Back to Journals » OncoTargets and Therapy » Volume 13
A Systemic Review on the Regulatory Roles of miR-34a in Gastrointestinal Cancer
Received 14 October 2019
Accepted for publication 22 December 2019
Published 3 April 2020 Volume 2020:13 Pages 2855—2872
DOI https://doi.org/10.2147/OTT.S234549
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Jiehong Kong, Weipeng Wang
Center for Drug Metabolism and Pharmacokinetics, College of Pharmaceutical Sciences, Soochow University, Suzhou 215123, People’s Republic of China
Correspondence: Weipeng Wang
Center for Drug Metabolism and Pharmacokinetics, College of Pharmaceutical Sciences, Soochow University, Wenjing Road, Suzhou Industrial Park, Yunxuan Building #1339, Suzhou 215123, People’s Republic of China
Email [email protected]
Abstract: MicroRNAs (miRNAs) are a class of endogenous non-coding single-stranded small-molecule RNAs that regulate gene expression by repressing target messenger RNA (mRNA) translation or degrading mRNA. miR-34a is one of the most important miRNAs participating in various physiological and pathological processes. miR-34a is abnormally expressed in a variety of tumors. The roles of miR-34a in gastrointestinal cancer (GIC) draw lots of attention. Numerous studies have demonstrated that dysregulated miR-34a is closely related to the proliferation, differentiation, migration, and invasion of tumor cells, as well as the diagnosis, prognosis, treatment, and chemo-resistance of tumors. Thus, we systematically reviewed the abnormal expression and regulatory roles of miR-34a in GICs including esophageal cancer (EC), gastric cancer (GC), colorectal cancer (CRC), hepatocellular carcinoma (HCC), pancreatic cancer (PC), and gallbladder cancer (GBC). It may provide a profile of versatile roles of miR-34a in GICs.
Keywords: miR-34a, gastrointestinal cancer, proliferation, migration, prognosis
Introduction
GICs are the most common malignant tumors in the world, including EC, GC, CRC, HCC, GBC, PC, etc. The incidence and mortality of GICs are extensively high. Recent studies have shown that the incidence and mortality of EC, GC, HCC, CRC, and PC rank among the top ten of malignant tumors in the Chinese population.1,2 Moreover, EC, GC, HCC, and CRC are four leading causes of cancer deaths, accounting for about 60% of all cancer deaths.1,2 At present, GICs are commonly treated with surgeons, chemical drugs, radiation therapy, and/or immunotherapy. However, the therapeutic responses are still limited. Therefore, it is of great significance to find new strategies to treat GICs.
miRNAs are non-coding single-stranded small-molecule RNAs with about 20–25 nucleotides in length.3–5 miRNAs regulate target gene expression through complete or incomplete complementarity with the 3ʹ-untranslated region (3ʹ-UTR) of target mRNA to inhibit or degrade the target mRNA, thereby participating in a series of biological processes including cell proliferation and apoptosis, hematopoiesis process, fat metabolism, development, and so on.6–8 miR-34a is one of the most closely concerned miRNAs and is widely expressed in various tissues including brain,9 myocardium,10 lung,11 and liver.12 However, miR-34a is abnormally expressed in a variety of tumors. For instance, miR-34a is down-regulated in breast cancer, prostate cancer, glioblastoma, EC, HCC and the other solid tumors, but it is up-regulated in chronic lymphoblastic leukemia13–16 (Table 1). miR-34a is directly regulated by p53, and is inactivated by CpG methylation in a wide range of cancers.16,17 A number of studies have demonstrated that miR-34a plays pivotal roles in cell proliferation, differentiation, migration, invasion, and survival by regulating a number of target genes involved in the mitogen-activated protein kinase (MAPK)/RAS proto-oncogene, GTPase (RAS), WNT/β-catenin, and the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathways in GICs13,18,19 (Table 2 and Figure 1). Thus miR-34a showed potent anti-tumor activity and contributed to chemotherapy in GICs (Table 3).
 |
Table 1 miR-34a Expression in GICs |
 |
Table 2 The Targets of miR-34a identified in GICs |
 |
Table 3 miR-34a-Based Treatment and Chemoresistance in GICs |
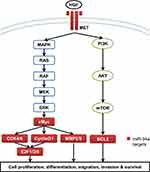 |
Figure 1 The target genes of miR-34a involved in MAPK/RAS and PI3K/AKT pathways. |
Recently, more and more studies on miR-34a had been reported. Although several research groups have reviewed miR-34a from different sides,13,18,20-29 the roles of miR-34a in GICs have not yet been systematically reviewed. Here, we present a global review of miR-34a in GICs.
miR-34a in EC
EC seriously threats human health with high morbidity and mortality.30 EC is mainly divided into esophageal squamous cell carcinoma (ESCC), esophageal adenocarcinoma, and undifferentiated carcinoma. In China, ESCC is a main type of EC with the highest incidence.31 The average five-year survival rate of ESCC is only about 10%, indicating defects in its treatment and prognosis.32 Therefore, it is necessary to ascertain the molecular mechanism and possible prognostic biomarkers of EC. At present, a lot of studies have focused on the regulatory roles of miR-34a in the proliferation, differentiation, migration, invasion, and prognosis of EC.
miR-34a Expression in EC
To investigate relationship between miR-34a expression and the progression of EC, the expression of miR-34a in 78 EC tissues and 25 pairs of EC and non-tumor esophageal tissues was examined by Cui et al.33 They found that the levels of miR-34a in 92% (23/25) of tumor tissues were significantly lower than those in non-tumor tissues. Moreover, the low-expression of miR-34a is closely related to the progression of EC. This is consistent with a previous study demonstrating that miR-34a is down-regulated in randomly selected 8 human EC tissues.34 Down-regulation of miR-34a was further confirmed in another study, in which miR-34a expression in 50 EC tissues was measured and the down-regulated miR-34a was correlated with clinical pathology.35 In addition, the results obtained from the Gene Expression Omnibus (GEO) database (accession number: GSE43732) showed a significant decrease in miR-34a levels in EC tissues compared with non-tumor tissues.36 These studies provide evidences to show that miR-34a is down-regulated in EC, and is closely related to the progression of EC.
The Role of miR-34a in the Proliferation and Differentiation of EC
There is a close relationship between abnormal expression of miR-34a and the proliferation and differentiation of EC. To explore the role of miR-34a in the development of EC, Shi et al34 investigated the expression level and DNA methylation of miR-34a in EC samples. They found that miR-34a was down-regulated in EC, and the low-expression of miR-34a was related to the abnormal CpG methylation of its promoter. miR-34a induced apoptosis and G0/G1 phase arrest by targeting several cell cycle genes or oncogenes, such as cyclin D1 (CCND1), cyclin E2 (CCNE2), cyclin dependent kinase 4 (CDK4), cyclin dependent kinase 6 (CDK6), E2F transcription factor 3 (E2F3), B cell lymphoma 2 (BCL2), sirtuin1 (SIRT1), and V-myc avian myelocytomatosis viral related oncogene (MYCN).17,37,38 The genes CCND1, CCNE2, CDK4, CDK6 and E2F3 are mainly related to the cell cycle. They participate in the G1-to-S phase transition of the cell cycle. miR-34a disrupts cell cycle by binding to the 3ʹ-UTR of these target genes. Since the genes BCL2, SIRT1, and MYCN are mainly related to cell senescence and apoptosis, miR-34a impacts cell proliferation and senescence by binding to their 3ʹ-UTRs. Therefore, low-expression of miR-34a promoted the proliferation and anti-apoptosis of EC cells, and consequent progression of EC. In addition, miR-34a also affected the proliferation and differentiation of EC by targeting phospholipase C (PLCE1).33 PLCE1 is a novel subtype of the PLC family, encoding phospholipase C enzyme, which regulates cell proliferation and differentiation through hydrolyzing membrane phosphatidylinositol 4, 5 diphosphate (PIP2) and producing inositol triphosphate (IP3) and diacylglycerol (DAG).39,40 miR-34a inhibited PLCE1 expression by directly binding to PLCE1 3ʹ-UTR. Thereby, the repressed PLCE1 promoted cell apoptosis, inhibited cell migration and enhanced the anti-tumor activity of miR-34a. However, low-expression of miR-34a in EC resulted in over-expression of PLCE1 and promotion of proliferation and migration of EC cells. These findings suggest that abnormal expression of miR-34a promotes the progression of EC by regulating target genes related to cell cycle, apoptosis, and senescence.
The Role of miR-34a in the Migration and Invasion of EC
Recent studies have found that methylation of miR-34a CpG island mediated miR-34a silencing contributes to the development of human EC. Specific DNA methylation markers at miR-34a CpG site were associated with EC metastasis.41,42 Higher methylation of miR-34a CpG locus resulted in higher risk of EC metastasis. Furthermore, low-expression of miR-34a led to upregulation of its target genes, such as matrix metalloproteinase-2 (MMP2), matrix metalloproteinase-9 (MMP9), type III fibronectin domain protein 3B (FNDC3B), and nuclear transcription factor Yin Yang 1 (YY1), thereby promoting the migration and invasion of EC cells. In these genes, MMP2 and MMP9 are involved in cancer invasion and metastasis by degrading type IV collagen, and FNDC3B and YY1 participate in the process of cell proliferation and differentiation. In addition, miR-34a promoted the metastasis and invasion of ESCC through epigenetic mechanisms. Previous studies have shown that long non-coding RNA (lncRNA) MNX1-AS1 is significantly up-regulated in ESCC, and high-expression of MNX1-AS1 is related to lymph node metastasis in ESCC. miR-34a is a direct target of MNX1-AS1. Knockdown of MNX1-AS1 enhanced miR-34a expression. Reinforced miR-34a inhibited cancer cell migration through suppression of MNX1-AS1, indicating that miR-34a is an important downstream effector of MNX1-AS1 in ESCC cell migration.43 These studies provided evidences to demonstrate that miR-34a inhibited migration and invasion of EC cells.44,45
The Role of miR-34a in the Prognosis of EC
Consistent results have shown that miR-34a is significantly down-regulated in 111 EC tissues, and the low-expression of miR-34a is related to the degree of tumor differentiation, lymph node status, and clinical stage.42,46 Meanwhile, Kaplan-Meier analysis and Log rank test showed that miR-34a expression was reversely correlated with the overall survival of patients with EC. Multivariate COX (proportional hazards model) regression analysis showed that miR-34a expression was an independent prognostic factor for EC. Moreover, methylation of miR-34a CpG sites was negatively correlated with miR-34a expression, and hypermethylation of miR-34a CpG sites was significantly associated with advanced stage (III/IV) and lymph node metastasis in EC. These results indicate that miR-34a has important clinical significance and prognostic value in EC and it may be considered as a potential biomarker for the prognosis of EC.
The Role of miR-34a in the Treatment of EC
At present, few studies on the relationship between miR-34a and the treatment and chemotherapy resistance of EC were exerted. Dai et al established a new therapeutic strategy that combines doxorubicin-loaded poly(ε-caprolactone) (PCL)-Pluronic micelles and miR-34a to combat EC.47 In vitro and in vivo combination of doxorubicin-loaded PCL-pluronic micelles and miR-34a achieved a significantly synergistic therapeutic effect over the single treatment, suggesting that miR-34a can be used to improve the anti-cancer activities of chemical drugs in the treatment of EC. However, studies on the roles of miR-34a in the treatment of EC are still needed to be further investigated.
miR-34a in GC
GC is the fourth most common cancer in the world with a high mortality rate and a low 5-year survival rate.48 In China, the incidence of GC is the highest in GICs, and is the second in malignant tumors.49 Recently, more and more studies have revealed important role of miR-34a in the proliferation, differentiation, migration, invasion, treatment, and prognosis of GC.
miR-34a Expression in GC
At present, studies have revealed that miR-34a is abnormally expressed in GC. For instance, Wu et al50 detected the expression level of miR-34a in 12 pairs of primary GC tissues and adjacent non-tumor tissues by using quantitative real-time PCR (qPCR) method. The results showed that miR-34a expression was decreased in 66.7% (8/12) cancer tissues compared with non-tumor tissues. The average expression level of miR-34a in the GC tissues was lower than that in the normal tissues. Meanwhile, several studies also confirmed that miR-34a was down-regulated in GC tissues, and was associated with tumor size, cell differentiation, distant metastasis, lymph node metastasis, recurrence and tumor lymph node metastasis (TNM) staging in patients with GC.51,52 Additionally, the results obtained from the Cancer Genome Atlas (TCGA) database demonstrated that low-expression of miR-34a was correlated with worse clinical outcome in GC patients.53 These findings indicate a positive relationship between abnormal expression of miR-34a and the development of GC.
The Role of miR-34a in the Proliferation and Differentiation of GC
Recent studies have shown that miR-34a inhibits the proliferation of GC cells by suppressing platelet-derived growth factor receptor (PDGFR) and mesenchymal epithelial cell transformation (MET) through PI3K/AKT pathway.54–56 miR-34a directly bond to PDGFR 3ʹ-UTR and inhibited PDGFR expression in GC. The decreased expression of PDGFR led to repression of tumor angiogenesis and inhibition of the proliferation and differentiation of tumor cells. Since miR-34a is lowly expressed in GC, PDGFR is highly expressed, resulting in enhancement of the proliferation and migration of GC cells. Polycytosine binding protein 2 (PCBP2), an RNA-binding protein, is an oncogenic protein and is highly expressed in GC.57–59 Hu et al found that PCBP2 promoted GC growth by inhibiting the expression of miR-34a.60 PCBP2 inhibited the apoptosis of GC cells by inhibiting the expression of miR-34a, and maintained the survival of GC cells, thereby promoted the development of GC. Another study reported that SIRT7 promoted the growth of GC cells and inhibited the apoptosis of GC cells by down-regulating the level of miR-34a.61 SIRT7, a NAD1-dependent class III histone deacetylase, binds to the promoter of miR-34a and deacetylates H3K18ac, thereby inhibiting the expression of miR-34a. As a result, the down-regulation of miR-34a inhibited the apoptosis of GC cells and restored the proliferation ability of GC cells, and consequently promoted the proliferation and differentiation of GC. These findings provide a new perspective for the mechanism explaining miR-34a-inhibited proliferation and differentiation of GC.
The Role of miR-34a in the Migration and Invasion of GC
It has been shown that GC inhibitor Gastrokine 1 (GKN1) inhibits the migration and invasion of GC cells by down-regulating Ras homolog gene family member A (RhoA).62 GKN1 inhibited epithelial-mesenchymal transition (EMT) by repressing the production of reactive oxygen species and PI3K/AKT signaling, as well as nuclear factor kappa-B (NF-ҡB)-dependent MMPs.63,64 RhoA, a key member of the Rho family of small GTP-binding proteins, is a major regulator of actin-dependent cell contraction and cell motility, and is closely linked to the signaling pathways involved in cell proliferation and migration.65–68 Further investigation demonstrated that the expression of miR-34a was positively correlated with the expression of GKN1, which was negatively correlated with RhoA. GKN1 also inhibited the expression of V-Myc avian myelocytomatosis viral oncogene homolog (c-Myc) in GC, which is a direct target of miR-34a.69 GKN1-induced miR-34a inhibited the expression of c-Myc by binding to its 3ʹ-UTR. However, c-Myc is an important transcription factor of RhoA.70 Down-regulation of c-Myc expression inhibited RhoA expression, which in turn inactivated the PI3K/AKT signaling pathway and consequent EMT process, as well as the migration and invasion of GC cells. In addition, miR-34a also inhibited the migration and invasion of GC cells by targeting oncogenes TGIF2, E2F3, and/or CDK6.51,71 These findings suggest that miR-34a plays a key role in the development of GC and can be considered as a therapeutic target for blocking metastasis and invasion of GC cells.
The Role of miR-34a in the Diagnosis and Prognosis of GC
It is reported that miR-34a is closely related to the diagnosis and prognosis of GC. Zhang et al72 showed that the down-regulation of miR-34a in GC was associated with high occurrence rate and low overall survival of patients with GC. Another study showed that the expression of miR-34a was found to be closely related to tumor size, cell differentiation, distant metastasis, lymph node metastasis, and occurrence of GC.73,74 Down-regulation of miR-34a was associated with Lauren grading and was positively correlated with overall survival of GC patients. Meanwhile, multivariate Cox regression analysis showed that miR-34a expression was an independent prognostic indicator of GC. These discoveries suggest that miR-34a may be used as a diagnosis and prognostic indicator for GC.
The Role of miR-34a in the Treatment and Chemoresistance of GC
At present, the main method to treat GC is chemotherapy based on cisplatin (DDP), but it is prone to cause drug resistance problems. Recent studies have found that miR-34a is associated with drug resistance of DDP.75 Reinforced miR-34a enhanced the sensitivity of GC cells to DDP. Further investigation demonstrated that miR-34a regulated the proliferation and apoptosis of cancer cells by targeting MET, thus regulated the sensitivity of GC cells to DDP. Moreover, studies have shown that lncRNA HOTAIR is associated with DDP resistance in GC. The weakened expression of lncRNA HOTAIR up-regulated miR-34a and inhibited the PI3K/AKT and WNT/β-catenin signaling pathways, thereby inhibiting DDP resistance in GC.19 In addition, Jang et al76 developed a nanoscale stable gene delivery system to enhance the delivery efficiency of miR-34a in vivo by encapsulating the poly-L-lysine-graft-imidazole (PLI)/miR-34a complex in the PEGylation (polyethylene glycol) liposome vehicles. The increased miR-34a not only enhanced its negative regulation on target genes, thereby inhibiting the occurrence, development, migration, and invasion of tumors, but also restored drug sensitivity. Moreover, miR-34a enhanced the sensitivity of GC cells to luteolin by directly targeting hexokinase 1 (HK1).52 Luteolin exerts anti-tumor effects by inhibiting cell proliferation, promoting apoptosis, and inhibiting cancer cell metastasis and angiogenesis.77,78 miR-34a was down-regulated in luteolin-resistant GC cells. When miR-34a was up-regulated, the chemo-sensitivity of drug-resistant GC cells to luteolin was improved. Further investigation revealed that there was a binding-site of miR-34a in the 3ʹ-UTR of HK1, which is the first restriction enzyme functioned in glycolysis.79,80 miR-34a reprograms metabolic processes by targeting HK1 to increase the chemosensitivity of GC cells to luteolin. Suzuki et al also presented evidences to demonstrate that miR-34a inhibited the proliferation, migration, and invasion of gastrointestinal stromal tumor cells by targeting PDGFRA.81 Therefore, exogenously increasing miR-34a may attenuate chemoresistance and improve the therapeutic effect of GC.
miR-34a in CRC
CRC is the third most common malignant tumor, with a high incidence of more than a million new cases diagnosed every year in the world.82–84 CRC has an especially high mortality rate in China, mainly due to cancer invasion and metastasis.85 Studies have demonstrated that miR-34a is closely related to the proliferation, differentiation, migration, treatment, and prognosis of CRC.
miR-34a Expression in CRC
Abnormal expression of miR-34a has been identified in CRC. Several studies have shown that miR-34a is highly expressed in patients with CRC. For instance, miR-34a was measured in tumors and adjacent noncancerous tissues from 159 American and 113 Chinese colon cancer patients using qPCR method. The results showed that miR-34a expression was up-regulated in two different ethnic CRC patients, and there was no significant correlation between miR-34a expression and TNM staging.86 Wang et al also reported that miR-34a was highly expressed in CRC tissues, and miR-34a expression was correlated with TNM staging.87 Moreover, the results obtained from the GEO database (accession numbers: GSE28364 and GSE83924) showed that the expression of miR-34a was significantly increased in the adenoma and cancer tissues as compared with the normal tissues.88,89 However, several studies have identified that miR-34a is under-expressed in CRC tissues and is closely related to the progression of CRC.90–94 In addition, a recent study found that a polymorphism rs35301225 in miR-34a was associated with tumor size, tumor differentiation and metastasis in Chinese CRC patients.95 Jun et al investigated the relationship between the methylation status of miR-34 gene and the expression of miR-34a in paired CRC tumor and normal tissues. The results showed that the methylation level of miR-34a in tumor tissues was significantly higher than that in normal tissues (P = 0.012), indicating that the methylation of miR-34 gene is related to the pathogenesis of CRC.96 These studies have shown that the expression of miR-34a is indeed closely related to CRC.
The Role of miR-34a in the Proliferation and Differentiation of CRC
Studies have demonstrated close relationship between miR-34a and the proliferation and differentiation of CRC. Formin-like 2 (FMNL2), a member of the family of homogenic proteins, is a catalyst for linear actin polymerization and is involved in many basic actin-dependent processes, including cell differentiation, proliferation, and cytokinesis.97,98 E2F transcription factor 5 (E2F5) is an important member of the E2F-family transcription factors, and plays a crucial role in the regulation of the cell cycle, especially in early G1 events, including G0/G1 transition.99 miR-34a inhibited proliferation and induced cell cycle arrest and apoptosis of CRC cells by targeting FMNL2 and E2F5.100 In addition, miR-34a also inhibited the proliferation of CRC cells by binding to the 3ʹ-UTR of high mobility group box 1 (HMGB1).101 HMGB1 is a chromatin protein which can organizes the DNA and regulates transcription. Since HMGB1 is highly expressed and miR-34a is down-expressed in CRC, the binding ability of miR-34a with HMGB1 is weakened, which contributes to the proliferation and differentiation of CRC. In addition, miR-34a also affected the proliferation and differentiation of colon cancer cells by targeting CDK4, CDK6, CCNE2, E2F3, BCL2 and the other genes closely related to cell cycle and anti-apoptosis.20 These findings suggest that miR-34a plays an important role in the proliferation and differentiation of CRC.
The Role of miR-34a in the Migration and Invasion of CRC
It has been shown that the interleukin 6 receptor (IL-6R)/signal transducer and activator of transcription 3 (STAT3)/miR-34a loop promoted EMT-mediated CRC migration and invasion.102 Interleukin-6 (IL-6) is an important proinflammatory cytokine that can be produced by tumor cells. Elevated IL-6 level was closely associated with advanced tumors, tumor size, and tumor metastasis.103,104 IL-6 exerts a biological effect by binding to its receptor IL-6R, resulting in activation of the carcinogenic transcription factor STAT3 and consequent inhibition of miR-34a. Low-expression of miR-34a reduced its binding ability to IL-6R and further activated STAT3. Thus, the activated IL-6R/STAT3/miR-34a loop was required for EMT, migration, and invasion of CRC cells. Additionally, activation of the transcription factor p53 inhibited the expression of IL-6R and the activation of STAT3, resulting in increased expression of miR-34a and disruption of the homeostasis of the IL-6R/STAT3/miR-34a loop, thereby interfering with IL-6-induced migration and invasion, and preventing EMT-mediated metastasis and invasion of CRC.105,106 miR-34a also inhibited CRC metastasis by regulating Notch signaling pathway.107 It has been found that miR-34a inhibited the expression of notch homolog 1 (Notch1) and ligand of Notch1 (JAG1) by directly binding to their 3ʹ-UTRs, thus promoting EMT, invasion, and migration of cancer cells.108–110 Meanwhile, the down-regulation of Notch1 and JAG1 decreased the expression of vimentin and fibronectin, and consequently weakened the migration and invasion of CRC cells. Lai et al observed that miR-34a inhibited the migration and invasion of CRC cells by regulating the SIRT1/p53 pathway.111 Studies have demonstrated that miR-34a participates in EMT through transcription factors and p53 signaling pathways. For instance, activation of p53 induced miR-34a expression and downregulated zinc finger transcription factor 1 (Snail1) and zing finger e box-binding homeobox 1 (ZEB1), which consequently inhibited the migration and invasion of CRC cells. While Snail1 and ZEB1 bond to the e-box in the miR-34a promoter, resulting in inhibition of miR-34a and forming a double negative feedback loop to regulate EMT. In addition to inhibiting Snail1, miR-34a also negatively regulated Slug, ZEB1, zinc finger protein 281 (ZNF281), etc., thereby stabilizing the epithelial phenotype.112–114 Additionally, several studies have shown that lncRNAs promote metastasis and invasion of CRC cells through competitively binding to miR-34a and activation of Wnt/β-catenin signaling pathway, such as HNF1A-AS1, GAPLINC, NEAT1, and XIST.115–118 These findings indicate that different molecular mechanisms were involved in the miR-34a-mediated inhibition of invasion and migration of CRC.
The Role of miR-34a in the Diagnosis and Prognosis of CRC
Aberrant miRNAs including miR-34a have been identified as potential prognostic biomarkers of CRC.119,120 In CRC, low-expression of miR-34a was reversely correlated with the expression of proto-oncogene c-Met, Snail1, and β-catenin, which were closely related to the distant metastasis of CRC. Multivariate COX regression analysis revealed that miR-34a expression was an independent prognostic factor for CRC recurrence. Furthermore, clinical data showed that miR-34a methylation was detected in 45.1% of 94 primary CRCs and was associated with liver metastasis and lymph node metastasis. Wu et al further confirmed that there was a close relationship between methylation of miR-34a and lymph node metastasis in CRC patients.121 These results indicate that miR-34a may be considered as a potential biomarker for the prognostic of CRC.
The Role of miR-34a in the Treatment and Chemoresistance of CRC
In recent years, many studies have found that miR-34a participated in chemotherapeutic drug-resistance of CRC. For instance, miR-34a induced the resistance of CRC cells to oxaliplatin by inhibiting macrophage autophagy via transforming growth factor-β (TGF-β)/drosophila mothers against decapentaplegic 4 (Smad4) pathway.122 miR-34a increased the sensitivity of CRC cells to 5-fluorouracil (5-FU) by inhibiting the expression and activity of lactate dehydrogenase A (LDHA), indicating that miR-34a-mediated inhibition of glucose metabolism may be a therapeutic target for patients with chemotherapy-resistant colon cancer.123 Moreover, a natural compound resveratrol increased the expression of miR-34a in cancer cells and down-regulated the target genes E2F3 and SIRT1, thus inhibiting the growth of cancer cells.124 Therefore, resveratrol exerted significant anti-tumor effects. It has been reported that Notch1 and Notch homolog 2 (Notch2) participate in various biological processes such as cell apoptosis and proliferation, and BCL2 is involved in cell apoptosis. Spica prunellae increased the level of miR-34a and enhanced its binding ability with target genes Notch1, Notch2, and BCL2, thereby inhibiting the growth of CRC cells.125 Curcumin difluoride (CDF), a new dietary ingredient curcumin analog, significantly inhibits cell growth and induces apoptosis in cancer cells resistant to 5-fluorouracil and oxaliplatin. The mechanistic study showed that CDF restored the expression of miR-34a in CRC cells by removing the methylation of miR-34a promoter.94 In addition, studies have revealed that lncRNA KCNQ1OT1 enhances the chemical metabolism of oxaliplatin in CRC by targeting the miR-34a/autophagy related 4B cysteine peptidase (ATG4B) pathway.126 Immunotherapy is a promising method for treating tumors in recent years, but it is prone to inducing tumor immune rejection. At present, several studies have shown that miR-34a affects the expression of the interferon stimulating genes in tumor microenvironment. Koelzer et al found that activation of E2F1 was an adverse event during dendritic cell (DC) maturation. miR-34a-mediated down-regulation of E2F1 and consequent death associated protein kinase 2 (DAPK2) and Sp1 transcription factor (Sp1) led to immaturity of DCs, therefore suppressing the immune response.127
miR-34a in HCC
HCC is the fifth most common malignancy in the world and the second leading cause of cancer-related deaths worldwide.128,129 In China, the incidence of HCC appears to be increasing every year, and the mortality rate ranks at the third in GICs. Recent studies have demonstrated that miR-34a is closely related to the proliferation, differentiation, migration, treatment, and prognosis of HCC.
miR-34a Expression in HCC
miR-34a has been identified to be abnormally expressed in HCC. For instance, Sun et al detected the expression of miR-34a in 60 HCC tissues, and found that miR-34a was lowly expressed in tumor tissues as compared with normal tissues.130 The expression of miR-34a in HCC tissues was related to clinical stage and lymph node metastasis. This is consistent with a recent study that the expression of miR-34a was sequentially reduced in normal tissues, liver cirrhosis (LC) tissues, and HCC tissues.131 Moreover, Xie et al confirmed that miR-34a was down-regulated in HCC tissues, and the proportion of methylation in the promoter region of miR-34a was higher in tumor tissues than that in non-tumor tissues.132 However, no correlation between the methylation and expression of miR-34a and the clinicopathological features of patients was observed. Fang et al analyzed the expression of miR-34a in GEO datasets (accession numbers: GSE10694, GSE22058, GSE21362, GSE67882) and found that miR-34a was downregulated in HCC tissues with no heterogeneity.133 These findings indicate that down-expression of miR-34a is involved in the progression of HCC.
The Role of miR-34a in the Proliferation of HCC
It has been found that miR-34a suppressed the proliferation and induced apoptosis of HCC cells by regulating the expression of histone deacetylase 1 (HDAC1).130 miR-34a inhibited HDAC1 expression by directly binding to its 3ʹ-UTR. Inhibition of HDAC1 restored its target genes by inducing histone and non-histone deacetylation.134 Down-regulated miR-34a led to over-expression of HDAC1 and silence of some key genes that regulate cell cycle and apoptosis, thereby promoting the proliferation and inhibiting the apoptosis of cancer cells. In addition, miR-34a regulated the telomerase activity in human HCC by targeting the forkhead box protein M1 (FoxM1)/c-Myc pathway, thereby inducing cell senescence and involving in the development of HCC.135 Another study demonstrated that the expression level of miR-34a was negatively correlated with telomerase index including telomere length and telomerase activity. miR-34a inhibited telomerase activity and telomere length in HCC cells, resulting in senescence-like phenotype and influence of cell survival. Both FoxM1 and c-Myc are involved in the activation of telomerase reverse transcriptase (hTERT) transcription and avoiding cell senescence.136,137 Therefore, miR-34a played an anti-tumor role by regulating the telomeres pathway in the senescence process of cells. Moreover, Jiang et al investigated the relationship between the risk of HCC and a polymorphism rs1057317 in the binding-site of miR-34a in the Toll-like receptor 4 (TLR4) gene.138 They found that rs1057317 was significantly associated with the risk of developing HCC, suggesting that the miR-34a/TLR4 axis plays an important role in the development of HCC. In addition, lncRNAs were observed to regulate the expression of miR-34a and to participate in the process of HCC. For instance, lncRNA CCAT2 promoted the growth of HCC tumors through a positive feedback loop of CCAT2-miR-34a-FOXM1.139 These studies have shown that miR-34a is closely related to the proliferation of HCC.
The Role of miR-34a in the Migration and Invasion of HCC
Many studies have examined the roles of miR-34a in the migration and invasion of HCC. It has been found that the expression level of miR-34a in the metastatic/invasive HCC tumor tissues is lower than that in the non-invasive HCC tumor tissues, suggesting that miR-34a is associated with the migration and invasion of HCC cells. Further investigation showed that miR-34a inhibited the migration and invasion of HCC by down-regulating c-Met.140 c-Met is a hepatocyte growth factor (HGF) receptor that activates MAPK pathway by binding to HGF, leading to constitutive activation of downstream components of the MAPK pathway, thereby inducing the migration and invasion of cancer cells.141–143 Moreover, miR-34a affected the non-glutamate decomposition of glutaminase 2 (GLS2) through the Dicer-miR-34a-Snail1 pathway, which promotes miR-34a maturation by interacting with Dicer and stabilizing Dicer.144 Up-regulated miR-34a inhibited EMT by suppressing Snail1 expression, ultimately inhibiting the migration and invasion of HCC. LncRNA-MUF, acting as the competitive endogenous RNA of miR-34a, restored Snail1 and promoted EMT, thus participated in the metastasis and invasion of HCC.145 These findings indicate that miR-34a influences the migration and invasion of HCC through different regulatory mechanisms.
The Role of miR-34a in the Diagnosis and Prognosis of HCC
Currently, several studies have shown the diagnosis and prognostic value of miR-34a expression in HCC patients.146,147 The low-expression of miR-34a was associated with vascular invasion and advanced TNM stage.146 Meanwhile, Kaplan-Meier analysis revealed that decreased miR-34a expression was associated with poor overall survival of HCC patients. Multivariate analysis showed that miR-34a expression was an independent prognostic factor for HCC.146 Additionally, Xiang et al showed that low-expression of miR-34a in serum and intratumoral tissues was an independent risk factor for bone metastasis in HCC patients.147 These findings suggest that miR-34a might be used as an indicator of diagnosis and prognosis of HCC.
The Role of miR-34a in the Treatment of HCC
Sorafenib, a multi-kinase inhibitor, is a first-line targeted chemotherapy for HCC. Sorafenib induces cell apoptosis by upregulating p53 pro-apoptotic effector p53 upregulated modulator of apoptosis (PUMA) and BCL2-associated X protein (Bax) and inhibiting anti-apoptotic BCL2 in tumor cells, thereby exerting antitumor effects. It has been reported that miR-34a influenced the therapeutic efficacy of sorafenib in HCC.148 Low-expression of miR-34a was significantly negatively correlated with high-expression of BCL2 in HCC tissues. Bioinformatics and dual luciferase reporter assays have shown that miR-34a inhibited BCL2 expression by directly binding to its 3ʹ-UTR. Therefore, miR-34a enhanced sorafenib-induced apoptosis of cancer cells by repressing the expression of BCL2. Moreover, a strong synergistic interaction between miR-34a and erlotinib, a tyrosine kinase inhibitor, was observed in the treatment of HCC.149 These findings provide new clinical strategies for the treatment of HCC.
miR-34a in PC
PC is a highly malignant tumor, which is difficult to be diagnosed and treated. About 90% of PCs are ductal adenocarcinomas originating from the ductal epithelium, named pancreatic ductal adenocarcinoma (PDAC). The morbidity and mortality of PC have significantly increased in recent years and have become the leading cause of cancer-related deaths.150 PC has the characteristics of hardly early diagnosis, low cure rate, and poor prognosis.151,152 At present, many studies have examined the relationship between miR-34a and PC.
miR-34a Expression in PC
It has been reported that the expression levels of miR-34a in plasma and tissues of patients with PDAC were significantly lower than those of benign pancreatic lesions and healthy subjects.153 The levels of miR-34a were also significantly associated with the clinicopathological features including tumor size, TNM stage, invasion, overall survival, and lymph node metastasis. Sun et al reported that miR-34a was down-regulated in PDAC tissues compared with normal tissues and was decreased along with TNM staging.154 Meanwhile, the expression level of miR-34a was closely related to the prognosis of patients with PDAC. However, miR-34a was found to be up-regulated in the serum of patients with PDAC.155 The results obtained from the GEO database (accession numbers: GSE32688 and GSE15471) also indicated that miR-34a expression in PC was up-regulated.156,157
The Role of miR-34a in the Proliferation of PC
Several studied have explored the relationship between miR-34a and the proliferation of PC cells. Kent et al found that reinforced miR-34a in two PDAC cell lines significantly inhibited the proliferation of cancer cells.158 Moreover, the expression levels of endogenous miR-34a in various PDAC cell lines were low or even absent, suggesting a negative relationship between miR-34a and proliferation of PC cells. In addition, studies have reported that miR-34a played a key role in the growth and apoptosis of PC cells, mainly through the regulation of Notch-1 signaling pathway, which is closely related to the cell growth and apoptosis.159 miR-34a also inhibited the proliferation of cancer cells by binding to lncRNAs.154 The lncRNA X inactive specific transcript (XIST) plays an important role in the development of PC, and promotes the proliferation, migration and invasion of cancer cells. Interestingly, miR-34a is down-regulated and XIST is up-regulated in PC cells. miR-34a is a target gene of XIST, and is negatively correlated with XIST expression, suggesting a crucial role of miR-34a in the proliferation of PC.154 miR-34a also participated in PC cell proliferation by targeting CD44 and CDK6.20 These results confirm that miR-34a plays a crucial role in the proliferation of PC.
The Role of miR-34a in the Migration and Invasion of PC
EMT is very important for the migration of cancer cells. Studies have revealed that absence of miR-34a led to migration, invasion, and anti-apoptosis of PC cells.160 Further investigation demonstrated that miR-34a inhibited tumor migration and invasion by repressing the expression of Snail1, a target gene of miR-34a. Snail1 gene products a zinc finger protein, which promotes EMT by inducing the transcription of mesenchymal genes (such as N-cadherin) and inhibiting the transcription of epithelial genes (such as E-cadherin), thereby resulting in migration and invasion of cancer cells.161,162 Overexpression of miR-34a suppressed the expression of Snail1, which in turn up-regulated E-cadherin. Moreover, the HDAC inhibitor Vorinostat (SAHA) inhibited the expression of EMT inducers Zeb-1, Snail, and Slug by up-regulating the expression of miR-34a, thereby attenuating the migration and invasion of PC cells.163 These results illustrated key roles of miR-34a in the migration and invasion of PC cells.
The Role of miR-34a in the Diagnosis and Prognosis of PC
It was found that the expression level of miR-34a was significantly associated with tumor size, TNM stage, invasion, overall survival, and lymph node metastasis of PC.153 Alemar et al determined the expression of miR-34a in the tissues of normal pancreas, chronic pancreatitis, and PDAC. The results showed that the expression level of miR-34a was closely related to the clinicopathological features of patients, suggesting that miR-34a may be used as a biomarker for the diagnosis and prognosis of PDAC.155 Furthermore, CpG methylation of miR-34a was also employed as a diagnostic indicator for the prognosis of patients with PC.164 Akamatsu et al165 found that the expression level of miR-34a in serum could be used as a biomarker to distinguish between PDAC and autoimmune pancreatitis (AIP). These studies indicate that miR-34a and its methylation levels may be used as indicators for diagnosis and prognosis in patients with PC.
The Role of miR-34a in the Treatment of PC
A recent study on the relationship between miR-34a and the treatment of PC showed that lipid-based nanoparticles encapsulating miR-34a exhibited anti-tumor effects on PC.166 Moreover, Feng et al found that simultaneous overexpression of miR-34a and miR-126 had strong anti-tumor effects in PC.167 In addition to using miRNAs to enhance anti-tumor effects, another study reported that the combination of miR-34a and polo like kinase 1 (PLK1) siRNA delivered by biodegradable amphiphilic polyglutamate amine polymer nanocarrier (APA) markedly enhanced anti-tumor effects of miR-34a in the treatment of PC.168 These studies demonstrated potential clinical applications of miR-34a in the treatment of PC.
miR-34a in GBC
GBC is one of the most common malignant gastrointestinal tumors in the world, accounting for 80–95% of global biliary carcinoma, and is also the main cause of global biliary malignant tumor-related death.169–171 GBC is a highly aggressive and fatal disease whose incidence is closely related to geography, race, and gender.170,172 Because of the lack of specific symptoms of GBC or reliable sensitive markers, it is difficult to diagnose GBC at an early stage, resulting in low survival rate. It has been reported that miR-34a is down-regulated in GBC,173 but few studies focus on the relationship between miR-34a and the prognosis of GBC.
Jin et al measured miR-34a expression and telomere length in 77 gallbladder adenocarcinomas and 36 non-tumor tissues.173 They found that miR-34a expression was significantly decreased in GBC tissues with long telomere length and poor survival rates and prognosis of GBC patients. Forced miR-34a expression attenuated the colony forming ability of CD44+ CD133+ GBC stem cell-like cells in vitro and inhibited the growth of xenograft tumors in vivo, indicating that miR-34a acted as a tumor suppressor in GBC. Simultaneously, the relationship between miR-34a and telomere length was further studied, and it was found that miR-34a reduced telomere length by down-regulating the expression of protein phosphatase 1 regulatory subunit 10 (PNUTS). PNUTS mediates the regulation of chromatin structure during cell transition from mitosis to interphase. Therefore, highly expressed miR-34a shortened the length of telomerase and inhibited the development of GBC by inhibiting the expression of PNUTS. These findings suggest that low-expression of miR-34a and long telomere length may be used as biomarkers of poor prognosis in patients with GBC and that miR-34a gene is a potential target for the treatment of GBC.
Summary
miR-34a is dysregulated in GICs with consistent low-expression in EC, GC, HCC, and GBC, but contradictory expression in CRC and PC, demonstrating complex regulatory roles of miR-34a in GICs. Numerous studies have revealed that miR-34a plays a critical role in the proliferation, anti-apoptosis, cell cycle arrest, migration, invasion, and treatment of GIC cells, through targeting a number of oncogenes, such as BCL2, CCND1, CCNE2, CD44, CDK4, CDK6, E2F1, E2F3, E2F5, FOXM1, MET, MMP2, MMP9, MYC, NOTCH1, SIRT1, SNAIL1, STAT3, etc. These indicate that miR-34a exerts important roles in the development, metastasis, and prognosis of GIC patients via signal pathways including MAPK/Ras, Wnt/β-Catenin, and PI3K/Akt. Thus, researchers have proposed augmenting miR-34a expression as a candidate therapeutic strategy and developed a liposome-capsuled miR-34a mimic (MRX34) for treating cancer patients in clinic.20,174-176 Although acceptable anti-tumor activity in patients was achieved, the clinical trial was terminated because of severe immune-related adverse effects. Hence more studies on the immune-regulatory role of miR-34a in GICs are still needed to be further elucidated. Even though, due to the potent tumor-suppressive roles of miR-34a in GICs, new strategies with elimination of miR-34a-mediated immune-related adverse events might be beneficial to cancer therapy, for instance, formulation of miR-34a with antibody or nanoparticle for improving the targeting activity, decreasing the dosage, or combination with other anti-cancer drugs. This review systematically outlined the regulatory activities of miR-34a in GICs and provided evidences to support miR-34a as a promising target for cancer therapy.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 81773044), Science and Technology Special Project of Clinical Medicine in Jiangsu Province (BL2014046), Social Development Project of Jiangsu Province (BE2019657), Qinglan Project of Jiangsu Province, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63–71. doi:10.1016/j.canlet.2017.04.024
3. Berezikov E, Cuppen E, Plasterk RH. Approaches to microRNA discovery. Nat Genet. 2006;38(Suppl):S2–S7. doi:10.1038/ng1794
4. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi:10.1016/0092-8674(93)90529-y
5. Shyu AB, Wilkinson MF, van Hoof A. Messenger RNA regulation: to translate or to degrade. EMBO J. 2008;27(3):471–481. doi:10.1038/sj.emboj.7601977
6. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi:10.1016/s0092-8674(04)00045-5
7. Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460(7254):479–486. doi:10.1038/nature08170
8. Lai EC. Micro RNAs are complementary to 3ʹ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet. 2002;30(4):363–364. doi:10.1038/ng865
9. Gao H, Zhao H, Xiang W. Expression level of human miR-34a correlates with glioma grade and prognosis. J Neurooncol. 2013;113(2):221–228. doi:10.1007/s11060-013-1119-1
10. Boon RA, Iekushi K, Lechner S, et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495(7439):107–110. doi:10.1038/nature11919
11. Wang X, Dong K, Gao P, et al. microRNA-34a sensitizes lung cancer cell lines to DDP treatment independent of p53 status. Cancer Biother Radiopharm. 2013;28(1):45–50. doi:10.1089/cbr.2012.1218
12. Callegari E, Elamin BK, Sabbioni S, Gramantieri L, Negrini M. Role of microRNAs in hepatocellular carcinoma: a clinical perspective. Onco Targets Ther. 2013;6:1167–1178. doi:10.2147/ott.S36161
13. Li XJ, Ren ZJ, Tang JH. MicroRNA-34a: a potential therapeutic target in human cancer. Cell Death Dis. 2014;5(7):e1327. doi:10.1038/cddis.2014.270
14. Saito Y, Nakaoka T, Saito H. microRNA-34a as a therapeutic agent against human cancer. J Clin Med. 2015;4(11):1951–1959. doi:10.3390/jcm4111951
15. Baer C, Claus R, Frenzel LP, et al. Extensive promoter DNA hypermethylation and hypomethylation is associated with aberrant microRNA expression in chronic lymphocytic leukemia. Cancer Res. 2012;72(15):3775–3785. doi:10.1158/0008-5472.Can-12-0803
16. Lodygin D, Tarasov V, Epanchintsev A, et al. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7(16):2591–2600. doi:10.4161/cc.7.16.6533
17. He L, He X, Lim LP, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447(7148):1130–1134. doi:10.1038/nature05939
18. Misso G, Di Martino MT, De Rosa G, et al. Mir-34: a new weapon against cancer? Mol Ther Nucleic Acids. 2014;3:e194. doi:10.1038/mtna.2014.47
19. Cheng C, Qin Y, Zhi Q, Wang J, Qin C. Knockdown of long non-coding RNA HOTAIR inhibits cisplatin resistance of gastric cancer cells through inhibiting the PI3K/Akt and Wnt/beta-catenin signaling pathways by up-regulating miR-34a. Int J Biol Macromol. 2018;107(PtB):2620–2629. doi:10.1016/j.ijbiomac.2017.10.154
20. Agostini M, Knight RA. miR-34: from bench to bedside. Oncotarget. 2014;5(4):872–881. doi:10.18632/oncotarget.1825
21. Chen X, Zhou JY, Zhou JY. MicroRNA-34a: role in cancer and cardiovascular disease. Curr Drug Targets. 2014;15(4):361–373. doi:10.2174/1389450115666140120102935
22. Chua CEL, Tang BL. miR-34a in neurophysiology and neuropathology. J Mol Neurosci. 2019;67(2):235–246. doi:10.1007/s12031-018-1231-y
23. Ghandadi M, Sahebkar A. MicroRNA-34a and its target genes: key factors in cancer multidrug resistance. Curr Pharm Des. 2016;22(7):933–939. doi:10.2174/1381612822666151209153729
24. Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17(2):193–199. doi:10.1038/cdd.2009.56
25. Imani S, Wu RC, Fu J. MicroRNA-34 family in breast cancer: from research to therapeutic potential. J Cancer. 2018;9(20):3765–3775. doi:10.7150/jca.25576
26. Li L. Regulatory mechanisms and clinical perspectives of miR-34a in cancer. J Cancer Res Ther. 2014;10(4):805–810. doi:10.4103/0973-1482.146084
27. Maroof H, Salajegheh A, Smith RA, Lam AK. Role of microRNA-34 family in cancer with particular reference to cancer angiogenesis. Exp Mol Pathol. 2014;97(2):298–304. doi:10.1016/j.yexmp.2014.08.002
28. Slabakova E, Culig Z, Remsik J, Soucek K. Alternative mechanisms of miR-34a regulation in cancer. Cell Death Dis. 2017;8(10):e3100. doi:10.1038/cddis.2017.495
29. Zhang DG, Zheng JN, Pei DS. P53/microRNA-34-induced metabolic regulation: new opportunities in anticancer therapy. Mol Cancer. 2014;13(1):115. doi:10.1186/1476-4598-13-115
30. Chen S, Li T, Zhao Q, Xiao B, Guo J. Using circular RNA hsa_circ_0000190 as a new biomarker in the diagnosis of gastric cancer. Clin Chim Acta. 2017;466:167–171. doi:10.1016/j.cca.2017.01.025
31. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349(23):2241–2252. doi:10.1056/NEJMra035010
32. Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus. 2009;22(1):1–8. doi:10.1111/j.1442-2050.2008.00901.x
33. Cui XB, Peng H, Li RR, et al. MicroRNA-34a functions as a tumor suppressor by directly targeting oncogenic PLCE1 in Kazakh esophageal squamous cell carcinoma. Oncotarget. 2017;8(54):92454–92469. doi:10.18632/oncotarget.21384
34. Shi H, Zhou S, Liu J, et al. miR-34a inhibits the in vitro cell proliferation and migration in human esophageal cancer. Pathol Res Pract. 2016;212(5):444–449. doi:10.1016/j.prp.2016.02.019
35. Asadi M, Shanehbandi D, Mohammadpour H, Hashemzadeh S, Sepehri B. Expression level of miR-34a in tumor tissue from patients with esophageal squamous cell carcinoma. J Gastrointest Cancer. 2018;50:304–307. doi:10.1007/s12029-018-0060-0
36. Chen Z, Li J, Tian L, et al. MiRNA expression profile reveals a prognostic signature for esophageal squamous cell carcinoma. Cancer Lett. 2014;350(1–2):34–42. doi:10.1016/j.canlet.2014.04.013
37. Sun F, Fu H, Liu Q, et al. Downregulation of CCND1 and CDK6 by miR-34a induces cell cycle arrest. FEBS Lett. 2008;582(10):1564–1568. doi:10.1016/j.febslet.2008.03.057
38. Wei JS, Song YK, Durinck S, et al. The MYCN oncogene is a direct target of miR-34a. Oncogene. 2008;27(39):5204–5213. doi:10.1038/onc.2008.154
39. Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70(1):281–312. doi:10.1146/annurev.biochem.70.1.281
40. Shibatohge M, Kariya K, Liao Y, et al. Identification of PLC210, a Caenorhabditis elegans phospholipase C, as a putative effector of Ras. J Biol Chem. 1998;273(11):6218–6222. doi:10.1074/jbc.273.11.6218
41. Chen X, Hu H, Guan X, et al. CpG island methylation status of miRNAs in esophageal squamous cell carcinoma. Int J Cancer. 2012;130(7):1607–1613. doi:10.1002/ijc.26171
42. Cui X, Zhao Z, Liu D, et al. Inactivation of miR-34a by aberrant CpG methylation in Kazakh patients with esophageal carcinoma. J Exp Clin Cancer Res. 2014;33(1):20. doi:10.1186/1756-9966-33-20
43. Chu J, Li H, Xing Y, et al. LncRNA MNX1-AS1 promotes progression of esophageal squamous cell carcinoma by regulating miR-34a/SIRT1 axis. Biomed Pharmacother. 2019;116:109029. doi:10.1016/j.biopha.2019.109029
44. Yang L, Song X, Zhu J, et al. Tumor suppressor microRNA-34a inhibits cell migration and invasion by targeting MMP-2/MMP-9/FNDC3B in esophageal squamous cell carcinoma. Int J Oncol. 2017;51(1):378–388. doi:10.3892/ijo.2017.4015
45. Nie J, Ge X, Geng Y, et al. miR-34a inhibits the migration and invasion of esophageal squamous cell carcinoma by targeting Yin Yang-1. Oncol Rep. 2015;34(1):311–317. doi:10.3892/or.2015.3962
46. Lin X, Xu XY, Chen QS, Huang C. Clinical significance of microRNA-34a in esophageal squamous cell carcinoma. Genet Mol Res. 2015;14(4):17684–17691. doi:10.4238/2015.December.21.41
47. Dai S, Ye Z, Wang F, et al. Doxorubicin-loaded poly(epsilon-caprolactone)-Pluronic micelle for targeted therapy of esophageal cancer. J Cell Biochem. 2018;119(11):9017–9027. doi:10.1002/jcb.27159
48. Duffy MJ, Lamerz R, Haglund C, et al. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. Int J Cancer. 2014;134(11):2513–2522. doi:10.1002/ijc.28384
49. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi:10.1002/ijc.25516
50. Wu H, Huang M, Liu Y, Shu Y, Liu P. Luteolin induces apoptosis by up-regulating miR-34a in human gastric cancer cells. Technol Cancer Res Treat. 2015;14(6):747–755. doi:10.7785/tcrt.2012.500434
51. Hu Y, Pu Q, Cui B, Lin J. MicroRNA-34a inhibits tumor invasion and metastasis in gastric cancer by targeting Tgif2. Int J Clin Exp Pathol. 2015;8(8):8921–8928.
52. Zhou Y, BZ D, YP L, HB W. MiR-34a, as a suppressor, enhance the susceptibility of gastric cancer cell to luteolin by directly targeting HK1. Gene. 2018;644:56–65. doi:10.1016/j.gene.2017.10.046
53. Zhou Y, Huang T, Siu HL, et al. IGF2BP3 functions as a potential oncogene and is a crucial target of miR-34a in gastric carcinogenesis. Mol Cancer. 2017;16(1):77. doi:10.1186/s12943-017-0647-2
54. Dierov J, Xu Q, Dierova R, Carroll M. TEL/platelet-derived growth factor receptor beta activates phosphatidylinositol 3 (PI3) kinase and requires PI3 kinase to regulate the cell cycle. Blood. 2002;99(5):1758–1765. doi:10.1182/blood.v99.5.1758
55. Feng H, Liu KW, Guo P, et al. Dynamin 2 mediates PDGFRalpha-SHP-2-promoted glioblastoma growth and invasion. Oncogene. 2012;31(21):2691–2702. doi:10.1038/onc.2011.436
56. Peng Y, Guo JJ, Liu YM, Wu XL. MicroRNA-34A inhibits the growth, invasion and metastasis of gastric cancer by targeting PDGFR and MET expression. Biosci Rep. 2014;34(3):3. doi:10.1042/bsr20140020
57. Cao W, Fan R, Wang L, et al. Expression and regulatory function of miRNA-34a in targeting survivin in gastric cancer cells. Tumour Biol. 2013;34(2):963–971. doi:10.1007/s13277-012-0632-8
58. Osawa S, Shimada Y, Sekine S, et al. MicroRNA profiling of gastric cancer patients from formalin-fixed paraffin-embedded samples. Oncol Lett. 2011;2(4):613–619. doi:10.3892/ol.2011.313
59. Stanitz E, Juhasz K, Toth C, Gombos K, Natali PG, Ember I. Evaluation of MicroRNA expression pattern of gastric adenocarcinoma associated with socioeconomic, environmental and lifestyle factors in northwestern hungary. Anticancer Res. 2013;33(8):3195–3200.
60. Hu CE, Liu YC, Zhang HD, Huang GJ. The RNA-binding protein PCBP2 facilitates gastric carcinoma growth by targeting miR-34a. Biochem Biophys Res Commun. 2014;448(4):437–442. doi:10.1016/j.bbrc.2014.04.124
61. Zhang S, Chen P, Huang Z, et al. Sirt7 promotes gastric cancer growth and inhibits apoptosis by epigenetically inhibiting miR-34a. Sci Rep. 2015;5(1):9787. doi:10.1038/srep09787
62. Yoon JH, Choi WS, Kim O, et al. Gastrokine 1 inhibits gastric cancer cell migration and invasion by downregulating RhoA expression. Gastric Cancer. 2017;20(2):274–285. doi:10.1007/s10120-016-0617-1
63. Xing R, Cui JT, Xia N, Lu YY. GKN1 inhibits cell invasion in gastric cancer by inactivating the NF-kappaB pathway. Discov Med. 2015;19(103):65–71.
64. Yoon JH, Kang YH, Choi YJ, et al. Gastrokine 1 functions as a tumor suppressor by inhibition of epithelial-mesenchymal transition in gastric cancers. J Cancer Res Clin Oncol. 2011;137(11):1697–1704. doi:10.1007/s00432-011-1051-8
65. Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009;1796(2):91–98. doi:10.1016/j.bbcan.2009.03.003
66. Li H, Peyrollier K, Kilic G, Brakebusch C. Rho GTPases and cancer. Biofactors. 2014;40(2):226–235. doi:10.1002/biof.1155
67. Ridley AJ, Schwartz MA, Burridge K, et al. Cell migration: integrating signals from front to back. Science. 2003;302(5651):1704–1709. doi:10.1126/science.1092053
68. Thumkeo D, Watanabe S, Narumiya S. Physiological roles of Rho and Rho effectors in mammals. Eur J Cell Biol. 2013;92(10–11):303–315. doi:10.1016/j.ejcb.2013.09.002
69. Yamamura S, Saini S, Majid S, et al. MicroRNA-34a modulates c-Myc transcriptional complexes to suppress malignancy in human prostate cancer cells. PLoS One. 2012;7(1):e29722. doi:10.1371/journal.pone.0029722
70. Chan CH, Lee SW, Li CF, et al. Deciphering the transcriptional complex critical for RhoA gene expression and cancer metastasis. Nat Cell Biol. 2010;12(5):457–467. doi:10.1038/ncb2047
71. Lujambio A, Calin GA, Villanueva A, et al. A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci U S A. 2008;105(36):13556–13561. doi:10.1073/pnas.0803055105
72. Zhang H, Li S, Yang J, Liu S, Gong X, Yu X. The prognostic value of miR-34a expression in completely resected gastric cancer: tumor recurrence and overall survival. Int J Clin Exp Med. 2015;8(2):2635–2641.
73. Hui WT, Ma XB, Zan Y, Wang XJ, Dong L. Prognostic significance of MiR-34a expression in patients with gastric cancer after radical gastrectomy. Chin Med J. 2015;128(19):2632–2637. doi:10.4103/0366-6999.166019
74. Yao Y, Suo AL, Li ZF, et al. MicroRNA profiling of human gastric cancer. Mol Med Rep. 2009;2(6):963–970. doi:10.3892/mmr_00000199
75. Zhang Z, Kong Y, Yang W, et al. Upregulation of microRNA-34a enhances the DDP sensitivity of gastric cancer cells by modulating proliferation and apoptosis via targeting MET. Oncol Rep. 2016;36(4):2391–2397. doi:10.3892/or.2016.5016
76. Jang E, Kim E, Son HY, et al. Nanovesicle-mediated systemic delivery of microRNA-34a for CD44 overexpressing gastric cancer stem cell therapy. Biomater. 2016;105:12–24. doi:10.1016/j.biomaterials.2016.07.036
77. Chakrabarti M, Ray SK. Anti-tumor activities of luteolin and silibinin in glioblastoma cells: overexpression of miR-7-1-3p augmented luteolin and silibinin to inhibit autophagy and induce apoptosis in glioblastoma in vivo. Apoptosis. 2016;21(3):312–328. doi:10.1007/s10495-015-1198-x
78. Sun DW, Zhang HD, Mao L, et al. Luteolin inhibits breast cancer development and progression in vitro and in vivo by suppressing notch signaling and regulating MiRNAs. Cell Physiol Biochem. 2015;37(5):1693–1711. doi:10.1159/000438535
79. Smith TA. Mammalian hexokinases and their abnormal expression in cancer. Br J Biomed Sci. 2000;57(2):170–178.
80. Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206(Pt 12):2049–2057. doi:10.1242/jeb.00241
81. Isosaka M, Niinuma T, Nojima M, et al. A screen for epigenetically silenced microRNA genes in gastrointestinal stromal tumors. PLoS One. 2015;10(7):e0133754. doi:10.1371/journal.pone.0133754
82. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. doi:10.1016/j.ejca.2012.12.027
83. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi:10.3322/caac.21395
84. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387
85. Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695. doi:10.1016/j.cell.2006.11.001
86. Hiyoshi Y, Schetter AJ, Okayama H, et al. Increased microRNA-34b and −34c predominantly expressed in stromal tissues is associated with poor prognosis in human colon cancer. PLoS One. 2015;10(4):e0124899. doi:10.1371/journal.pone.0124899
87. Wang M, Zhang P, Li Y, et al. The quantitative analysis by stem-loop real-time PCR revealed the microRNA-34a, microRNA-155 and microRNA-200c overexpression in human colorectal cancer. Med Oncol. 2012;29(5):3113–3118. doi:10.1007/s12032-012-0241-9
88. Nagy ZB, Wichmann B, Kalmar A, et al. Colorectal adenoma and carcinoma specific miRNA profiles in biopsy and their expression in plasma specimens. Clin Epigenetics. 2017;9(1):22. doi:10.1186/s13148-016-0305-3
89. Reid JF, Sokolova V, Zoni E, et al. miRNA profiling in colorectal cancer highlights miR-1 involvement in MET-dependent proliferation. Mol Cancer Res. 2012;10(4):504–515. doi:10.1158/1541-7786.Mcr-11-0342
90. Li Y, Zeng C, Hu J, et al. Long non-coding RNA-SNHG7 acts as a target of miR-34a to increase GALNT7 level and regulate PI3K/Akt/mTOR pathway in colorectal cancer progression. J Hematol Oncol. 2018;11(1):89. doi:10.1186/s13045-018-0632-2
91. Luo Y, Chen JJ, Lv Q, et al. Long non-coding RNA NEAT1 promotes colorectal cancer progression by competitively binding miR-34a with SIRT1 and enhancing the Wnt/beta-catenin signaling pathway. Cancer Lett. 2018;440-441:11–22. doi:10.1016/j.canlet.2018.10.002
92. Nugent M, Miller N, Kerin MJ. Circulating miR-34a levels are reduced in colorectal cancer. J Surg Oncol. 2012;106(8):947–952. doi:10.1002/jso.23174
93. Orosz E, Kiss I, Gyongyi Z, Varjas T. Expression of circulating miR-155, miR-21, miR-221, miR-30a, miR-34a and miR-29a: comparison of colonic and rectal cancer. In Vivo. 2018;32(6):1333–1337. doi:10.21873/invivo.11383
94. Roy S, Levi E, Majumdar AP, Sarkar FH. Expression of miR-34 is lost in colon cancer which can be re-expressed by a novel agent CDF. J Hematol Oncol. 2012;5(1):58. doi:10.1186/1756-8722-5-58
95. Jiang H, Ge F, Hu B, Wu L, Yang H, Wang H. rs35301225 polymorphism in miR-34a promotes development of human colon cancer by deregulation of 3ʹUTR in E2F1 in Chinese population. Cancer Cell Int. 2017;17(1):39. doi:10.1186/s12935-017-0402-1
96. Jun HH, Kwack K, Lee KH, et al. Association between TP53 genetic polymorphisms and the methylation and expression of miR-34a, 34b/c in colorectal cancer tissues. Oncol Lett. 2019;17(5):4726–4734. doi:10.3892/ol.2019.10092
97. Li Y, Zhu X, Zeng Y, et al. FMNL2 enhances invasion of colorectal carcinoma by inducing epithelial-mesenchymal transition. Mol Cancer Res. 2010;8(12):1579–1590. doi:10.1158/1541-7786.Mcr-10-0081
98. Zhu XL, Zeng YF, Guan J, et al. FMNL2 is a positive regulator of cell motility and metastasis in colorectal carcinoma. J Pathol. 2011;224(3):377–388. doi:10.1002/path.2871
99. Kothandaraman N, Bajic VB, Brendan PN, et al. E2F5 status significantly improves malignancy diagnosis of epithelial ovarian cancer. BMC Cancer. 2010;10(1):64. doi:10.1186/1471-2407-10-64
100. Lu G, Sun Y, An S, et al. MicroRNA-34a targets FMNL2 and E2F5 and suppresses the progression of colorectal cancer. Exp Mol Pathol. 2015;99(1):173–179. doi:10.1016/j.yexmp.2015.06.014
101. Chandrasekaran KS, Sathyanarayanan A, Karunagaran D. Downregulation of HMGB1 by miR-34a is sufficient to suppress proliferation, migration and invasion of human cervical and colorectal cancer cells. Tumour Biol. 2016;37(10):13155–13166. doi:10.1007/s13277-016-5261-1
102. Rokavec M, Oner MG, Li H, et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2014;124(4):1853–1867. doi:10.1172/jci73531
103. Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70 Suppl 1:i104–i108. doi:10.1136/ard.2010.140145.
104. Studebaker AW, Storci G, Werbeck JL, et al. Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res. 2008;68(21):9087–9095. doi:10.1158/0008-5472.Can-08-0400
105. Schwitalla S, Ziegler PK, Horst D, et al. Loss of p53 in enterocytes generates an inflammatory microenvironment enabling invasion and lymph node metastasis of carcinogen-induced colorectal tumors. Cancer Cell. 2013;23(1):93–106. doi:10.1016/j.ccr.2012.11.014
106. Spehlmann ME, Manthey CF, Dann SM, et al. Trp53 deficiency protects against acute intestinal inflammation. J Immunol. 2013;191(2):837–847. doi:10.4049/jimmunol.1201716
107. Zhang X, Ai F, Li X, et al. MicroRNA-34a suppresses colorectal cancer metastasis by regulating Notch signaling. Oncol Lett. 2017;14(2):2325–2333. doi:10.3892/ol.2017.6444
108. Du R, Sun W, Xia L, et al. Hypoxia-induced down-regulation of microRNA-34a promotes EMT by targeting the Notch signaling pathway in tubular epithelial cells. PLoS One. 2012;7(2):e30771. doi:10.1371/journal.pone.0030771
109. Wang Y, Wu B, Chamberlain AA, et al. Endocardial to myocardial notch-wnt-bmp axis regulates early heart valve development. PLoS One. 2013;8(4):e60244. doi:10.1371/journal.pone.0060244
110. Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23(5):1155–1165. doi:10.1038/sj.emboj.7600069
111. Lai M, Du G, Shi R, et al. MiR-34a inhibits migration and invasion by regulating the SIRT1/p53 pathway in human SW480 cells. Mol Med Rep. 2015;11(5):3301–3307. doi:10.3892/mmr.2015.3182
112. Hahn S, Hermeking H. ZNF281/ZBP-99: a new player in epithelial-mesenchymal transition, stemness, and cancer. J Mol Med. 2014;92(6):571–581. doi:10.1007/s00109-014-1160-3
113. Hahn S, Jackstadt R, Siemens H, Hunten S, Hermeking H. SNAIL and miR-34a feed-forward regulation of ZNF281/ZBP99 promotes epithelial-mesenchymal transition. EMBO J. 2013;32(23):3079–3095. doi:10.1038/emboj.2013.236
114. Siemens H, Jackstadt R, Hunten S, et al. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle. 2011;10(24):4256–4271. doi:10.4161/cc.10.24.18552
115. Fang C, Qiu S, Sun F, et al. Long non-coding RNA HNF1A-AS1 mediated repression of miR-34a/SIRT1/p53 feedback loop promotes the metastatic progression of colon cancer by functioning as a competing endogenous RNA. Cancer Lett. 2017;410:50–62. doi:10.1016/j.canlet.2017.09.012
116. Luo Y, Ouyang J, Zhou D, et al. Long noncoding RNA GAPLINC promotes cells migration and invasion in colorectal cancer cell by regulating miR-34a/c-MET signal pathway. Dig Dis Sci. 2018;63(4):890–899. doi:10.1007/s10620-018-4915-9
117. Luo Y, Chen JJ, Lv Q, et al. Long non-coding RNA NEAT1 promotes colorectal cancer progression by competitively binding miR-34a with SIRT1 and enhancing the Wnt/beta-catenin signaling pathway. Cancer Lett. 2019;440–441:11–22. doi:10.1016/j.canlet.2018.10.002
118. Sun N, Zhang G, Liu Y. Long non-coding RNA XIST sponges miR-34a to promotes colon cancer progression via Wnt/beta-catenin signaling pathway. Gene. 2018;665:141–148. doi:10.1016/j.gene.2018.04.014
119. Gao J, Li N, Dong Y, et al. miR-34a-5p suppresses colorectal cancer metastasis and predicts recurrence in patients with stage II/III colorectal cancer. Oncogene. 2015;34(31):4142–4152. doi:10.1038/onc.2014.348
120. Siemens H, Neumann J, Jackstadt R, et al. Detection of miR-34a promoter methylation in combination with elevated expression of c-Met and beta-catenin predicts distant metastasis of colon cancer. Clin Cancer Res. 2013;19(3):710–720. doi:10.1158/1078-0432.Ccr-12-1703
121. Wu XD, Song YC, Cao PL, et al. Detection of miR-34a and miR-34b/c in stool sample as potential screening biomarkers for noninvasive diagnosis of colorectal cancer. Med Oncol. 2014;31(4):894. doi:10.1007/s12032-014-0894-7
122. Sun C, Wang F-J, Zhang H-G, et al. miR-34a mediates oxaliplatin resistance of colorectal cancer cells by inhibiting macroautophagyvia transforming growth factor-β/Smad4 pathway. World J Gastroenterol. 2017;23(10):1816–1827. doi:10.3748/wjg.v23.i10.1816
123. Li X, Zhao H, Zhou X, Song L. Inhibition of lactate dehydrogenase A by microRNA-34a resensitizes colon cancer cells to 5-fluorouracil. Mol Med Rep. 2015;11(1):577–582. doi:10.3892/mmr.2014.2726
124. Kumazaki M, Noguchi S, Yasui Y, et al. Anti-cancer effects of naturally occurring compounds through modulation of signal transduction and miRNA expression in human colon cancer cells. J Nutr Biochem. 2013;24(11):1849–1858. doi:10.1016/j.jnutbio.2013.04.006
125. Fang Y, Zhang L, Feng J, Lin W, Cai Q, Peng J. Spica Prunellae extract suppresses the growth of human colon carcinoma cells by targeting multiple oncogenes via activating miR-34a. Oncol Rep. 2017;38(3):1895–1901. doi:10.3892/or.2017.5792
126. Li Y, Li C, Li D, Yang L, Jin J, Zhang B. lncRNA KCNQ1OT1 enhances the chemoresistance of oxaliplatin in colon cancer by targeting the miR-34a/ATG4B pathway. Onco Targets Ther. 2019;12:2649–2660. doi:10.2147/ott.S188054
127. Koelzer VH, Sokol L, Zahnd S, et al. Digital analysis and epigenetic regulation of the signature of rejection in colorectal cancer. Oncoimmunology. 2017;6(4):e1288330. doi:10.1080/2162402x.2017.1288330
128. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi:10.1016/s0140-6736(12)61728-0
129. Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatol. 2015;61(1):191–199. doi:10.1002/hep.27388
130. Sun TY, Xie HJ, Li Z, et al. miR-34a regulates HDAC1 expression to affect the proliferation and apoptosis of hepatocellular carcinoma. Am J Transl Res. 2017;9(1):103–114.
131. Feili X, Wu S, Ye W, Tu J, Lou L. MicroRNA-34a-5p inhibits liver fibrosis by regulating TGF-beta1/Smad3 pathway in hepatic stellate cells. Cell Biol Int. 2018;42(10):1370–1376. doi:10.1002/cbin.11022
132. Xie K, Liu J, Chen J, et al. Methylation-associated silencing of microRNA-34b in hepatocellular carcinoma cancer. Gene. 2014;543(1):101–107. doi:10.1016/j.gene.2014.03.059
133. Ren FH, Yang H, He RQ, et al. Analysis of microarrays of miR-34a and its identification of prospective target gene signature in hepatocellular carcinoma. BMC Cancer. 2018;18(1):12. doi:10.1186/s12885-017-3941-x
134. Ropero S, Esteller M. The role of histone deacetylases (HDACs) in human cancer. Mol Oncol. 2007;1(1):19–25. doi:10.1016/j.molonc.2007.01.001
135. Xu X, Chen W, Miao R, et al. miR-34a induces cellular senescence via modulation of telomerase activity in human hepatocellular carcinoma by targeting FoxM1/c-Myc pathway. Oncotarget. 2015;6(6):3988–4004. doi:10.18632/oncotarget.2905
136. Daniel M, Peek GW, Tollefsbol TO. Regulation of the human catalytic subunit of telomerase (hTERT). Gene. 2012;498(2):135–146. doi:10.1016/j.gene.2012.01.095
137. Wierstra I, Alves J. FOXM1c transactivates the human c-myc promoter directly via the two TATA boxes P1 and P2. FEBS J. 2006;273(20):4645–4667. doi:10.1111/j.1742-4658.2006.05468.x
138. Jiang ZC, Tang XM, Zhao YR, Zheng L. A functional variant at miR-34a binding site in toll-like receptor 4 gene alters susceptibility to hepatocellular carcinoma in a Chinese Han population. Tumour Biol. 2014;35(12):12345–12352. doi:10.1007/s13277-014-2547-z
139. Chen F, Bai G, Li Y, Feng Y, Wang L. A positive feedback loop of long noncoding RNA CCAT2 and FOXM1 promotes hepatocellular carcinoma growth. Am J Cancer Res. 2017;7(7):1423–1434.
140. Li N, Fu H, Tie Y, et al. miR-34a inhibits migration and invasion by down-regulation of c-Met expression in human hepatocellular carcinoma cells. Cancer Lett. 2009;275(1):44–53. doi:10.1016/j.canlet.2008.09.035
141. Leelawat K, Leelawat S, Tepaksorn P, et al. Involvement of c-Met/hepatocyte growth factor pathway in cholangiocarcinoma cell invasion and its therapeutic inhibition with small interfering RNA specific for c-Met. J Surg Res. 2006;136(1):78–84. doi:10.1016/j.jss.2006.05.031
142. Ma PC, Tretiakova MS, Nallasura V, Jagadeeswaran R, Husain AN, Salgia R. Downstream signalling and specific inhibition of c-MET/HGF pathway in small cell lung cancer: implications for tumour invasion. Br J Cancer. 2007;97(3):368–377. doi:10.1038/sj.bjc.6603884
143. Sipeki S, Bander E, Buday L, et al. Phosphatidylinositol 3-kinase contributes to Erk1/Erk2 MAP kinase activation associated with hepatocyte growth factor-induced cell scattering. Cell Signal. 1999;11(12):885–890. doi:10.1016/S0898-6568(99)00060-1
144. Kuo TC, Chen CK, Hua KT, et al. Glutaminase 2 stabilizes Dicer to repress Snail and metastasis in hepatocellular carcinoma cells. Cancer Lett. 2016;383(2):282–294. doi:10.1016/j.canlet.2016.10.012
145. Yan X, Zhang D, Wu W, et al. Mesenchymal stem cells promote hepatocarcinogenesis via lncRNA-MUF Interaction with ANXA2 and miR-34a. Cancer Res. 2017;77(23):6704–6716. doi:10.1158/0008-5472.Can-17-1915
146. Tian YW, Shen Q, Jiang QF, Wang YX, Li K, Xue HZ. Decreased levels of miR-34a and miR-217 act as predictor biomarkers of aggressive progression and poor prognosis in hepatocellular carcinoma. Minerva Med. 2017;108(2):108–113. doi:10.23736/s0026-4806.16.04616-4
147. Xiang ZL, Zhao XM, Zhang L, et al. MicroRNA-34a expression levels in serum and intratumoral tissue can predict bone metastasis in patients with hepatocellular carcinoma. Oncotarget. 2016;7(52):87246–87256. doi:10.18632/oncotarget.13531
148. Yang F, Li QJ, Gong ZB, et al. MicroRNA-34a targets Bcl-2 and sensitizes human hepatocellular carcinoma cells to sorafenib treatment. Technol Cancer Res Treat. 2014;13(1):77–86. doi:10.7785/tcrt.2012.500364
149. Zhao J, Kelnar K, Bader AG. In-depth analysis shows synergy between erlotinib and miR-34a. PLoS One. 2014;9(2):e89105. doi:10.1371/journal.pone.0089105
150. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039–1049. doi:10.1056/NEJMra1404198
151. Egawa S, Toma H, Ohigashi H, et al. Japan pancreatic cancer registry; 30th year anniversary: Japan pancreas society. Pancreas. 2012;41(7):985–992. doi:10.1097/MPA.0b013e318258055c
152. Luo J, Xiao L, Wu C, Zheng Y, Zhao N. The incidence and survival rate of population-based pancreatic cancer patients: Shanghai cancer registry 2004-2009. PLoS One. 2013;8(10):e76052. doi:10.1371/journal.pone.0076052
153. Long LM, Zhan JK, Wang HQ, Li S, Chen YY, Liu YS. The clinical significance of miR-34a in pancreatic ductal carcinoma and associated molecular and cellular mechanisms. Pathobiology. 2017;84(1):38–48. doi:10.1159/000447302
154. Sun Z, Zhang B, Cui T. Long non-coding RNA XIST exerts oncogenic functions in pancreatic cancer via miR-34a-5p. Oncol Rep. 2018;39(4):1591–1600. doi:10.3892/or.2018.6245
155. Alemar B, Izetti P, Gregorio C, et al. miRNA-21 and miRNA-34a are potential minimally invasive biomarkers for the diagnosis of pancreatic ductal adenocarcinoma. Pancreas. 2016;45(1):84–92. doi:10.1097/mpa.0000000000000383
156. Donahue TR, Tran LM, Hill R, et al. Integrative survival-based molecular profiling of human pancreatic cancer. Clin Cancer Res. 2012;18(5):1352–1363. doi:10.1158/1078-0432.Ccr-11-1539
157. Sanati S, Watson MA, Salavaggione AL, Humphrey PA. Gene expression profiles of ductal versus acinar adenocarcinoma of the prostate. Mod Pathol. 2009;22(10):1273–1279. doi:10.1038/modpathol.2009.103
158. Kent OA, Mullendore M, Wentzel EA, et al. A resource for analysis of microRNA expression and function in pancreatic ductal adenocarcinoma cells. Cancer Biol Ther. 2009;8(21):2013–2024. doi:10.4161/cbt.8.21.9685
159. Xia J, Duan Q, Ahmad A, et al. Genistein inhibits cell growth and induces apoptosis through up-regulation of miR-34a in pancreatic cancer cells. Curr Drug Targets. 2012;13(14):1750–1756. doi:10.2174/138945012804545597
160. Tang Y, Tang Y, Cheng YS. miR-34a inhibits pancreatic cancer progression through Snail1-mediated epithelial-mesenchymal transition and the Notch signaling pathway. Sci Rep. 2017;7(1):38232. doi:10.1038/srep38232
161. Lin Y, Li XY, Willis AL, Liu C, Chen G, Weiss SJ. Snail1-dependent control of embryonic stem cell pluripotency and lineage commitment. Nat Commun. 2014;5(1):3070. doi:10.1038/ncomms4070
162. Grande MT, Sanchez-Laorden B, Lopez-Blau C, et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med. 2015;21(9):989–997. doi:10.1038/nm.3901
163. Nalls D, Tang SN, Rodova M, Srivastava RK, Shankar S. Targeting epigenetic regulation of miR-34a for treatment of pancreatic cancer by inhibition of pancreatic cancer stem cells. PLoS One. 2011;6(8):e24099. doi:10.1371/journal.pone.0024099
164. Vogt M, Munding J, Gruner M, et al. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch. 2011;458(3):313–322. doi:10.1007/s00428-010-1030-5
165. Akamatsu M, Makino N, Ikeda Y, et al. Specific MAPK-associated MicroRNAs in serum differentiate pancreatic cancer from autoimmune pancreatitis. PLoS One. 2016;11(7):e0158669. doi:10.1371/journal.pone.0158669
166. Pramanik D, Campbell NR, Karikari C, et al. Restitution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol Cancer Ther. 2011;10(8):1470–1480. doi:10.1158/1535-7163.Mct-11-0152
167. Feng SD, Mao Z, Liu C, et al. Simultaneous overexpression of miR-126 and miR-34a induces a superior antitumor efficacy in pancreatic adenocarcinoma. Onco Targets Ther. 2017;10:5591–5604. doi:10.2147/ott.S149632
168. Gibori H, Eliyahu S, Krivitsky A, et al. Amphiphilic nanocarrier-induced modulation of PLK1 and miR-34a leads to improved therapeutic response in pancreatic cancer. Nat Commun. 2018;9(1):16. doi:10.1038/s41467-017-02283-9
169. Yang G, Zhang L, Li R, Wang L. The role of microRNAs in gallbladder cancer. Mol Clin Oncol. 2016;5(1):7–13. doi:10.3892/mco.2016.905
170. Sharma A, Sharma KL, Gupta A, Yadav A, Kumar A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: recent update. World J Gastroenterol. 2017;23(22):3978–3998. doi:10.3748/wjg.v23.i22.3978
171. Lazcano-Ponce EC, Miquel JF, Munoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51(6):349–364. doi:10.3322/canjclin.51.6.349
172. Chandra V, Kim JJ, Mittal B, Rai R. MicroRNA aberrations: an emerging field for gallbladder cancer management. World J Gastroenterol. 2016;22(5):1787–1799. doi:10.3748/wjg.v22.i5.1787
173. Jin K, Xiang Y, Tang J, et al. miR-34 is associated with poor prognosis of patients with gallbladder cancer through regulating telomere length in tumor stem cells. Tumour Biol. 2014;35(2):1503–1510. doi:10.1007/s13277-013-1207-z
174. Bader AG. miR-34 - a microRNA replacement therapy is headed to the clinic. Front Genet. 2012;3:120. doi:10.3389/fgene.2012.00120
175. Di Martino MT, Leone E, Amodio N, et al. Synthetic miR-34a mimics as a novel therapeutic agent for multiple myeloma: in vitro and in vivo evidence. Clin Cancer Res. 2012;18(22):6260–6270. doi:10.1158/1078-0432.CCR-12-1708
176. Cortez MA, Ivan C, Valdecanas D, et al. PDL1 Regulation by p53 via miR-34. J Natl Cancer Inst. 2016;108(1):1. doi:10.1093/jnci/djv303
177. Badea L, Herlea V, Dima SO, Dumitrascu T, Popescu I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterol. 2008;55(88):2016–2027.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
