Back to Journals » Journal of Inflammation Research » Volume 15
A Systemic Inflammation Response Score for Prognostic Prediction in Hepatocellular Carcinoma Patients After Hepatectomy
Authors Zhang D , Huo L, Pan Y , Yang Z, Zeng H, Wang X, Chen J , Wang J, Zhang Y , Zhou Z, Chen M, Hu D
Received 13 November 2022
Accepted for publication 21 December 2022
Published 29 December 2022 Volume 2022:15 Pages 6869—6881
DOI https://doi.org/10.2147/JIR.S397375
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Deyao Zhang,1,2,* Lanqing Huo,1,3,* Yangxun Pan,1,2,* Zhenyun Yang,1,2,* Huilan Zeng,1,2 Xin Wang,1,2 Jinbin Chen,1,2 Juncheng Wang,1,2 Yaojun Zhang,1,2 Zhongguo Zhou,1,2 Minshan Chen,1,2 Dandan Hu1,2
1Collaborative Innovation Center for Cancer Medicine, State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center, Guangzhou, 510060, People’s Republic of China; 2Department of Liver Surgery, Sun Yat-sen University Cancer Center, Guangzhou, 510060, People’s Republic of China; 3Department of Radiation Oncology, Sun Yat-sen University Cancer Center, Guangzhou, 510060, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Minshan Chen; Dandan Hu, Sun Yat-sen University Cancer Center, 651 Dongfeng East Road, Guangzhou, 510000, People’s Republic of China, Tel +86 13902241061 ; +86 18676630499, Fax +86 8734-3115 ; +86 8734-3115, Email [email protected]; [email protected]
Purpose: To investigate the value of preoperative systemic inflammation response (SIRS) score in predicting the prognosis of hepatocellular carcinoma (HCC) after hepatectomy.
Patients and Methods: The study analyzed 1001 patients with pathologically proven HCC who received curative resection at Sun Yat-sen University Cancer Center between March 2016 and May 2020. Patients were randomly divided into a training cohort (n = 751) and a validation cohort (n = 250). Clinicopathological characteristics were collected retrospectively. The SIRS score formula was based on the results of a multivariate cox analysis of hematological inflammation indexes in the training cohort. Then, a nomogram consisting of the SIRS score was constructed and the calibration plot, areas under the receiver operating characteristic (AUC) curve, and decision curve analysis (DCA) showed good predictive ability.
Results: Univariate and multivariate cox analysis revealed that the SIRS score is an independent prognostic factor for OS in HCC patients. A higher SIRS score was associated with a larger maximum lesion diameter, poor tumor differentiation, a greater possibility of vascular invasion, and a more advanced cancer stage. When the nomogram was used to predict 1-year, 3-year, and 5-year survival rates, the AUC in the training cohort was 0.763, 0.712, and 0.687, respectively; In the validation cohort, it was 0.715, 0.648, and 0.614, respectively. The AUC of this nomogram showed significantly better predictive performance than those of commonly used staging systems.
Conclusion: The preoperative SIRS score has good efficacy in predicting the prognosis of HCC patients undergoing hepatectomy, and nomograms based on the SIRS score can potentially guide individualized follow-up and adjuvant therapy.
Keywords: liver cancer, systemic inflammation response score, hepatocellular carcinoma, HCC, preoperative, Nomogram, prognosis
Introduction
Liver cancer is one of the most common malignant tumors, and its incidence ranks sixth, and morality ranks third worldwide in 2020 among 185 countries.1 Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer and accounts for 90% of all cases.2 The Barcelona Clinic Liver Cancer (BCLC) stage is the most widely used staging system that guides therapeutic strategy in clinical settings.3 Despite recent progress in targeted therapy and immunotherapy in HCC, surgical resection remains one of the most important curative treatment strategies. However, due to the underlying heterogeneity, patients with the same BCLC stage receive similar surgery but have different prognoses. It is essential to carry out more precise preoperative assessments of HCC patients. As more and more attention has been paid to precision medicine, liquid biopsy4 like circulating tumor DNA (ctDNA) detection5 and circulating cell-free DNA (cfDNA) detection6 has been used for the stratification of the prognosis of HCC patients. Although improving the accuracy of prediction, the high costs of these emerging technologies make their use limited. Therefore, it is of great importance to develop simple, inexpensive, reliable indicators for predicting prognosis in HCC patients.
Inflammation plays a key role in the tumor microenvironment, and it is considered to be the seventh “hallmark” of cancer.7,8 It is widely accepted that systemic inflammatory responses reflect the secretion of angiogenic, DNA damage, and tumor invasion through the up-regulation of cytokines.9,10 Chronic hepatitis B is the leading cause of morbidity and mortality of liver cancer in Asia,11 and long-term exposure to HBV infection induced liver inflammation, fibrogenesis, and oxidative stress resulting in cirrhosis and even to liver cirrhosis.12 In addition, inflammation-related indicators such as C-reactive protein,13 platelet lymphocyte ratio (PLR),14 neutrophil-lymphocyte ratio (NLR),15 lymphocyte to monocyte ratio (LMR),16 and systemic immune-inflammation index (SII)17 have been reported to be independent prognostic factors for HCC, but the underlying mechanism is very complex. Using a single existing inflammation indicator may raise the risk of bias, and using a combined model could reduce the potential error. Nevertheless, there were few reports about a comprehensive analysis of these inflammation indices in HCC patients. Therefore, this study aims to evaluate the prognostic value of systemic inflammation response score (SIRS) as an independent factor to integrate all significant inflammation indices and build a simple, inexpensive, and reliable prediction model in HCC patients.
Materials and Methods
Patients
We retrospectively analyzed 1001 HCC patients who received liver resection at Sun Yat-sen University Cancer Center between March 2016 and May 2020.
The inclusion criteria were as follows: (1) pathologically diagnosed as hepatocellular carcinoma (HCC), (2) naive treatment, (3) liver function Child-Pugh A or B, (4) Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, (5) received curative resection.
The exclusion criteria included (1) pathologically confirmed ICC or combined hepatocellular and intrahepatic cholangiocarcinoma, (2) other synchronous malignancies, (3) patients with distant metastasis (eg, lung, bone), (4) posttreatment survival time of less than 1 month, (5) lack of a follow-up assessment, (6) liver function Child-Pugh C, (7) received targeted therapy, immunotherapy, transarterial chemoembolization (TACE), or hepatic arterial infusion chemotherapy (HAIC) before surgery.
All enrolled patients were randomly separated into a training cohort or validation cohort in a 3:1 ratio.
Data Collection and Cut-Off
The lab test data were collected within three days before surgery. Serum samples were collected and clotted at room temperature, then centrifuged at 3500r/min for 10 min, which could be used to estimate the level of serum biomarkers. Albumin concentrations were measured using a Roche Cobas 702 Automatic Biochemical Analyzer. Albumin and total proteins were measured using colorimetry. Neutrophils, lymphocytes, monocytes, and platelet counts were evaluated using the XN-2000 automated hematology analyzer. The coefficient of variation of the two tests in our laboratory is ≤5%. Child-Pugh classifications and Barcelona Clinic Liver Cancer (BCLC) staging were calculated accordingly. Tumor grade, tumor number, and vascular invasion were recorded as described in the pathology report. The size of the tumor was recorded as the longest diameter on pathological size. The platelet-lymphocyte ratio (PLR), neutrophil-lymphocyte ratio (NLR), platelet-monocyte ratio (PMR), neutrophil-monocyte ratio (NMR), lymphocyte-monocyte ratio (LMR), lymphocyte-CRP ratio (LCR), neutrophil-albumin ratio (NAR), monocyte-albumin ratio (MAR), platelet-albumin ratio (PAR), CRP-albumin ratio (CAR), neutrophil×monocyte (N×M), neutrophil×platelet (N×P), neutrophil ×CRP (N×C), monocyte×platelet (M×P), monocyte×CRP (M×C), platelet×CRP (P×C), and lymphocyte×albumin (L×A) were calculated as follows: PLR=P/L; NLR=N/L; PMR=P/M; NMR=N/M; LMR=L/M; LCR=L/C; NAR=N/A; MAR=M/A; PAR=P/A; CAR=C/A; N×M=N*M; N×P=N*P; N×C=N*C; M×P=M*P; M×C=M*C; P×C=P*C; and L×A=L*A, where P, N, L, M, C, A represent the platelet (109/L) counts, neutrophil (109/L) counts, lymphocyte (109/L) counts, monocyte (109/L) counts, c-reactive protein (mg/L) counts, and albumin (g/L) counts, respectively, and continuous variables were converted to categorical variables. The best cut-off value for laboratory variables was identified using the surv_cutpoint function of survminer using overall survival as the target variable (Supplemental Figure 1). The cut-off values were as follows: ALB (45.9 g/L), C-reactive protein (CRP) (5.78 mg/L), PLR (106.03), NLR (1.30), PMR (357.14), NMR (10.94), LMR (3.27), LCR (0.25), NAR (0.09), MAR (0.01), PAR (4.84), CAR (0.13), N×M (1.36), N×P (1006.72), N×C (20.41), M×P (152.50), M×C (1.92), P×C (1162.04), and L×A (73.95).
Follow-Up and Data
Patients were followed every 3 months up to 2 years and every 6 months until year 5. Patients needed to receive radiological examinations such as dynamic computed tomography (CT) scans, magnetic resonance imaging (MRI), and chest radiography on time. Laboratory tests included blood routine, liver function, and liver tumor markers. Overall survival (OS) was calculated from the date of surgery to the date of death or the last follow-up time. The last follow-up was conducted in November 2022.
Statistical Analysis
We performed all Statistical analyses with SPSS 24.0 software version 26.0 (IBM Corporation, Armonk, NY, USA) and R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org). Two-tailed t-tests, ANOVA tests, or Spearman correlation tests were conducted for continuous variables and chi-squared tests were used for categorical variables. According to the cut-off value, we defined the result of hematological indexes below the cut-off value as 0 and above the cut-off value as 1. Univariate and multivariate cox regression analyses were performed to find prognostic indicators of OS. Three hematological indexes (PLR, NLR, and M×C) for SIRS were significantly associated with OS. The SIRS score of each patient was calculated according to the following formula: SIRS score =sum (each status of hematological index *corresponding regression coefficient). Patients were divided into SIRS-low and SIRS-high groups according to the median value of the SIRS score. And nomogram was constructed to predict OS at 1, 3, and 5 years. The performance characteristics of the predictive nomogram were evaluated by calibration plots. Receiver operating characteristics (ROC curves), decision-curve analysis (DCA), and the area under the curve (ACU) were calculated to analyze the predictive accuracy of the nomogram. The test level (alpha) was set at 0.05, and all tests were two-tailed.
Results
Clinical-Pathological Characteristics
A total of 1001 HCC patients who underwent hepatectomy were enrolled in this study. Patients were randomly separated into a training (n = 751) or validation cohort (n = 250) in a 3:1 ratio. There were no significant differences in terms of baseline clinicopathological characteristics between the training and validation groups. (Table 1). The training cohort was used to build the SIRS formula and prognostic model, and the validation cohort was used to assess its accuracy. There were 859 males and 142 females with a median age of 55.8 ± 11. 9 years in this study; The proportion of HBsAg-positive patients was 72.5%, and that of HCV-Ab was 2.3%; The majority of patients with tumor present were BCLC stage A (56.0%); The positive rates of alpha-fetoprotein (AFP), des-gamma-carboxyprothrombin (DCP), carcinoembryonic antigen (CEA), and cancer antigen 199 (CA19-9) were 55.1%, 71.7%, 12.5%, and 22.9% respectively.
 |
Table 1 Baseline Clinicopathological Characteristics of the Patients |
The Construction of Systemic Inflammation Score and Its Relationship with OS
In addition, we assessed the prognostic power of ALB, CRP, PLR, NLR, PMR, NMR, LMR, LCR, NAR, MAR, PAR, CAR, N×M, N×P, N×C, M×P, M×C, P×C, and L×A in 1001 patients, with a purpose to identify the association between patients’ survival and systemic inflammation score. The univariate cox analysis demonstrated that evaluated CRP, NLR, CAR, N×C, M×C, and P×C levels (p < 0.05), along with reduced PLR, LCR, PAR, N×P, and M×P (p < 0.05) were associated with poor outcome (Figure 1A). However, multivariate cox analysis showed that only PLR, NLR, and M×C were independent prognostic hematological factors for OS in HCC patients (p < 0.05) (Figure 1B). Thus, the SIRS score of each patient was based on three hematological indexes and their corresponding regression coefficient in the training group. Eventually, the SIRS score of each patient is calculated as the following formula: SIRS = −0.696*PLR + 0.660*NLR + 0.944*M×C (Figure 1C). The median of the SIRS score (median = 0.248) was defined as the demarcation value, the score of patients less than 0.248 was defined as the SIRS-low group, and those greater than 0.248 were defined as the SIRS-high group. Then a Kaplan-Meier analysis was performed (Figure 1D and E). Both in the training and validation cohort, patients in the SIRS-high group had a worse prognosis compared with patients in the SIRS-low group (p < 0.001).
SIRS Score is an Independent Prognostic Factor for OS
To further explore whether the SIRS score is an independent prognostic factor, the clinical characteristics variables and SIRS score were included in a univariate and multivariate Cox regression for OS. The univariate analyses showed that CRP, SIRS, Tumor size, AFP, CA19-9, DCP, Grade, Vascular invasion, and Child-Pugh score were associated with OS (p < 0.05). In the multivariate study, we demonstrated that SIRS (hazard ratio [HR] = 1.806, 95% confidence interval [CI]:1.268–2.573; p = 0.001), Tumor size (HR = 1.604, 95% CI: 1.145–2.246; p = 0.005), CA19-9 (HR = 1.625, 95% CI:1.181–2.236; p = 0.003), Grade (HR = 1.754, 95% CI:1.269–2.424; p = 0.001), and Vascular invasion (HR = 2.468, 95% CI:1.376–4.423; p = 0.001) were independent factors of OS (Table 2). These results suggested that SIRS remained a reliable prognostic factor in HCC patients after adjustment for other well-known clinicopathologic prognostic factors.
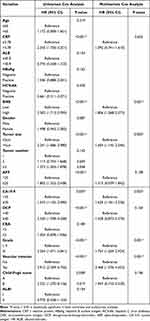 |
Table 2 Results of the Univariate Cox Regression Analysis and Multivariate Cox Regression Analysis for OS Among the Clinical Characteristics and SIRS |
The Association Between SIRS and the Clinical Characteristics
To understand the relationships between the clinical characteristics and SIRS, we took a further analysis (Table 3). The result showed that compared with patients in SIRS low group, patients in SIRS high group were more likely to have larger maximum lesion diameter (43.4% vs 27.9%, p < 0.001), poor tumor differentiation (46.7% vs 39.4%, p = 0.047), more proportion of vascular invasion (3.7% vs 1.7%, p = 0.040). Thus, the cancer stage was more advanced in the SIRS high group than in the SIRS low group (p = 0.023), which also explained the poorer prognosis of patients in the SIRS high group.
 |
Table 3 Relationship Between SIRS and Clinical Characteristics |
Nomogram Construction and Validation
We constructed a nomogram (Figure 2A) to quantify the results of multivariate cox regression in the training cohort. Vascular invasion, the variable with the largest absolute coefficient value, was set as a reference whose scale range ranged from 0 to 100 points. Compared to other variables, the risk point scores of high SIRS made up a high proportion of the nomogram (85 points). After summing up the total point score and locating it on the total point scale, a corresponding probability of the 1-year, 3-year, and 5-year survival rates was calculated for each individual. The calibration plot showed that the nomogram was well calibrated in the training cohort with bootstrap sampling (n = 679) and validation cohort (n = 227) (Figure 2B–G). The accuracy of the predicted nomogram was analyzed by the time-dependent AUC curves analysis (Figure 3). When the nomogram was used to predict 1-year, 3-year, and 5-year survival rates, the AUC in the training cohort was 0.763, 0.712, and 0.687, respectively (Figure 3A); In the validation cohort, it was 0.715, 0.648, and 0.614, respectively (Figure 3B). Then, ROC analyses also showed that in both the training and validation cohort, this model predicts a maximum AUC value compared with the SIRS, Tumor size, CA19-9, Tumor grade, Vascular invasion, and BCLC stage (Figure 3C–E). Finally, we plotted DCA curves to illustrate the discriminating superiority of nomograms with respect to 1-, 3-, and 5-year OS in the training cohort and validation cohort (Figure 4A and B).
 |
Figure 4 Decision-curve analysis (DCA) plot depicting the standardized net benefit. (A). Training cohort at 12, 36, and 60 months. (B). Validation cohort at 12, 36, and 60 months. |
Discussion
HCC is one of the most common malignant tumors with rising incidence and mortality rates around the world. The treatment for HCC differs based on the disease stage. Although the BCLC stage system has been the most widely used prediction and treatment staging system,18 there is still some controversy over the treatment algorithm, particularly in Asia.3 The reason can be sketched as follows. First, the BCLC stage system is established based on western patients, while the pathogenesis factors for HCC are different in Western and Eastern countries. Alcoholic liver disease is the main cause of liver cancer in Western countries,19 while hepatitis B virus (HBV) infection is the main cause of HCC in Eastern countries.11 In addition, liver cancer is also an inflammation-related disease, inflammation also plays an important role in the occurrence, invasion, and metastasis of liver cancer.20 In addition, the BCLC staging system pays more attention to the impact of tumor load on the prognosis of patients, and little attention has been placed on inflammation. Thus, we design this research to investigate whether the SIRS score could provide additional prognostic information for a traditional clinical staging system.
As shown in Table 1, lab tests of 1001 HCC patients were recorded as indicators of systemic inflammation. Then, univariate and multivariate analysis indicated that the SIRS score based on PLR, NLR, and M×C was an independent prognostic factor for HCC. Thus, a nomogram based on the results of multivariate cox analysis was successfully established. As shown in Figure 2, all the variables were calculated. This nomogram showed good calibration in the training and validation sets, as illustrated by calibration curves. At last, the ROC analysis was plotted (Figure 3). We found that the nomogram model had a better ability in predicting 1, 3, and 5-year survival in both cohorts in comparison with the BCLC stage system.
Indeed, systemic inflammation has been proven to correlate with prognosis in a variety of tumors.21 In our research, we found that the combination of preoperative platelet counts, neutrophil counts, lymphocyte counts, monocyte counts, and CRP levels were independent prognostic factors. Many recent studies have reported that neutrophils could stimulate tumor angiogenesis, stimulate tumor-cell proliferation, and promote tumor metastasis.22,23 Platelets, can promote angiogenesis,24 and, release plate-derived growth factor (PDGF) and transforming growth factor beta (TGF-β), which promote tumor cell proliferation and suppress apoptosis.25,26 Without a doubt, T lymphocytes are the main effectors of tumor cell destruction in many types of cancer,27,28 and decreased lymphocytic infiltration was reported to be associated with poor prognosis.29 In addition, tumor-associated macrophages (TAMs) were also reported to contribute to HCC progression.30 CRP is one of the acute phase reactants secreted by the liver, and its secretion was associated with the induction of inflammation-associated cytokines like IL-1, IL-6, or TNF-α.31 Sieghart et al reported that elevated CRP at diagnosis was associated with poor OS in HCC patients.32 However, the detailed mechanism of platelet, neutrophil, lymphocyte, monocyte, and CRP still warrants further study.
This nomogram was built with HCC patients whose survival outcomes were already known, and we aim to provide additional prognostic information over the current gold standard of patient prognosis prediction, the BCLC stage system. However, we acknowledge there are some limitations to our study. This is a single-center retrospective study investigating the SIRS score in HCC patients. With the development of individualized treatment, the diagnosis and treatment method of HCC may differ, and there could be some differences depending on the operator. In addition, the main reason why the prediction value of 5-year survival is not as accurate as the value of 1-year is that subsequent treatment modalities after recurrence may differ. So, we plan to enlarge our cohort and design a multicenter study further to analyze a more accurate clinical prediction model.
Conclusions
In conclusion, the preoperative SIRS score has good efficacy in predicting the prognosis of patients undergoing hepatectomy, and nomograms based on the SIRS score can potentially guide individualized follow-up and adjuvant therapy.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Ethics Approval and Informed Consent
Patient informed consent is exempt because the study used retrospective clinical data that were obtained after each patient agreed to treatment. Individuals cannot be identified based on the data presented. We declare to ensure the confidentiality of patient data. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethical Committee of Sun Yat-Sen University Cancer Center University (RDDA2020000350).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception and design, data acquisition, or data analysis and interpretation, participated in the drafting of the article or critically revising it for important intellectual content, agreed to submit to the current journal, gave final approval for the version to be published, and agreed to be accountable for all aspects of the work.
Funding
This work is funded by the National Natural Science Foundation of China (No. 81874070, 82103566).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Hindupur SK, Colombi M, Fuhs SR, et al. The protein histidine phosphatase LHPP is a tumour suppressor. Nature. 2018;555(7698):678–682. doi:10.1038/nature26140
3. Dhanasekaran R, Venkatesh SK, Torbenson MS, Roberts LR. Clinical implications of basic research in hepatocellular carcinoma. J Hepatol. 2016;64(3):736–745. doi:10.1016/j.jhep.2015.09.008
4. Yang M, Forbes ME, Bitting RL, et al. Incorporating blood-based liquid biopsy information into cancer staging: time for a TNMB system? Ann Oncol. 2018;29(2):311–323. doi:10.1093/annonc/mdx766
5. Xu RH, Wei W, Krawczyk M, et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat Mater. 2017;16(11):1155–1161. doi:10.1038/nmat4997
6. Zhang X, Wang Z, Tang W, et al. Ultrasensitive and affordable assay for early detection of primary liver cancer using plasma cell-free DNA fragmentomics. Hepatology. 2022;76(2):317–329. doi:10.1002/hep.32308
7. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
8. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. doi:10.1158/2159-8290.CD-21-1059
9. Marisi G, Cucchetti A, Ulivi P, et al. Ten years of sorafenib in hepatocellular carcinoma: are there any predictive and/or prognostic markers? World J Gastroenterol. 2018;24(36):4152–4163. doi:10.3748/wjg.v24.i36.4152
10. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. doi:10.1016/S1470-2045(14)70263-3
11. Bollinger RC, Thio CL, Sulkowski MS, McKenzie-White J, Thomas DL, Flexner C. Addressing the global burden of hepatitis B virus while developing long-acting injectables for the prevention and treatment of HIV. Lancet HIV. 2020;7(6):e443–e448. doi:10.1016/S2352-3018(19)30342-X
12. Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13(2):123–135. doi:10.1038/nrc3449
13. Suner A, Carr BI, Akkiz H, et al. Inflammatory markers C-reactive protein and PLR in relation to HCC characteristics. J Transl Res. 2019;5(3). doi:10.15761/JTS.1000260
14. Lin WF, Zhong MF, Zhang YR, et al. Prognostic role of platelet-to-lymphocyte ratio in hepatocellular carcinoma with different BCLC stages: a systematic review and meta-analysis. Gastroenterol Res Pract. 2018;2018:5670949. doi:10.1155/2018/5670949
15. Wang D, Bai N, Hu X, et al. Preoperative inflammatory markers of NLR and PLR as indicators of poor prognosis in resectable HCC. PeerJ. 2019;7:e7132. doi:10.7717/peerj.7132
16. Nouri-Vaskeh M, Mirza-Aghazadeh-Attari M, Pashazadeh F, et al. Prognostic impact of monocyte to lymphocyte ratio in clinical outcome of patients with hepatocellular carcinoma: a systematic review and meta-analysis. Galen Med J. 2020;9:e1948. doi:10.31661/gmj.v9i0.1948
17. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi:10.1158/1078-0432.CCR-14-0442
18. Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi:10.21037/hbsn-20-480
19. Avila MA, Dufour JF, Gerbes AL, et al. Recent advances in alcohol-related liver disease (ALD): summary of a gut round table meeting. Gut. 2020;69(4):764–780. doi:10.1136/gutjnl-2019-319720
20. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi:10.1016/j.cell.2010.01.025
21. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi:10.1038/nature10673
22. Cools-Lartigue J, Spicer J, McDonald B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123(8):3446–3458. doi:10.1172/JCI67484
23. Berkow RL, Wang D, Larrick JW, Dodson RW, Howard TH. Enhancement of neutrophil superoxide production by preincubation with recombinant human tumor necrosis factor. J Immunol. 1987;139(11):3783–3791.
24. Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. doi:10.1038/nrc3004
25. Yeung J, Li W, Holinstat M. Platelet signaling and disease: targeted therapy for thrombosis and other related diseases. Pharmacol Rev. 2018;70(3):526–548. doi:10.1124/pr.117.014530
26. Mezouar S, Mege D, Darbousset R, et al. Involvement of platelet-derived microparticles in tumor progression and thrombosis. Semin Oncol. 2014;41(3):346–358. doi:10.1053/j.seminoncol.2014.04.010
27. Escors D, Gato-Cañas M, Zuazo M, et al. The intracellular signalosome of PD-L1 in cancer cells. Signal Transduct Target Ther. 2018;3:26. doi:10.1038/s41392-018-0022-9
28. Zheng C, Zheng L, Yoo JK, et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell. 2017;169(7):1342–1356.e1316. doi:10.1016/j.cell.2017.05.035
29. Liu T, Tan J, Wu M, et al. High-affinity neoantigens correlate with better prognosis and trigger potent antihepatocellular carcinoma (HCC) activity by activating CD39(+)CD8(+) T cells. Gut. 2021;70(10):1965–1977. doi:10.1136/gutjnl-2020-322196
30. Bao D, Zhao J, Zhou X, et al. Mitochondrial fission-induced mtDNA stress promotes tumor-associated macrophage infiltration and HCC progression. Oncogene. 2019;38(25):5007–5020. doi:10.1038/s41388-019-0772-z
31. Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140(2):197–208. doi:10.1016/j.cell.2009.12.052
32. Sieghart W, Pinter M, Hucke F, et al. Single determination of C-reactive protein at the time of diagnosis predicts long-term outcome of patients with hepatocellular carcinoma. Hepatology. 2013;57(6):2224–2234. doi:10.1002/hep.26057
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



