Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 10 » Issue 1
A variant in 3'-untranslated region of KRAS compromises its interaction with hsa-let-7g and contributes to the development of lung cancer in patients with COPD
Authors Hu H, Zhang L, Teng G, Wu Y, Chen Y
Received 27 February 2015
Accepted for publication 9 April 2015
Published 17 August 2015 Volume 2015:10(1) Pages 1641—1649
DOI https://doi.org/10.2147/COPD.S83596
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Hua Hu,1 Linlin Zhang,1 Geling Teng,1 Yanhua Wu,1 Ying Chen2
1The Respiratory Department, Shandong Provincial Chest Hospital, 2The Clinical Laboratory of Shandong Provincial Chest Hospital, Jinan, Shandong, People’s Republic of China
Objective: The objective of the present study was to explore the molecular mechanism by which a single nucleotide polymorphism (rs712) interferes with interaction between 3'-untranslated region (3'-UTR) of KRAS and let-7g, and its association with development of lung cancer in the patients with COPD.
Materials and methods: In this study, we confirmed that KRAS is a target of let-7g in lung cancer cells, and that introduction of rs712 minor allele into 3'-UTR significantly compromised the miRNA/mRNA interaction by using a luciferase reporter system. Additionally, a total of 35 lung tissue samples were obtained (TT:17, TG:12, GG:6), and let-7g and KRAS expression levels were determined.
Results: We showed that let-7g level was similar between groups, and the concentration of KRAS in GG genotype group was significantly higher than in TT or GT genotype group. Meanwhile, we found COPD patients with GG genotype had significantly higher risk for lung cancer (odds ratio OR =6.83, P=0.0081), compared with TT and GT genotypes.
Conclusion: Our study demonstrated that KRAS 3'-UTR rs712 polymorphism interfered with miRNA/mRNA interaction, and showed that the minor allele was associated with an elevated risk for development of lung cancer in COPD.
Keywords: let-7g, chronic obstructive pulmonary disease, lung cancer, KRAS, single nucleotide polymorphism
Introduction
Lung cancer is the cause for more deaths than most other cancers worldwide. Tobacco exposure continues to be a major risk factor for lung cancer and is also closely associated with the pathogenesis of COPD.1 COPD alone also raises the risk of lung cancer independent of tobacco use. As a matter of fact, 50%–80% of patients with lung cancer have COPD.1 In particular, squamous cell carcinoma and adenocarcinoma are the most common histological subtypes among patients with COPD and among long-term smokers.2,3 Therefore, it is believed that lung cancer and COPD may share common etiological factors and similar molecular mechanisms.4
The discovery of microRNAs (miRNAs) in early 1990s revealed an unexpected level of gene expression regulation that proves to be relevant in regulating numerous physiological and pathological conditions, including carcinogenesis, cancer progression, and response to therapy.5 miRNAs are noncoding, evolutionally preserved, small-sized RNA that regulate gene expression through different mechanisms.6 As many as 2,000 human miRNAs have been reported (Sanger miRBase version 18) to be involved in the control of important physiological processes and in the pathogenesis of different diseases.6,7 Some miRNAs are transcribed from polycistronic primary transcripts, either through independent action or within a coordinated regulatory network. For lung cancer, miRNA profiling may be useful in the diagnosis, prediction of recurrence, and assessment of prognosis in different clinical scenarios.8,9 miRNA clusters have also been recognized as essential components of lung cancer pathways; for example, the miR-17-92 cluster regulates growth stimulatory signaling pathways in small-cell lung cancer.10 Better understanding of the molecular networks regulated by miRNAs may be potentially useful biomarkers for screening, diagnosis, and/or therapeutic targeting.
There is increasing evidence that single nucleotide polymorphisms (SNPs) residing in miRNA binding sites can lead to differential expression of target genes.11,12 The impact of miRNA binding site polymorphisms on cancer risk was reviewed by Chen et al in 2008.13 An SNP in the let-7 complementary binding site (LCS6) of the KRAS 3′-untranslated region (3′-UTR) is associated with an elevated risk of non-small-cell lung cancer,14 increased cetuximab responsiveness in KRAS wild-type metastatic colorectal cancer (CRC) patients,15 and reduced survival in oral cancer and late-stage CRC patients.15,16 An SNP in the let-7 binding site of the KRAS 3tage e metastatic s reviewed by Chen et al in 2008 sites can lead to differential expression17–19 and gastric cancer.17
We hypothesized that KRAS rs712 polymorphism may increase the susceptibility of lung cancer in the background of COPD. To test this hypothesis, we examined the relationship between the rs712 polymorphism and the incidence of lung cancer in the patients with COPD. In addition, we assessed the effect of KRAS rs712 polymorphism on the interaction between the let-7g and KRAS mRNA as well as the expression pattern of let-7g and KRAS in the lung tissue samples of each genotype.
Materials and methods
Patients and clinical specimens
A total of 554 patients with diagnosis of COPD, including 132 with lung cancer and 442 without lung cancer, were enrolled in this study. The included COPD patients were recruited from Shandong Provincial Chest Hospital. The disease was staged with the following criteria: classification of severity of airflow limitation in COPD. Global initiative for chronic Obstructive Lung Disease (GOLD) 1 (mild): forced expiratory volume in 1 second (FEV1) ≥80% predicted; GOLD 2 (moderate): 50%≤ FEV1 <80% predicted; GOLD 3 (severe): 30%≤ FEV1 <50% predicted; GOLD 4 (very severe): FEV1 <30% predicted. To evaluate the effect of smoking, we evaluated cumulative smoking dose by calculating pack-years index:
Pack-years = (Cigarettes per day/20) × Years smoked | (1) |
Among the recruited participants, resected specimens were available in 35 patients including 24 COPD patients who had received surgery for lung tumor resection (at least 2 cm away from tumor tissues), and eleven participants who had undergone volume reduction surgery. Institutional review board of Shandong Provincial Chest Hospital approved the research protocol. Five milliliters of peripheral blood was donated for genomic DNA extraction after signed written consent was provided by each participant.
Genotyping
Genomic DNA was extracted from peripheral blood using the extraction kit (Bioteke, Beijing, People’s Republic of China) as per the manufacturer’s protocol. Chromosome segments containing KRAS rs712 polymorphism were polymerase chain reaction (PCR) amplified using the primer set: 5′-TAATCTGGGTGTTGATGA-3′and 5′-GAGTGACCATGACTAATA-3′, and genotyping was performed using direct sequencing.
Quantification of miRNA and mRNA expression levels
Real-time qPCR was conducted using Power SYBR Fast PCR Master Mix and Step-one plus real-time PCR machine (Applied Biosystems, Foster City, CA, USA). The human U6 small nuclear RNA was used as an internal control. The transcription levels of let-7g, KRAS, and ATK were quantified by the SYBR green-I Master PCR Mix with specific real-time PCR primer sets. All reactions were done in duplicate. Expression levels of miRNA and mRNA were quantified based on the 2ΔΔCt relative quantification method. The primer sets used for let-7g were: forward 5′-TGAGGTAGTTTGTACAGTT-3′, reverse 5′-TTGACCGGTCAGGTTCAG-3′; KRAS: forward 5′-tgaggactggggagggcttt-3′, reverse 5′-aggcatcatcaacaccctgtct-3′; AKT: forward 5′-TTTGGGAAGGTGATCCTGGTG-3′, reverse 5′-GGTCGTGGGTCTGGAATGAGT-3′; U6: forward 5′-CTCGCTTCGGCAGCACA-3′, reverse 5′-AACTCTTCACTAATTTGCTG-3′.
Western blot
Lung tissue samples were homogenized and lyzed in radioimmunoprecipitation assay (RIPA) lysis buffer that contains protease inhibitor cocktail (Roche, Indianapolis, IN, USA). The same amount of proteins were resolved on SDS–PAGE and transferred onto nitrocellular membranes. The membranes were blocked with 5% (wt/vol) nonfat milk, washed in Tris-buffered saline Tween 20 solution, and incubated with primary antibody (anti-KRAS antibody, anti-AKT antibody, and anti-p-AKT antibody) at 4°C overnight. After rinsing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Bio-Rad Laboratories Inc., Hercules, CA, USA) at dilutions of 1:10,000 for 1 hour at room temperature, and immunoreactive bands were visualized with Pierce SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Waltham, MA, USA). The relative band intensities were quantified through densitometry using NIH ImageJ software (National Institutes of Health, Bethesda, MD, USA) and normalized with image densities of actin. All antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Let-7g target validation by UTR luciferase reporter assay
Two primers were designed to amplify the KRAS 3′-UTR chromosomal segment that contained let-7g binding site and KRAS 3′-UTR rs712 polymorphism (forward: 5′-ATGACTGAATATAAACTTGTGGTAG-3′; reverse: 5′-ACTAGATAAAACACAGAATAGGGAT-3′), and the PCR product was inserted into a modified version of pcDNA3.1 with a firefly luciferase inserted. The mutant construct of KRAS 3′-UTR was generated by site-directed mutagenesis.
The luciferase assay with wild-type or mutant KRAS 3′-UTR was performed in two lung cancer cell lines H226 (squamous lung cancer) and H522 (lung adenocarcinoma), respectively. Approximately, 4×104 cells were seeded onto 12-well plates, and after the cells reached 80%–90% confluence, each well was transfected with 300 ng of 3′-UTR reporter vectors, 1,200 ng of let-7g mimics (5′-GAGGUAGUAGUUUGUACAGUU-3′), and 40 ng of phRL-TK (Promega, Fitchburg, WI, USA) with 4 μL of Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). At 2 days after transfection, the cells were harvested and luciferase activities were determined using the Dual-Luciferase Reporter Assay System (Promega).
Statistical analysis
The differences in the characteristics of COPD in patients with or without lung cancer were compared using the chi-square test (for categorical variables) and Student’s t-test (for continuous variables). The risk of the KRAS rs712 polymorphism for lung cancer in COPD was estimated by calculating the odds ratio (OR) and 95% confidence intervals (CI). All statistical analyses were performed using SPSS statistical software package version 21.0 (SPSS, Inc., Chicago, IL, USA). All tests were two-sided and statistical significance was considered if P≤0.05.
Results
Characteristics of the participants
The demographic and clinicopathological parameters of the participants are described in Table 1. The COPD patients with and without lung cancer were statistically matched regarding age (P=0.215), sex (P=0.0582), and smoking status (P=0.0856) and smoking index (P=0.06). However, COPD severity in COPD patients with lung cancer was significantly higher than in those without lung cancer (P<0.001). Additionally, all these variables were included in the multivariate logistic regression analysis to evaluate the potential effects on the association between KRAS 3RAS rs712 polymorphism and the risk of development of lung cancer in COPD patients.
  | Table 1 Clinicopathological characteristics of the participants in this study |
Association analysis between KRAS 3′-UTR rs712 polymorphism and risk of development of lung cancer in COPD
Genotype frequency of KRAS 3′-UTR rs712 polymorphism among the COPD patients with or without lung cancers and their associations with lung cancer risk in COPD are presented in Table 2. The frequencies of the TT, TG, and GG genotypes were 65.87%, 31.27%, and 2.86%, respectively, among those COPD patients without lung cancer, and 54.54%, 28.79%, and 16.67%, respectively, among those with lung cancer (P=0.0282). When we used the TT genotype as the reference, we noted that the CC genotype was associated with a statistically significantly increased risk of lung cancer in COPD (adjusted OR =7.07, 95% CI =3.34–14.97). Also, a similar trend was observed in the GG genotype compared with the combined TT/TG genotypes (adjusted OR =6.83, 95% CI =3.27–14.24).
  | Table 2 Statistical analysis of the association between the rs712 polymorphism genotype and the presence of lung cancer in COPD patient |
Effect of the KRAS 3′-UTR rs712 polymorphism on KRAS expression in lung cancer cells
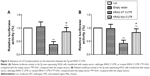
It has been previously shown that KRAS is a target of let-7g in human cells. Based on the computational analysis, the KRAS 3′-UTR rs712 polymorphism was found to be located in the flanking sequence close to the predicted “binding site” for hsa-let-7g (Figure 1). To test whether hsa-let-7g targets KRAS 3ASgets 1 lung cancer cells, we constructed reporter vectors carrying wild-type or mutant KRAS 3′-UTR, as described in Figure 1. Subsequently, we used them for transient transfection with the lung cancer cells together with let-7g mimics or scramble controls. As shown in Figure 2, only the luciferase activity from the cells cotransfected with wild-type KRAS 3′-UTR and let-7g mimics was significantly lower than the control, and activity in all other groups were comparable. The results confirmed that KRAS is a validated target of let-7g in lung cancer cells, that rs712 polymorphism compromised the interaction between let-7g and KRAS 3′-UTR, and that the nucleotide substitution completely abrogates the miRNA/mRNA interaction in lung cancer cells.
  | Figure 1 Schematic sequence comparison of KRAS 3′-UTR and primary let-7g and location of rs712 polymorphism in the 3′-UTR of KRAS. |
Determination of expression patterns of let-7g and KRAS in lung tissues with different genotypes
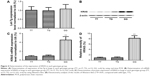
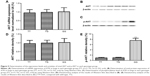
The lung tissues of three different genotypes (TT, n=17; TG, n=12; GG, n=6) were used to further explore the impacts of the polymorphism on the interaction between let-7g and KRAS 3′-UTR. Using immunohistochemistry analysis, we found that the expression of KRAS was comparable between the TT and TG groups, both of which were substantially lower than GG group (Figure 3). Additionally, to characterize the role and effect of KRAS 3′-UTR rs712 polymorphism on the miRNA/mRNA interaction in lung cancer cells which plays a central role in the development of lung cancer in the patients with COPD, we quantified the expression of let-7g and KRAS by using real-time PCR as well as Western blot. As shown in Figure 4, while the expression levels of let-7g were similarly distributed among each genotype group, the mRNA and protein expression levels of KRAS in GG genotype group were significantly higher than in TT and TG groups. To further confirm the effect of KRAS 3′-UTR rs712 polymorphism on the signaling pathway, we further examined the expression levels of ATK as well as the phosphorylation status of the protein. As shown in Figure 5, even though the mRNA and total protein expression levels of ATK were comparable among each genotype group, the phosphorylated ATK was significantly higher in GG group than in TT or TG group.
  | Figure 3 Determination of expression of KRAS in lungs of each genotype group by using immunohistochemistry. |
Discussion
The occurrence of lung cancer in patients with COPD has been thought to reflect the role of the common etiological agent, cigarettes. High lung cancer incidence rates among nonsmokers in COPD clinics challenge this idea and prompt epidemiological studies to address this question. Little progress has been made toward identifying common mechanistic links for these heterogeneous diseases, as both lung cancer and COPD are characterized by multiple sub-phenotypes.18 Despite the increased attention, the nature of the link between COPD and lung cancer remains unclear. This is, in a large part, due to the seemingly opposite nature of the two diseases. Emphysema is characterized by the destruction of matrix structures, epithelial cell death, and vanishing blood supply (alveolar capillary dropout). We hypothesized that some molecular defect may be responsible for such changes of opposite direction.
miRNAs can act as transacting factors to suppress translation of or induce mRNA degradation of target genes; they are found to regulate gene expression in various cancers. The let-7 miRNA family functions as tumor suppressors in many cancers including GC, and their expressions are downregulated in various other cancers.19–21 Let-7 was reported to target and downregulate RAS by binding to specific sites in the 3′-UTR of the KRAS mRNA and exert its tumor-suppressing function.22,23 Over-expression of let-7g can inhibit the growth of transplanted lung cancer in nude mice model.23 The expression of let-7 is associated with cancer survival in the anti-KRAS monoclonal antibodies. Earlier studies have revealed that let-7f can inhibit tumor invasion and metastasis in human gastric cancer.19
KRAS, also known as V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog, acts as an intracellular signal transducer. The oncogenic KRAS mutation is an essential part of the development of many human cancers.24–26 KRAS has been reported to be negatively regulated by the let-7 miRNA family.22,27 Transfection of human colon cancer cells with let-7g precursor miRNA leads to the inhibition of cell growth and downregulation of KRAS, which suggests that let-7 miRNA may be involved in the growth of colon cancer cells.27 In this study, we confirmed that KRAS is a target of let-7g in two lung cancer cell lines. The human KRAS 3′-UTR contains multiple putative let-7 complementary sites (LCS), which enables the let-7 regulation of KRAS activity.22 Two polymorphisms have been so far studied in multiple cancer types. Chin et al for the first time reported that the KRAS LCS6 variant allele was significantly associated with an elevated risk of non-small-cell lung cancer among moderate smokers.28 The variant allele was reported to confer an elevated risk for triple negative breast cancer29 and breast cancer in families with the BRCA1 mutation,30 but such finding was not noted for invasive epithelial ovarian cancer.31 Another well-studied polymorphism is rs712 polymorphism. Kranenburg reported that the KRAS rs712 polymorphism was associated with an increased risk of CRC and the clinical features of the disease.24 AKT is one of the most important downstream effectors along the signaling pathway, and phosphorylation is a necessary step to activate the function of AKT.32 In this study, we recruited 554 COPD patients with (n=132) and without (n=422) lung cancer, and we did not detect KRAS LCS6 variant in our sample pool; therefore, we focused on rs712 polymorphism. By using luciferase reporter system, we found that introduction of rs712 minor allele into 3′-UTR significantly compromised the miRNA/mRNA interaction. Additionally, to verify such relationship in human samples, we collected 35 human tissue samples genotyped as TT (17), TG (12), GG (6), and let-7g and KRAS expression levels were determined. We showed that let-7g level was similar between groups, and the concentration of KRAS in GG genotype group was significantly higher than in TT or GT genotype group. Meanwhile, we found COPD patients with GG genotype had significantly higher risk for lung cancer (OR =6.83, P=0.0081), compared with TT and GT genotypes.
Overall, our study, together with previous reports,33 provides the evidence that the rs712 polymorphism compromised the interaction between let-7g and 3′-UTR of KRAS, and was associated with the development of lung cancer in COPD. This polymorphism could be a biomarker to predict the development of lung cancer in COPD and may be of preventive and therapeutic relevance in the clinical practice.
Disclosure
The authors report no conflicts of interest in this work.
References
Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34:380–386. | ||
de Torres JP, Marin JM, Casanova C, et al. Lung cancer in patients with chronic obstructive pulmonary disease – incidence and predicting factors. Am J Respir Crit Care Med. 2011;184:913–919. | ||
Purdue MP, Gold L, Jarvholm B, et al. Impaired lung function and lung cancer incidence in a cohort of Swedish construction workers. Thorax. 2007;62:51–56. | ||
Young RP, Hopkins RJ. COPD and lung cancer linked at a molecular genetic level. Chest. 2011;140:266–267. | ||
Bentwich I, Avniel A, Karov Y, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–770. | ||
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. | ||
Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev. 2011;91:827–887. | ||
Landi MT, Zhao Y, Rotunno M, et al: MicroRNA expression differentiates histology and predicts survival of lung cancer. Clin Cancer Res. 2010;16:430–441. | ||
Yanaihara N, Caplen N, Bowman E, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. | ||
Hayashita Y, Osada H, Tatematsu Y, et al. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–9632. | ||
Chin LJ, Ratner E, Leng S, et al. A SNP in a let-7 microRNA complementary site in the KRAS 3′ untranslated region increases non-small cell lung cancer risk. Cancer Res. 2008;68:8535–8540. | ||
Gao Y, He Y, Ding J, et al. An insertion/deletion polymorphism at miRNA-122-binding site in the interleukin-1alpha 3′ untranslated region confers risk for hepatocellular carcinoma. Carcinogenesis. 2009;30:2064–2069. | ||
Chen K, Song F, Calin GA, et al. Polymorphisms in microRNA targets: a gold mine for molecular epidemiology. Carcinogenesis. 2008;29:1306–1311. | ||
Christensen BC, Moyer BJ, Avissar M, et al. A let-7 microRNA-binding site polymorphism in the KRAS 3′ UTR is associated with reduced survival in oral cancers. Carcinogenesis. 2009;30:1003–1007. | ||
Graziano F, Canestrari E, Loupakis F, et al. Genetic modulation of the Let-7 microRNA binding to KRAS 3′-untranslated region and survival of metastatic colorectal cancer patients treated with salvage cetuximab-irinotecan. Pharmacogenomics J. 2010;10:458–464. | ||
Wang WY, Chien YC, Wong YK, et al. Effects of KRAS mutation and polymorphism on the risk and prognosis of oral squamous cell carcinoma. Head Neck. 2012;34:663–666. | ||
Li ZH, Pan XM, Han BW, et al. A let-7 binding site polymorphism rs712 in the KRAS 3′ UTR is associated with an increased risk of gastric cancer. Tumour Biol. 2013;34:3159–3163. | ||
Punturieri A, Szabo E, Croxton TL, et al. Lung cancer and chronic obstructive pulmonary disease: needs and opportunities for integrated research. J Natl Cancer Inst. 2009;101:554–559. | ||
Liang S, He L, Zhao X, et al. MicroRNA let-7f inhibits tumor invasion and metastasis by targeting MYH9 in human gastric cancer. PLoS One. 2011;6:e18409. | ||
Takamizawa J, Konishi H, Yanagisawa K, et al: Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. | ||
Chang SS, Jiang WW, Smith I, et al. MicroRNA alterations in head and neck squamous cell carcinoma. Int J Cancer. 2008;123:2791–2797. | ||
Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. | ||
He XY, Chen JX, Zhang Z, et al. The let-7a microRNA protects from growth of lung carcinoma by suppression of k-Ras and c-Myc in nude mice. J Cancer Res Clin Oncol. 2010;136:1023–1028. | ||
Kranenburg O. The KRAS oncogene: past, present, and future. Biochim Biophys Acta. 2005;1756:81–82. | ||
Bos JL, Fearon ER, Hamilton SR, et al. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–297. | ||
Boughdady IS, Kinsella AR, Haboubi NY, et al. K-ras gene mutations in adenomas and carcinomas of the colon. Surg Oncol. 1992;1:275–282. | ||
Akao Y, Nakagawa Y, Naoe T. let-7 microRNA functions as a potential growth suppressor in human colon cancer cells. Biol Pharm Bull. 2006;29:903–906. | ||
Chin LJ, Ratner E, Leng S, et al. A SNP in a let-7 microRNA complementary site in the KRAS 3′ untranslated region increases non-small cell lung cancer risk. Cancer Res. 2008;68:8535–8540. | ||
Paranjape T, Heneghan H, Lindner R, et al. A 3′-untranslated region KRAS variant and triple-negative breast cancer: a case-control and genetic analysis. Lancet Oncol. 2011;12:377–386. | ||
Hollestelle A, Pelletier C, Hooning M, et al. Prevalence of the variant allele rs61764370 T>G in the 3′UTR of KRAS among Dutch BRCA1, BRCA2 and non-BRCA1/BRCA2 breast cancer families. Breast Cancer Res Treat. 2011;128:79–84. | ||
Pharoah PD, Palmieri RT, Ramus SJ, et al. The role of KRAS rs61764370 in invasive epithelial ovarian cancer: implications for clinical testing. Clin Cancer Res. 2011;17:3742–3750. | ||
Collins MA, Pasca MM. Kras as a key oncogene and therapeutic target in pancreatic cancer. Front Physiol. 2014;4:407. | ||
Kim M, Chen X, Chin LJ, et al. Extensive sequence variation in the 3′ untranslated region of the KRAS gene in lung and ovarian cancer cases. Cell Cycle. 2014;13:1030–1040. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.