hiam D, Craig J, et al. Epigenetic changes in healthy human skeletal muscle following exercise - a systematic review. Epigenetics. 2019;14:633–648. doi:10.1080/15592294.2019.1614416
40. Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi:10.1136/bmj.e7586
41. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi:10.1136/bmj.g1687
42. Frigiola A, Amedro P, Gavotto A, et al. Impact of a centre and home-based cardiac rehabilitation program on the quality of life of teenagers and young adults with congenital heart disease: the QUALI-REHAB study rationale, design and methods. Int J Cardiol. 2019;288:70–71. doi:10.1016/j.ijcard.2019.03.006
43. Budts W, Börjesson M, Chessa M, et al. Physical activity in adolescents and adults with congenital heart defects: individualized exercise prescription. Eur Heart J. 2013;34:3669–3674. doi:10.1093/eurheartj/eht433
44. Kelley ML, Heffer RW, Gresham FM, et al. Development of a modified treatment evaluation inventory. J Psychopathol Behav Assess. 1989;11:235–247. doi:10.1007/BF00960495
45. Whitehead AL, Julious SA, Cooper CL, et al. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res. 2016;25:1057–1073. doi:10.1177/0962280215588241
46. Domin A, Spruijt-metz D, Theisen D, et al. Smartphone-based interventions for physical activity promotion: scoping review of the evidence over the last 10 years. JMIR Mhealth Uhealth. 2021;9:e24308. doi:10.2196/24308
47. Williams CA, Wadey C, Pieles G, et al. Physical activity interventions for people with congenital heart disease. Cochrane Database Syst Rev. 2020;10:CD013400. doi:10.1002/14651858.CD013400.pub2
48. Fessenden T. Recruiting and screening candidates for user research projects; 2021. Available from: https://www.nngroup.com/articles/recruiting-screening-research-candidates/.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
