Back to Journals » OncoTargets and Therapy » Volume 12
A temperature-sensitive phase-change hydrogel of tamoxifen achieves the long-acting antitumor activation on breast cancer cells
Authors Meng D, Lei H, Zheng X , Han Y, Sun R, Zhao D, Liu R
Received 13 January 2019
Accepted for publication 16 April 2019
Published 24 May 2019 Volume 2019:12 Pages 3919—3931
DOI https://doi.org/10.2147/OTT.S201421
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Takuya Aoki
Du Meng,1,* Hongwei Lei,2,* Xiaoqiang Zheng,3 Yaxuan Han,4 Ronggang Sun,5 Dongli Zhao,1 Rui Liu1
1Department of Radio Oncology, The First Affiliated Hospital of Xi’an Jiaotong University School of Medicine, Xi’an, Shaanxi Province, 710061, People’s Republic of China; 2Department of Radio Oncology, The Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning Province, 116027, People’s Republic of China; 3Department of Medical Oncology, The First Affiliated Hospital of Xi’an Jiaotong University School of Medicine, Xi’an, Shaanxi Province, 710061, People’s Republic of China; 4Department of Oncology, The Xi’an Chest Hospital, Xi’an, Shaanxi Province, 710000, People’s Republic of China; 5Department of Radio Oncology, The People’s Hospital of YangZhong City, YangZhong, Jiangsu Province, 212200, People’s Republic of China
*These authors contributed equally to this work
Background: Breast cancer is one of the foremost threats to female health nowadays. Tamoxifen, an antagonist of estrogen receptor-α (ERα), is the first choice for endocrine-dependent breast cancer (ERα-positive breast cancer) treatment. However, ERα has an important function in the normal physical regulation of estrogen, and current oral administration of tamoxifen has potential side effects on normal endocrine secretion. In the present work, we aim to develop novel approaches to increase the antitumor effect of tamoxifen on breast cancer cells and decrease the potential side effects in the human body during treatment.
Methods: A temperature-sensitive phase-change hydrogel for tamoxifen (Tam-Gel) was generated. After establishing subcutaneous tumors formed by MCF-7, an ERα-positive breast cancer cell line, in nude mice, an intratumoral injection of Tam-Gel was performed to examine whether Tam-Gel facilitated the slow-release or antitumor effect of tamoxifen. A metastatic breast cancer model was established using the intrahepatic growth of MCF-7 cells in immunodeficient rats.
Results: Tam-Gel can transform from liquid to hydrogel at room temperature. An intratumoral injection of Tam-Gel facilitated the slow-release or antitumor effect of tamoxifen. Once Tam-Gel, but not Tam-Sol, was administered by intratumoral injection, it significantly decreased the uptake of radionuclide probes (18F-fluoroestradiol or 18F-fluorodeoxyglucose) by cells in rats’ livers and the intrahepatic growth of MCF-7 cells in rats’ livers.
Conclusion: A novel slow-release system was successfully prepared to facilitate the long-term release of tamoxifen in breast cancer tissues, and achieved an antitumor effect in the long term.
Keywords: breast cancer, estrogen receptor-α, tamoxifen nanoparticle, temperature-sensitive phase-change hydrogel
Introduction
Breast cancer is one of the foremost threats to female health nowadays.1–3 Among the pathological types, endocrine-dependent breast cancer (estrogen receptor-α [ERα]-positive breast cancer) is the most common subtype of breast cancer and accounts for most cases. Thus, ERα has been confirmed to be the primary target for endocrine-dependent breast cancer.4–7 Tamoxifen, an oral antagonist of ERα, is the first choice for ERα-positive breast cancer treatment.8–11 Although the application of an oral antagonist of ERα could significantly prolong survival and improve the quality of life of patients with breast cancer, there are still some problems: 1) ERα is not only a therapeutic target of endocrine-dependent breast cancer but also an important regulator of human physical processes, and oral administration of tamoxifen has potential side effects on cardiovascular and muscular systems;12–15 and 2) administration of tamoxifen orally or by an intravenous drip will enable the systemic distribution of tamoxifen in the body and thus limit the concentration of the drug in the breast cancer lesion.16–20 Therefore, it is valuable to develop novel approaches to increase the safety and efficacy of an oral antagonist of ERα for endocrine-dependent breast cancer treatment.
Although the existing antitumor treatment strategy is based on oral or intravenous drip therapy strategies, the precise administration of antitumor drugs in localized tumor tissues has been achieved by several image-guided interventional techniques to avoid various toxic and side effects caused by antitumor drugs.21–23 Therefore, preparing slow-release and long-acting formulations of antitumor drugs can not only achieve long-term antitumor effects by single administration but also help to avoid the side effects of the drug.24,25 In the present work, a temperature-sensitive phase-change hydrogel of tamoxifen was established. Tam-hydrogel (Tam-Gel) was injected intratumorally in established subcutaneous MCF-7 tumors or intrahepatic MCF-7 tumors. The slow release of tamoxifen in the blood and tumor tissues was measured. Tumor growth was identified by measuring tumor volumes, tumor weight and nodule area, or by micro-positron emission tomography (micro-PET) screening.
Material and methods
Cell lines and agents
MCF-7 cells (an ERα-positive breast cancer cell line) were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, People’s Republic of China), an organization possessing biological samples typical to the Chinese government, a gift from Dr Feng Fan maintained under conditions described in the previous work.26 Tamoxifen (cat no S1192) was purchased from the Selleck Corporation (Houston, TX, USA). Poly(lactic-co-glycolic acid)– polyethylene glycol (PLGA-PEG) was purchased from Dai-Gang Corporation (Jinan City, Shandong Province, People's Republic of China). The results of infrared detection and nuclear magnetic detection at similar physiological temperatures of PLGA-PEG are shown in Figures S1 and S2. All experiments, protocols and use of cell lines were approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiao Tong University.
 | Figure S1 The infrared detection of PLGA-PEG. |
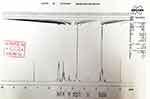 | Figure S2 >Nuclear magnetic detection of PLGA-PEG. |
Preparation of tamoxifen formulations
To prepare tamoxifen-hydrogel (Tam-Gel), tamoxifen citrate powder was accurately dissolved in acetone, and the prepared acetone solution was slowly added dropwise to a 5% aqueous solution of glucose, under stirring, to obtain a suspension of tamoxifen nanoparticles (nanosuspension). The amount of acetone used in preparing tamoxifen was 1 mL of acetone per 1 mg of drug. Acetone was removed from nanosuspension using a dialysis method, and the suspension was freeze-dried to remove water, resulting in nanoparticle freeze-dried powder. The solution of PLGA-PEG-PLGA in PBS was prepared at 10°C and added to the nanoparticle freeze-dried powder. The mixture was stirred at 10°C to prepare the tamoxifen hydrogel (named Tam-Gel). Acetone was removed from the suspension of tamoxifen nanoparticles (nanosuspension) in PBS; this was named Tam-Sol and used as a control. The final concentration of tamoxifen in its Tam-Sol or Tam-Gel form was almost 2 mg/mL. To examine the phase transition of Tam-Gel from liquid to hydrogel, the apparent viscosity η (Pa/s) of Tam-Gel with different concentrations of PLGA-PEG-PLGA as a function of temperature (°C) was examined. The phase-transition temperature (T1/2) was calculated based on apparent viscosity–temperature curves. The phase transition of hydrogels with indicated PLGA-PEG-PLGA amounts (5.0, 7.5, 10.0, 12.5 and 15.0%) was examined. The effect of pH values on the phase transition of hydrogels was also examined under indicated pH values (7.4, 7.2, 7.0, 6.8 and 6.6).
After the tamoxifen had been dissolved in an organic solvent (eg, DMSO), the tamoxifen solution was obtained by dilution with PBS to form the Tam-Sol formulation, used as a control. The concentrations of tamoxifen in Tam-Gel and Tam-Sol were basically the same, by controlling the amount of PBS added to the formulations.
In vitro and in vivo release of tamoxifen from Tam-Gel
For the in vitro release of tamoxifen from Tam-Gel, Tam-Gel was incubated in sterilized PBS. Samples of PBS were collected at indicated time-points and extracted with acetonitrile (ACN). Next, the tamoxifen in the samples was detected by liquid chromatography–tandem mass spectrometry (LC-MS/MS), as described in previous publications.27,28
For the in vivo release of tamoxifen from Tam-Gel, subcutaneous tumor animal models were used. All animal experiments included in the present work were permitted by the Institutional Animal Care and Use Committee of The First Affiliated Hospital of Xi’an Jiaotong University School of Medicine and were carried out in accordance with the UK Animals (Scientific Procedures) Act of 1986 and associated guidelines. MCF-7 cells were injected into nude mice (1×107 cells per animal) to form subcutaneous tumors.29,30 The tumor volumes were calculated as: Tumor length × Tumor width × Tumor width/2. When the volume of the subcutaneous tumors reached almost 1200–1500 mm3, the tamoxifen formulations (Tam-Sol and Tam-Gel) were injected into these tumors. Nude mice were harvested at indicated time-points, and tumor tissues were collected. Collected tumor tissues were lysed, and the tamoxifen remaining in the tumor tissues was extracted with ACN for LC-MS/MS examination following the methods described previously.27,28
In vivo antitumor effects of tamoxifen formulations
For the subcutaneous tumor model, MCF-7 cells were injected into nude mice. When tumor volumes reached almost 1000–1200 mm3, Tam-Sol or Tam-Gel was injected into the tumor tissues. Each tumor could be injected with about 50 μL of tamoxifen formulations. After 3–4 weeks’ growth, tumors were harvested, and the tumor weights or volumes were measured.
For the intrahepatic model in immunodeficient rats, MCF-7 cells were first seeded into nude mice to form subcutaneous tumors. Next, subcutaneous tumor tissues formed by MCF-7 cells were harvested and microblocks of subcutaneous tumor tissues with equal weights were prepared via weighing using a precision balance. Then, microblocks of subcutaneous tumor tissues with equal weights were seeded into immunodeficient rats’ livers by open laparotomy (Table S1). After 5–6 weeks’ growth, intrahepatic tumors formed in the rats’ livers. Formulations of tamoxifen, Tam-Sol or Tam-Gel were injected into the MCF-7 lesions in the rats’ livers by open laparotomy (50 μL per injection). At each time-point, mice were injected intravenously with 200 μCi (7.4 MBq) of 18F-radiolabeled fluorodeoxyglucose (18F-FDG) or 100 μCi (3.7 MBq) of 18F-radiolabeled fluoroestradiol (18F-FES) and examined by a positron emission tomography/computed tomography (PET/CT) scanner (Philips, Amsterdam, The Netherlands) following previously published methods.31–33 Ci is a unit of nuclear intensity: 1 Curie (Ci) equals 3.7×1010 Becquerels (Bq); so, in this study, 200 μCi=7.4 MBq and 100 μCi=3.7 MBq. Results from images or liver organs were quantifiably analyzed by ImageJ software following previous methods.34,35
Ethics statement
The use of cell lines was approved by the Ethics Committee of The First Affiliated Hospital of Xi’an Jiaotong University School of Medicine. The use of animals was permitted by the Institutional Animal Care and Use Committee of The First Affiliated Hospital of Xi’an Jiaotong University School of Medicine and were carried out in accordance with the UK Animals (Scientific Procedures) Act of 1986 and associated guidelines.
Statistical analysis
Statistical analysis was carried out by a two-way ANOVA without Bonferroni correction using SPSS statistics software (IBM Corp., Armonk, NY, USA). The phase-transition temperature (T1/2) of Tam-Gel or half-life (t1/2 value) of tamoxifen in Tam-Gel or the tumor tissues was calculated with Origin statistics software (version 8.5; OriginLab Corp., Northampton, MA, USA). A P-value <0.05 was considered to indicate a statistically significant difference between groups.
Results
Preparation of the temperature-sensitive phase-transition hydrogel of tamoxifen
First, the temperature-sensitive phase transition of tamoxifen was prepared, and its physicochemical properties were identified. Table 1 shows the T1/2 values of Tam-Gel containing the indicated concentrations of PLGA-PEG-PLGA. The T1/2 value of Tam-Gel containing 10% PLGA-PEG-PLGA was nearest to body temperature (37.14±0.66°C), so the 10% concentration of PLGA-PEG-PLGA was used in the next steps of the experiments. Table 2 shows the T1/2 values of Tam-Gel containing 10% PLGA-PEG-PLGA at the indicated pH values. The results show that the changes in pH did not affect the T1/2 of hydrogel within the selected pH range.
 | Table 1 Phase-transition (T1/2) values of Tam-Gel at indicated concentrations of PLGA-PEG-PLGA |
 | Table 2 Phase-transition (T1/2) values of Tam-Gel at indicated pH values |
Next, Tam-Gel images from the transmission electron microscope were obtained. As shown in Figure 1, the particle diameter of the tamoxifen nanoparticles was almost 120 nm and the particles were uniformly distributed in the PLGA-PEG-PLGA hydrogel. Therefore, we successfully prepared a tamoxifen temperature-sensitive phase-change hydrogel (Tam-Gel).
In vitro release of tamoxifen from Tam-Gel
To examine whether Tam-Gel could achieve the slow release of tamoxifen, the in vitro release of tamoxifen from Tam-Gel to PBS was examined by LC-MS/MS experiments. As shown in Figure 2, Tam-Gel achieved the slow release of tamoxifen from the temperature-sensitive phase-transition hydrogel of tamoxifen (Tam-Gel). Tamoxifen was sustained in Tam-Gel for more than 400 hours and the half-life (t1/2) of tamoxifen release from Tam-Gel (sustained in hydrogel) was 194.41±12.60 hours. Table 3 shows the t1/2 values of tamoxifen release from Tam-Gel at the indicated pH values. The results indicate that the changes in pH did not affect the t1/2 of tamoxifen release from Tam-Gel within the selected pH range (pH 7.4–6.6). Therefore, the preparation of tamoxifen in the Tam-Gel formulation achieved the slow release of in vitro tamoxifen.
 | Table 3 Half-life (t1/2) times of tamoxifen release from Tam-Gel at indicated pH values |
In vivo release of tamoxifen from tumor tissues injected with tamoxifen formulations
To examine whether the Tam-Gel preparation could achieve the in vivo slow release or long-term sustained features of tamoxifen in tumor tissues, MCF-7 cells were subcutaneously injected into nude mice to form subcutaneous tumors. Once the volumes of the subcutaneous tumors had reached 1200–1500 mm3, tamoxifen formulations (Tam-Gel or Tam-Sol) were injected into the subcutaneous tumors, and the tumor tissues were harvested at the indicated time-points for LC-MS/MS analysis. As shown in Figure 3, after intratumoral injection of Tam-Sol, tamoxifen was quickly released and almost completely cleared from the tumor tissues within 48 hours. The t1/2 value of tamoxifen in tumor tissues injected with tamoxifen (Tam-Sol) was 13.08±1.14 hours.
Moreover, the intratumoral injection of Tam-Gel achieved slower and more sustained release of tamoxifen in tumor tissues compared to the Tam-Sol injection group. The t1/2 value of tamoxifen in tumor tissues injected with tamoxifen (Tam-Gel) was 137.95±11.72 hours. Moreover, after the injection of Tam-Gel in the tumor tissues, tamoxifen could still be detected in the tumor tissues at the 500 hour time-point (Figure 3). Therefore, the intratumoral injection of Tam-Gel achieved the slow release and long sustainability of tamoxifen in tumor tissues formed by MCF-7 cells.
Long-acting antitumor effect of a single administration of Tam-Gel in tumor tissues
To further examine the antitumor effect of Tam-Gel on breast cancer cells, the subcutaneous model or intrahepatic model of MCF-7 was used. As shown in Figure 4, a single administration/one-dose administration of Tam-Gel, but not Tam-Sol or the solvent control, inhibited the subcutaneous growth of MCF-7 cells in nude mice.
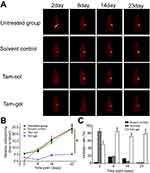
The in vivo inhibition of activation of ERα by tamoxifen was examined by micro-PET screening of 18F-FES absorption. Single administration of Tam-Gel, but not Tam-Sol or the solvent control, inhibited the intrahepatic growth of MCF-7 cells in immunodeficient rats (Figures 5 and 6). The intrahepatic growth of MCF-7 cells in immunodeficient rats’ livers was examined by micro-PET screening of rats after the injection of nuclear probes. First, the absorption of estrogen nuclear probes (18F-FES) by MCF-7 cells was examined. As shown in Figure 5, the intratumoral injection of the solvent control did not inhibit the absorption of 18F-FES by the MCF-7 cells. A single administration of Tam-Sol by intratumoral injection inhibited the absorption of estrogen by MCF-7 cells only at day 2 but not at subsequent time-points, whereas one administration of Tam-Gel could inhibit the absorption of 18F-FES by MCF-7 cells at the indicated time-points (from day 2 to 23) for a long time.
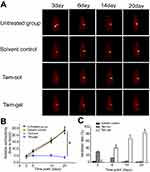
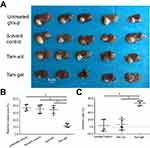
Next, the in vivo inhibition of the MCF-7 cells’ growth was examined by micro-PET screening of the 18F-FDG absorption (Figures 6 and 7). The intrahepatic growth of the MCF-7 cells was examined by micro-PET screening of rats that received 18F-FDG nuclear probes or images of rats’ livers with tumor lesions formed by MCF-7 cells (Figures 6 and 7). As shown in Figure 6, a single administration of Tam-Gel, but not Tam-Sol or the solvent control, inhibited the intensity of 18F-FDG nuclear probes in the liver region of rats for a long time. At day 24, rats were harvested. Images of their livers with the formed lesions are shown in Figure 7. The results indicated that only Tam-Gel, but not Tam-Sol or the solvent control, inhibited the MCF-7 tumor volumes in rats’ livers. Therefore, intratumoral injection of Tam-Gel achieved the long-acting antitumor activation of tamoxifen on MCF-7 cells.
Discussion
Breast cancer is the biggest threat to female health and one of the foremost causes of death from cancers affecting females.36,37 In fact, among the three main subtypes of breast cancer, about 70% of all breast cancer patients suffer from endocrine-related/dependent breast cancer and have ERα-positive tumors.38,39 Therefore, ERα is the prime target for breast cancer treatment.40,41 Antagonists of ERα, for example, tamoxifen and fulvestrant, have been widely used to treat patients with breast cancer.42,43 In the presence of tamoxifen, ERα binds preferentially and recruits the transcriptional corepressors, N-CoR and SMRT.44 Recruitment of these corepressors induced by tamoxifen not only decreases the expression of ERα’s downstream genes but also inhibits the proliferation of breast cancer cells.39,44 Although antitumor treatment of endocrine-dependent breast cancer has made great progress, considerably prolonging the survival of patients and improving their prognosis, current therapeutic strategies, such as oral administration of tamoxifen citrate tablets, still have many problems. These problems include: 1) oral administration of tamoxifen leads to a wide distribution of the drug in various organs of the human body; the effective concentration of the drug in tumor lesions is low, and the drug utilization is also insufficient; and 2) estrogen receptors not only are key regulators of normal endocrine regulation but also play significant roles in other important physiological processes, such as in osteoclasts; therefore, oral administration of estrogen receptor antagonists could interfere with the normal physiological regulation of various organs in the body while treating breast cancer, and induce side effects.
To avoid these problems, this study prepared a new pharmaceutical formulation of tamoxifen, a temperature-sensitive phase-change hydrogel of tamoxifen (Tam-Gel), and achieved direct administration in tumor tissues. The solubility of tamoxifen is poor, and the preparation of tamoxifen as a nanoparticle not only improved its chemical properties but also accommodated large amounts of tamoxifen in small volumes. Tamoxifen nanoparticles were prepared into gels using PLGA-PEG-PLGA. Tam-Gel appears as a liquid state at room temperature and undergoes a phase change to convert to a hydrogel state at body temperature. After the Tam-Gel hydrogel was injected into the tumor tissue, the drug was released by the dissolution of the gel into the tumor tissue, thereby achieving the sustained release of the drug in the tumor tissue. PLGA-PEG-PLGA is a novel organic polymer material with good safety and is fully biodegradable.45–47 It also has the chemistry of sol-gel conversion and could be used to prepare temperature-sensitive phase-change hydrogels. At body temperature, the biopolymer chains in PLGA-PEG formulations undergo stretching and cross-linking, eventually undergoing phase transformation, converting from liquid to hydrogel.48,49 The formulation has different ratios of PLGA-PEG, and its phase-transition temperatures are also different. In the present work, the phase-transition temperature of PLGA-PEG hydrogel is near body temperature. In a gel prepared with PLGA-PEG-PLGA, the amount of PLGA-PEG-PLGA is small, the mechanical strength of the gel is good, the drug is slowed down and the sustained release time is long.50,51 Therefore, the gel prepared in this study can achieve drug limitation in tumor tissues, avoiding potential toxic side effects on various organs of the body.
Current breast cancer research mainly uses breast cancer cells or patient-derived cells/tissues to establish a subcutaneous tumor model in nude mice;52,53 although the subcutaneous tumor model is widely used as a classic in vivo research method, subcutaneous tumors cannot mimic the microenvironment of human cancers, and recent studies have focused on establishing in situ tumor models (establishing a tumor tissue model in a malignant, tumor-derived organ). However, the in situ tumor models of breast cancer cells are still similar to the subcutaneous tumors. Therefore, this study used immunodeficient rats to establish the lesions caused by MCF-7 cells in the livers of the animals. Establishment of an intrahepatic tumor model in rats can mimic the liver metastasis of breast cancer and the growth of MCF-7 cells in organs. Compared with the nude mouse tumor model, the immunodeficient rats have a larger body volume. The intrahepatic tumor tissue obtained in this study can achieve intratumoral drug administration and will be widely used in the future for ultrasound-guided abdominal puncture and other precise drug-delivery methods.
In the present work, tamoxifen was used. Tamoxifen has often been administered orally to patients. There has been some research focusing on the drug-delivery system of tamoxifen nanoparticles.54,55 However, there have been no reports on the formulation of tamoxifen to maintain the drug in breast cancer. Medical image-guided interventional therapeutic strategies, such as CT-guided abdominal puncture and digital subtraction angiography-guided transcatheter arterial chemoembolization, can directly inject antitumor drugs into tumor lesions, and also achieve long-term effects of a single administration after the injection of a sustained-release preparation of the drug into tumor tissue.56,57 This strategy could not only relieve the economic burden on patients but also alleviate the side effects of the drugs.21 Currently, the choice of drugs in interventional therapeutic strategies is limited, and Tam-Gel can be accurately administered by ultrasound-guided or CT-guided percutaneous puncture. Fulvestrant (ICI-182780), another antagonist of estrogen receptors, has also been widely used as first-line or second-line therapy for locally advanced or metastatic breast cancer.58,59 Fulvestrant is also the foremost therapeutic choice for post-menopausal patients with breast cancer.60,61 In the future, we will try to establish various new pharmaceutical formulations of fulvestrant to achieve more effective antitumor treatments for breast cancer. Moreover, there are other strategies to prepare controlled-release or slow-release systems.62–64 The drug loading amount in the present work is almost 2 mg/mL. It will be valuable to develop novel controlled-release or slow-release systems for drugs by other strategies.
Conclusion
In this study, a novel slow-release system was successfully prepared that facilitates the long-term release of tamoxifen in breast cancer tissues and achieves an antitumor effect in the long term.
Acknowledgment
We thank Dr Xiaojie Xu, Department of Medical Molecular Biology, Beijing Institute of Biotechnology, Beijing, People's Republic of China for her kind advice.
Author contributions
All authors made substantial contributions to the design and conception; and the acquisition, analysis or interpretation of data. All authors took part in either drafting or revising the manuscript. All authors gave final approval of the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vaidya JS, Bulsara M, Wenz F, Tobias JS, Joseph D, Baum M. Targeted radiotherapy for early breast cancer. Lancet. 2018;391:26–27. doi:10.1016/S0140-6736(17)33316-0
2. Allemani C, Matsuda T, Di Carlo V, et al.;
3. Rimawi M, Ferrero JM, de la Haba-Rodriguez J, et al.;
4. Razavi P, Chang MT, Xu G, et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell. 2018;34:427–438.e6. doi:10.1016/j.ccell.2018.08.008
5. Zhang W, Yu W, Cai G, et al. A new synthetic derivative of cryptotanshinone KYZ3 as STAT3 inhibitor for triple-negative breast cancer therapy. Cell Death Dis. 2018;9:1098. doi:10.1038/s41419-018-1111-y
6. Meng D, Lei M, Han Y, et al. MicroRNA-645 targets urokinase plasminogen activator and decreases the invasive growth of MDA-MB-231 triple-negative breast cancer cells. Onco Targets Ther. 2018;11:7733–7743. doi:10.2147/OTT.S187221
7. Shah N, Mohammad AS, Saralkar P, et al. Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases. Pharmacol Res. 2018;132:47–68. doi:10.1016/j.phrs.2018.03.021
8. Zhu Z, Li Y, Yang X, Pan W, Pan H. The reversion of anti-cancer drug antagonism of tamoxifen and docetaxel by the hyaluronic acid-decorated polymeric nanoparticles. Pharmacol Res. 2017;126:84–96. doi:10.1016/j.phrs.2017.07.011
9. Goetz MP, Suman VJ, Reid JM, et al. First-in-human Phase I study of the tamoxifen metabolite Z-endoxifen in women with endocrine-refractory metastatic breast cancer. J Clin Oncol. 2017;35:3391–3400. doi:10.1200/JCO.2017.73.3246
10. Davies C, Pan H, Peto R. 10 vs 5 years of adjuvant tamoxifen: exclusion of 1/402 centres in ATLAS. Lancet. 2017;389:1884. doi:10.1016/S0140-6736(17)31003-6
11. Rocca A, Maltoni R, Bravaccini S, Donati C, Andreis D. Clinical utility of fulvestrant in the treatment of breast cancer: a report on the emerging clinical evidence. Cancer Manag Res. 2018;10:3083–3099. doi:10.2147/CMAR.S137772
12. Li J, Li B, Jiang Q, et al. Do genetic polymorphisms of the vitamin D receptor contribute to breast/ovarian cancer? A systematic review and network meta-analysis. Gene. 2018;677:211–227. doi:10.1016/j.gene.2018.07.070
13. Khan MM. Translational significance of selective estrogen receptor modulators in psychiatric disorders. Int J Endocrinol. 2018;2018:9516592. doi:10.1155/2018/1528437
14. Sharma D, Kumar S, Narasimhan B. Estrogen alpha receptor antagonists for the treatment of breast cancer: a review. Chem Cent J. 2018;12:107. doi:10.1186/s13065-018-0472-8
15. Meitzen J, Meisel RL, Mermelstein PG. Sex differences and the effects of estradiol on striatal function. Curr Opin Behav Sci. 2018;23:42–48. doi:10.1016/j.cobeha.2018.03.007
16. Xie H, Tian S, Yu H, et al. A new apatinib microcrystal formulation enhances the effect of radiofrequency ablation treatment on hepatocellular carcinoma. Onco Targets Ther. 2018;11:3257–3265. doi:10.2147/OTT.S165000
17. Wang Y, Tang G. A novel long-sustaining system of apatinib to long-termly inhibit the proliferation of hepatocellular carcinoma cells. Onco Targets Ther. 2018;11:8529–8541. doi:10.2147/OTT.S188209
18. De Placido S, Gallo C, De Laurentiis M, et al. Adjuvant anastrozole versus exemestane versus letrozole, upfront or after 2 years of tamoxifen, in endocrine-sensitive breast cancer (FATA-GIM3): a randomised, phase 3 trial. Lancet Oncol. 2018;19:474–485. doi:10.1016/S1470-2045(18)30116-5
19. Dehghani F, Farhadian N, Golmohammadzadeh S, Biriaee A, Ebrahimi M, Karimi M. Preparation, characterization and in-vivo evaluation of microemulsions containing tamoxifen citrate anti-cancer drug. Eur J Pharm Sci. 2017;96:479–489. doi:10.1016/j.ejps.2016.09.033
20. Peris-Vicente J, Carda-Broch S, Esteve-Romero J. Quantification of tamoxifen in pharmaceutical formulations using micellar liquid chromatography. Anal Sci. 2014;30:925–930.
21. Park CG, Hartl CA, Schmid D, Carmona EM, Kim HJ, Goldberg MS. Extended release of perioperative immunotherapy prevents tumor recurrence and eliminates metastases. Sci Transl Med. 2018;10:
22. Hyun MH, Lee YS, Kim JH, et al. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: a meta-analysis of high-quality studies. Hepatology. 2018;68:977–993. doi:10.1002/hep.29883
23. Lo GH. The use of steroid for transcatheter arterial chemoembolization: to relieve symptoms or to mask adverse events? Hepatology. 2018;68:1207. doi:10.1002/hep.30112
24. Majumder P, Baxa U, Walsh STR, Schneider JP. Design of a multicompartment hydrogel that facilitates time-resolved delivery of combination therapy and synergized killing of glioblastoma. Angew Chem Int Ed Engl. 2018;57:15040–15044. doi:10.1002/anie.201806483
25. Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1:
26. Zhang F, Feng F, Yang P, et al. Four-and-a-half-LIM protein 1 down-regulates estrogen receptor α activity through repression of AKT phosphorylation in human breast cancer cell. Int J Biochem Cell Biol. 2012;44:320–326. doi:10.1016/j.biocel.2011.11.002
27. Johänning J, Heinkele G, Precht JC, et al. Highly sensitive simultaneous quantification of estrogenic tamoxifen metabolites and steroid hormones by LC-MS/MS. Anal Bioanal Chem. 2015;407:7497–7502. doi:10.1007/s00216-015-8907-8
28. Rama Raju KS, Taneja I, Singh SP, et al. Simultaneous determination of centchroman and tamoxifen along with their metabolites in rat plasma using LC-MS/MS. Bioanalysis. 2015;7:967–979. doi:10.4155/bio.14.253
29. An L, Li DD, Chu HX, et al. Terfenadine combined with epirubicin impedes the chemo-resistant human non-small cell lung cancer both in vitro and in vivo through EMT and notch reversal. Pharmacol Res. 2017;124:105–115. doi:10.1016/j.phrs.2017.07.021
30. Ji Q, Xu X, Li L, et al. miR-216a inhibits osteosarcoma cell proliferation, invasion and metastasis by targeting CDK14. Cell Death Dis. 2017;8:e3103. doi:10.1038/cddis.2017.518
31. Xu X, Fan Z, Kang L, et al. Hepatitis B virus X protein represses miRNA-148a to enhance tumorigenesis. J Clin Invest. 2013;123:630–645. doi:10.1172/JCI64265
32. Feng F, Jiang Q, Cao S, et al. Pregnane X receptor mediates sorafenib resistance in advanced hepatocellular carcinoma. Biochim Biophys Acta Gen Subj. 2018;1862:1017–1030. doi:10.1016/j.bbagen.2018.01.011
33. Li L, Liang Y, Kang L, et al. Transcriptional regulation of the warburg effect in cancer by SIX1. Cancer Cell. 2018;33:368–385.e7. doi:10.1016/j.ccell.2018.01.010
34. Li J, Zhao J, Wang H, et al. MicroRNA-140-3p enhances the sensitivity of hepatocellular carcinoma cells to sorafenib by targeting pregnenolone X receptor. Onco Targets Ther. 2018;11:5885–5894. doi:10.2147/OTT.S179509
35. Shao Z, Li Y, Dai W, et al. ETS-1 induces sorafenib-resistance in hepatocellular carcinoma cells via regulating transcription factor activity of PXR. Pharmacol Res. 2018;135:188–200. doi:10.1016/j.phrs.2018.08.003
36. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
37. Fan W, Xie J, Xia J, et al. RUVBL1-ITFG1 interaction is required for collective invasion in breast cancer. Biochim Biophys Acta Gen Subj. 2017;1861:1788–1800. doi:10.1016/j.bbagen.2017.03.016
38. Biava PM, Nicolini A, Ferrari P, Carpi A, Sell S. A systemic approach to cancer treatment: tumor cell reprogramming focused on endocrine-related cancers. Curr Med Chem. 2014;21:1072–1081.
39. Cui J, Germer K, Wu T, et al. Cross-talk between HER2 and MED1 regulates tamoxifen resistance of human breast cancer cells. Cancer Res. 2012;72:5625–5634. doi:10.1158/0008-5472.CAN-12-1305
40. Johmura Y, Maeda I, Suzuki N, et al. Fbxo22-mediated KDM4B degradation determines selective estrogen receptor modulator activity in breast cancer. J Clin Invest. 2018;
41. Wang L, Zhao L, Jia X, et al. Aminophenols increase proliferation of thyroid tumor cells by inducing the transcription factor activity of estrogen receptor α. Biomed Pharmacother. 2018;109:621–628. doi:10.1016/j.biopha.2018.10.168
42. Cun J, Yang Q. Bioinformatics-based interaction analysis of miR-92a-3p and key genes in tamoxifen-resistant breast cancer cells. Biomed Pharmacother. 2018;107:117–128. doi:10.1016/j.biopha.2018.07.158
43. Kornblum N, Zhao F, Manola J, et al. Randomized Phase II trial of fulvestrant plus everolimus or placebo in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer resistant to aromatase Inhibitor therapy: results of PrE0102. J Clin Oncol. 2018;36:1556–1563. doi:10.1200/JCO.2017.76.9331
44. Peterson TJ, Karmakar S, Pace MC, Gao T, Smith CL. The silencing mediator of retinoic acid and thyroid hormone receptor (SMRT) corepressor is required for full estrogen receptor alpha transcriptional activity. Mol Cell Biol. 2007;27:5933–5948. doi:10.1128/MCB.00237-07
45. El-Zaafarany GM, Soliman ME, Mansour S, et al. A tailored thermosensitive PLGA-PEG-PLGA/emulsomes composite for enhanced oxcarbazepine brain delivery via the nasal route. Pharmaceutics. 2018;10:
46. Pan A, Wang Z, Chen B, et al. Localized co-delivery of collagenase and trastuzumab by thermosensitive hydrogels for enhanced antitumor efficacy in human breast xenograft. Drug Deliv. 2018;25:1495–1503. doi:10.1080/10717544.2018.1474971
47. Shi Z, Li SK, Charoenputtakun P, Liu CY, Jasinski D, Guo P. RNA nanoparticle distribution and clearance in the eye after subconjunctival injection with and without thermosensitive hydrogels. J Control Release. 2018;270:14–22. doi:10.1016/j.jconrel.2017.11.028
48. GuhaSarkar S, Banerjee R. Intravesical drug delivery: challenges, current status, opportunities and novel strategies. J Control Release. 2010;148:147–159. doi:10.1016/j.jconrel.2010.08.031
49. Mukerjee A, Ranjan AP, Vishwanatha JK. Combinatorial nanoparticles for cancer diagnosis and therapy. Curr Med Chem. 2012;19:3714–3721.
50. Santoveña A, Monzón C, Alvarez-Lorenzo C, et al. Structure-performance relationships of temperature-responsive PLGA-PEG-PLGA gels for sustained release of bone morphogenetic protein-2. J Pharm Sci. 2017;106:3353–3362. doi:10.1016/j.xphs.2017.07.007
51. Dimchevska S, Geskovski N, Koliqi R, et al. Efficacy assessment of self-assembled PLGA-PEG-PLGA nanoparticles: correlation of nano-bio interface interactions, biodistribution, internalization and gene expression studies. Int J Pharm. 2017;533:389–401. doi:10.1016/j.ijpharm.2017.05.054
52. Feng Z, Xia Y, Gao T, et al. The antipsychotic agent trifluoperazine hydrochloride suppresses triple-negative breast cancer tumor growth and brain metastasis by inducing G0/G1 arrest and apoptosis. Cell Death Dis. 2018;9:1006. doi:10.1038/s41419-018-1111-y
53. Zhou EY, Knox HJ, Reinhardt CJ, Partipilo G, Nilges MJ, Chan J. Near-infrared photoactivatable nitric oxide donors with integrated photoacoustic monitoring. J Am Chem Soc. 2018;140:11686–11697. doi:10.1021/jacs.8b05514
54. Patel SH, O‘Hara L, Atanassova N, et al. Low-dose tamoxifen treatment in juvenile males has long-term adverse effects on the reproductive system: implications for inducible transgenics. Sci Rep. 2017;7:8991. doi:10.1038/s41598-017-09016-4
55. Dhaundiyal A, Jena SK, Samal SK, Sonvane B, Chand M, Sangamwar AT. Alpha-lipoic acid-stearylamine conjugate-based solid lipid nanoparticles for tamoxifen delivery: formulation, optimization, in-vivo pharmacokinetic and hepatotoxicity study. J Pharm Pharmacol. 2016;68:1535–1550. doi:10.1111/jphp.12644
56. Xie H, Yu H, Tian S, et al. What is the best combination treatment with transarterial chemoembolization of unresectable hepatocellular carcinoma? a systematic review and network meta-analysis. Oncotarget. 2017;8:100508–100523. doi:10.18632/oncotarget.20119
57. Feng F, Jiang Q, Jia H, et al. Which is the best combination of TACE and Sorafenib for advanced hepatocellular carcinoma treatment? A systematic review and network meta-analysis. Pharmacol Res. 2018;135:89–101. doi:10.1016/j.phrs.2018.06.021
58. Zhang K, Hong R, Xu F, et al. Clinical value of circulating ESR1 mutations for patients with metastatic breast cancer: a meta-analysis. Cancer Manag Res. 2018;10:2573–2580. doi:10.2147/CMAR.S173193
59. Du Y, Li N, Jiao X, Li K, Yan S. The predictive ability of plasma ESR1 mutations for the efficacy of endocrine therapy in hormone-receptor-positive advanced breast cancer. Onco Targets Ther. 2018;11:6023–6029. doi:10.2147/OTT.S171465
60. Rusz O, Kószó R, Dobi Á, et al. Clinical benefit of fulvestrant monotherapy in the multimodal treatment of hormone receptor and HER2 positive advanced breast cancer: a case series. Onco Targets Ther. 2018;11:5459–5463. doi:10.2147/OTT.S170736
61. Zhang T, Feng F, Zhao W, et al. Effect of first-line endocrine therapy in patients with hormone-sensitive advanced breast cancer: a network meta-analysis. Onco Targets Ther. 2018;11:2647–2656. doi:10.2147/OTT.S165681
62. Nosrati H, Salehiabar M, Davaran S, Danafar H, Manjili HK. Methotrexate-conjugated L-lysine coated iron oxide magnetic nanoparticles for inhibition of MCF-7 breast cancer cells. Drug Dev Ind Pharm. 2017;27:1–9.
63. Nosrati H, Adinehvand R, Manjili HK, Rostamizadeh K, Danafar H. Synthesis, characterization, and kinetic release study of methotrexate loaded mPEG-PCL polymersomes for inhibition of MCF-7 breast cancer cell line. Pharm Dev Technol. 2019;24:89–98. doi:10.1080/10837450.2018.1425433
64. Nosrati H, Rashidi N, Danafar H, Manjili HK. Anticancer activity of tamoxifen loaded tyrosine decorated biocompatible Fe3O4 magnetic nanoparticles against breast cancer cell lines. J Inorg Organomet Polym Mater. 2018;28:1178–1186. doi:10.1007/s10904-017-0758-7
Supplementary materials
 | Table S1 Weights of tumor tissues seeded into rats' livers |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.