Back to Journals » Research and Reports in Urology » Volume 12
A Systematic Review on the Investigation of SARS-CoV-2 in Semen
Authors Gonzalez DC , Khodamoradi K, Pai R, Guarch K, Connelly ZM , Ibrahim E, Arora H, Ramasamy R
Received 21 September 2020
Accepted for publication 9 November 2020
Published 1 December 2020 Volume 2020:12 Pages 615—621
DOI https://doi.org/10.2147/RRU.S277679
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jan Colli
Daniel C Gonzalez,1 Kajal Khodamoradi,1 Raghav Pai,1 Kristopher Guarch,2 Zachary M Connelly,3 Emad Ibrahim,1 Himanshu Arora,1 Ranjith Ramasamy1
1Department of Urology, Miller School of Medicine, University of Miami, Miami, FL 33136, USA; 2Florida International University, Miami, FL 33199, USA; 3Louisiana State University Health Shreveport, Shreveport, LA 71103, USA; 4The Interdisciplinary Stem Cell Institute, University of Miami, Miller School of Medicine, Miami, FL 33136, USA
Correspondence: Ranjith Ramasamy
Department of Urology, Miller School of Medicine, University of Miami, 1120 NW 14th St, #1551, Miami, FL 33136, USA
Tel +1 (305)-243-7200
Email [email protected]
Background: Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a virus that is present in most bodily fluids. However, whether SARS-CoV-2 is present in the semen remains underexplored. Thus, we systematically reviewed the existing studies on the presence of SARS-CoV-2 in semen.
Methods: A literature search of the PubMed, Embase, Cochrane, Web of Science, Google Scholar, and Ovid databases was performed for articles from the dates of their inception to August 2020 using the following keywords: COVID-19, SARS-CoV2, seminal, semen, and sperm. After excluding non-human studies and articles that were not in the English language, we identified 19 relevant studies. The full text of the articles were reviewed and a total of eight articles remained after applying our selection criteria.
Results: After reviewing the presence of SARS-CoV-2 in the eight different studies using semen samples, only one reported the presence of the virus. Six out of 160 total semen samples with SARS-CoV-2 positive demonstrated the presence of viral RNA, of which 2 were from males in the recovery phase and 4 from the acute phase of the infection.
Conclusion: The novel nature of SARS-CoV-2 has limited the number and size of studies on semen. Nevertheless, the current literature, while limited, has confirmed the presence of SARS-CoV-2 in semen in one out of the eight reported studies and totaling 4.3% of the population screened. Taken together, the risk of the presence of SARS-CoV-2 in semen appears to be extremely low and likely negligible in recovered men. Future studies need to focus on whether complete viral particles can be seen in semen and the possibility of sexual transmission.
Keywords: COVID-19, coronavirus, semen, semen parameters
Introduction
As severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a novel virus strain, the impact on male reproduction is one of the many aspects that are still unknown. Notably, SARS-CoV-2 has been detected in respiratory fluids, saliva, gastrointestinal tract samples, blood, feces, and urine.1–3 This suggests that infection routes other than respiratory droplets are theoretically possible. The main entry point of SARS-CoV-2 appears to be mediated by its glycoprotein spikes (S protein), which requires priming via TMPRSS2 (transmembrane protease serine 2) to facilitate viral and cellular membrane fusion.4 Angiotensin-converting enzyme 2 (ACE2) protein has been identified as the viral receptor. In particular, within the testis, ACE2 expression has been documented on seminiferous ducts cells, as well as on spermatogonia, Leydig, and Sertoli cells. ACE2 receptor expression in testicular tissue, as well as TMPRSS2 in prostate epithelial cells, has fostered a curiosity about the possible implications for viral shedding into semen.5–7
Many viruses have been isolated in semen, including Mumps, Zika, Ebola, and Cytomegalovirus. Therefore, it is tempting to speculate whether SARS-CoV-2 is present in semen and if there is potential sexual transmission.8 The broad range of different virus families lends evidence that viral spread into the reproductive tract may be associated with a high viral load in blood, as is the case with SARS-CoV and the possibility of causing orchitis.9 While such an occurrence has not yet been reported for the current SARS-CoV-2, it notably shares 80% sequence homology with SARS-CoV.10 Evidence regarding viral seeding and viral entry into the cells of the male reproductive tract and semen after SARS–CoV-2 infection is not well understood, and mixed results have been reported.11–13 Despite these important findings, this topic has not yet been the subject of a systematic review, and we believe that such a review is warranted as it will help to better elucidate the presence of SARS-CoV-2 in semen. Herein, the aim of this study was to systematically review the available data on the transmission of SARS-CoV-2 in semen and results in sexual transmission.
Materials and Methods
The current systematic review was designed and conducted according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.14 A literature search of PubMed, Embase, Cochrane, Web of Science, Google Scholar and Ovid was conducted by two independent authors (D.G. and R.P). The literature search was limited to English publications or publications translated into English. The search was restricted to related articles from December 1, 2020, to August 30, 2020.
To review the presence of SARS-CoV-2 in the semen, the following search strategy was implemented using these keywords: (“COVID 19” OR “coronavirus” OR “SARS-CoV2” OR “severe acute respiratory syndrome-coronavirus 2” OR “ severe acute respiratory syndrome coronavirus 2” OR SARS CoV2”) AND (“semen” OR “seminal” OR “sperm”). Both authors independently screened titles and abstracts which appeared relevant to the topic with respect to the inclusion criteria. The full text of eligible articles was obtained and evaluated by each reviewer independently, and disagreement was resolved by discussion. Authors were not blind to journal names, authors, or institutions. All editorials, case reports, reviews, systematic reviews, in vitro animal studies, or material not directly related to the topic were excluded. To further ensure a comprehensive search, authors manually scanned the references of the included articles and suitability was determined. Articles were selected if they met any of the following inclusion criteria: conducted in human, identification of SARS-CoV-2 in semen, discussion of SARS-CoV-2 in semen, or discussion of sexual transmission. Two independent reviewers extracted data from the full-text papers of eligible studies, including the name of the first author, publication month and year, city and country of the study, whether patients were acutely infected or recovered, sample size, presence of virus in the semen, age of subjects, mean days until semen sample collected. A summary of the included studies is presented in Table 1.
 |
Table 1 Study Characteristics for SARS-CoV-2 Positive Individuals |
Results
Search Strategy Results
Our primary search identified a total number of 234 articles (including 73 duplicates). The title and abstract screening procedure resulted in the exclusion of publications for these reasons: irrelevant studies (n=92), editorial letter (n=16), and review articles (n= 34). The eligibility of 19 full texts was examined. After excluding papers, eight articles were finally included in the systematic review (Figure 1).
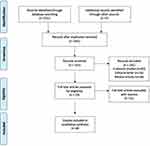 |
Figure 1 Flow diagram of literature search. |
Investigation of SARS-CoV-2 in Semen During the Acute Infection
Three studies encompassing a total of 35 patients investigated semen samples from infected patients during the acute phase of the disease.11–13 One study investigated and collected semen samples solely from acutely infected and hospitalized patients.11 Moreover, two other studies investigated samples from acutely infected and recovered patients.12,13 Two of the three studies involving a total of 20 patients with acute infection failed to detect the presence of SARS-CoV-2 viral RNA in semen samples.11,12 Although a study of 38 males by Li et al reported the detection of SARS-CoV-2 in semen samples in 6 out of 38 patients (15.8%).13
Li et al enrolled 38 patients with laboratory results for SARS-CoV-2 that were approved by positive results of SARS-CoV-2 in real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay of nasal and pharyngeal swabs in their study. They found the presence of SARS-CoV-2 in semen for 4 out of 15 patients (26.7%) in the acute stage of infection, and for 2 out of 23 patients (8.7%) in the recovery phase by RT-PCR. The median time to obtain a sample after the onset of symptoms was 10.5 days, with a range of 6 to 16 days.
A study by Kayaaslan et al investigated 16 male samples (average age 33.5 years) who were in the acute stage of the infection and hospitalized, demonstrating mild or moderate symptoms.11 Five of the 16 males had moderate disease, as classified by pneumonia or radiological evidence of ground glass opacities. The mean time to obtain a semen sample after a positive nasopharyngeal test was 1 day, with the latest provided at 7 days, and all 16 semen samples were negative for SARS-CoV-2 PCR (Figure 2).
 |
Figure 2 A scatter plot of study sample size over time (mean days) from diagnosis until semen sample collection. The size of each data point indicates the corresponding sample size. |
Holtmann et al reported their series of 18 men (average age 42.2 years), including 14 who had recovered from SARS-CoV-2 infection and 4 with acute moderate SARS-CoV-2 infection.12 Men who demonstrated a positive nasopharyngeal swab or Immunoglobulin (Ig) A and IgG antibodieswere defined as SARS-CoV-2 positive. The authors defined a moderate SARS-CoV-2 infection as males who required hospitalization with up to 6 L oxygen supplied to achieve >92% peripheral oxygenation. The mean time to obtain a semen sample after the end of symptoms was 32.7 days. No SARS-CoV-2 was detected by means of RT-PCR in the semen of both recovered and acutely infected SARS-CoV-2 men.
Investigation of SARS-CoV-2 in Semen During the Recovery Stage
Five studies investigated and collected semen samples from patients who recovered from a SARS-CoV-2 infection.15–20 Song et al tested for the presence of SARS-CoV-2 in the semen of 12 males within the recovery phase, defined as two consecutive negative quantitative real-time polymerase chain reactions (qRT-PCR).15 Detection of either SARS-CoV-2 RNA on pharyngeal swabs (qRT-PCR) or anti-2019-nCoV antibodies (both IgM and IgG) in serum (immunoassays) was considered to confirm a positive SARS-CoV-2 result. Semen samples were collected after a median 30 days from the confirmation of diagnosis (Figure 2). No SARS-CoV-2 RNA was detected in any of the semen samples.
In a cohort of 23 males with a recent infection or recovery from SARS-CoV-2, who their SARS-CoV-2 positive was confirmed by qRT–PCR amplification on pharyngeal swab specimens, Guo et al did not find the presence of SARS-CoV-2 in semen.16 Semen samples were collected after a median of 33.5 (IQR 27.5–33) days from the confirmation of diagnosis. The authors noted that in 12 males, SARS-CoV-2 was still present in the sputum and fecal specimens when the semen samples were collected, while in 11 patients the virus had been cleared.
Similarly, Pan et al tested a case series of 34 men who recovered from SARS-CoV-2 and a positive test was confirmed by qRT-PCR of pharyngeal swab samples. Their study confirmed the absence of the virus in all semen samples with qRT-PCR for viral amplification.17 The males were mostly affected by mild disease, and semen testing was performed on average at 31 (IQR 29–36) days from diagnosis.
Ma et al reported the absence of SARS-CoV-2 in the semen of 12 males (median age 31.5 years) who recovered from moderate SARS-CoV-2 and tested negative with nasopharyngeal PCR when samples were obtained.19 In all of 12 semen samples, SARS-CoV-2 was not found by qRT-PCR. The time between semen collection and disease onset ranged from 56 days to 109 days (with a median of 78.5 days).
Most recently, Rawlings et al tested a total of 6 males, aged between 28 and 45, and reported the absence of SARS-CoV-2 in all 6 semen samples.18 Paired saliva and semen samples were collected for a mean of 12 days, ranging from 6 to 17 days, after the onset of symptoms, and digital droplet-PCR was performed to quantify SARS-CoV-2 levels. Despite all males demonstrating viral shedding in oral secretions up to 792 copies/uL, all subjects displayed relatively mild symptoms.
In the above-aforementioned studies, there was no significant difference between negative and positive results for COVID-19 by age, urogenital disease history, days since onset, days since hospitalization, or days since the clinical recovery of patients.
Discussion
In this article, we have reviewed the presence of SARS-CoV-2 in semen within both recovered and acutely infected SARS-CoV-2 males. Of the eight studies on semen samples, one reported virus presence while the other seven studies denied it, raising doubts about SARS-CoV-2 presence in semen. Herein, we demonstrated that there have been 6 out of 160 total semen samples with SARS-CoV-2 present recorded thus far, of which 2 were from males in the recovery phase and 4 from the acute phase of the infection, suggesting that the chances of SARS-CoV-2 in semen are extremely low, especially among recovered men. It would be important to study this question among asymptomatic carriers where sexual transmission is most likely. Furthermore, if SARS-CoV-2 is present in semen, it would imperative to evaluate the duration that it is present.
The study by Li et al reported the detection of SARS-CoV-2 in semen samples in 6 out of 38 patients (15.8%), and to our knowledge, may be the only study reporting a positive result in semen.13 However, this study may have several noteworthy methodological limitations. The semen samples for SARS-CoV-2 were tested by qualitative RT-PCR, and thus neither the limits of detection nor the threshold values were described. Notably, the study did not describe which PCR primer was utilized to identify the SARS-CoV-2 RNA. Additionally, there lays heterogeneity with PCR primers utilized amongst the literature. While Song et al utilized RT‐qPCR or anti‐SARS‐CoV‐2 antibodies (both IgM and IgG) in serum by colloidal gold‐based immunoassays, Paoli et al in a case report utilized RT-PCR targeting E and S viral genes, which is different than the aforementioned authors.15,20 Perhaps to mitigate future methodological issues, the use of a quantitative PCR assay would be more useful to detect the virus as well as test its concentration in semen.
The results by Li et al need to be cautiously interpreted, as they focused on hospitalized patients with severe disease, from which the authors cited 12 comatose or dying subjects.13 A study by Chen et al reported that disease severity was related to viremia, and thus a higher chance to reach other organs and body fluids including the semen.21 Moreover, if samples are not taken in accordance with sterile conditions, the contamination of semen samples with the patients’ aerosol secretions may also cause a false-positive result.20 To avoid viral contamination from non-semen sources, semen samples should be obtained in accordance with the World Health Organization (WHO) guidelines. The process of semen sample collection includes passing urine, washing hands and penis with soap, drying hands and penis, and then ejaculating the semen by masturbation into a sterile and wide-mouthed container.22 The diversity among the results of the studies may be due to differences in disease stage or severity, differences in the timing of sample collection from symptom onset, as well as possible contamination of semen samples with aerosol or other body fluids from the patient.
Investigation of ACE2 expression patterns in adult human testis at the level of single-cell transcriptomes shows that ACE2 is predominantly enriched in Leydig and Sertoli cells, implying a possible direct effect on spermatozoa.23 If aerosol drops containing virus come in contact with liquid nitrogen, the virus will vitrify and contaminate the liquid nitrogen. Although the properties of SARS-CoV-2 interacting with liquid nitrogen and reproductive cells are not fully investigated, a realistic expectation may be that it can contaminate the operator upon warming or cross-contaminate samples with ACE2 receptor such as testis tissue.24 Moreover, it has been found that early embryos express high levels of ACE2, and has been proposed to be responsible for either reduced or total failure of fertilization during conventional IVF.25,26 Cautionary safety measures such as washing and storing semen in a separate liquid nitrogen cryostorage canister for SARS-CoV-2 patients could be a plausible choice.27,28 Taking these facts altogether and in order to mitigate the possibility of contamination, it is highly possible that the general precautions taken during IVF treatment with SARS-CoV-2 positive patients will change.
While overall results indicate the absence of SARS-CoV-2 in semen among milder and asymptomatic men, the cumulative number of subjects is still too low to consider this data conclusive. A major limitation of most studies investigating the possible presence of SARS-CoV-2 in semen is the collection of samples after patients recover, as the most potential period for transmission of the virus is the acute stage, where viremia may be detected and the virus may shed into the male genitourinary tract via an imperfect blood-testis barrier.13 Future research is needed to evaluate for the presence of SARS-CoV-2 in seminal fluid during an acute infection with severe symptoms as well as long-term follow-up of SARS-CoV-2 in semen, especially among asymptomatic SARS-CoV-2 patients.
Conclusion
We have reported the presence and absence of SARS-CoV-2 in semen. Taken together, the risk of the presence of SARS-CoV-2 in semen appears to be extremely low and likely negligible in recovered men. Understanding virus dynamics and knowing all the possible transmission routes help us to determine preventive measures that have to be taken. It is of particular importance to include long-term follow-up in men who have reported symptoms of scrotal discomfort or orchitis, as this could indicate a violation of the blood-testis barrier. Longitudinal assessments with repeated RNA detection with appropriate time intervals are necessary. Future studies need to focus on whether complete viral particles can be seen in semen and the possibility of sexual transmission.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020.
2. Peng L, Liu J, Xu W, et al. SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J Med Virol. 2020;92(9):1676–1680. doi:10.1002/jmv.25936
3. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected Pneumonia in Wuhan, China. JAMA. 2020.
4. Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5(4):562–569. doi:10.1038/s41564-020-0688-y
5. Douglas GC, O’Bryan MK, Hedger MP, et al. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145(10):4703–4711. doi:10.1210/en.2004-0443
6. Chen YW, Lee MS, Lucht A, et al. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am J Pathol. 2010;176(6):2986–2996. doi:10.2353/ajpath.2010.090665
7. Aversa A, Jannini EA. COVID-19, or the triumph of monogamy? Minerva Endocrinol. 2020;45(2):77–78. doi:10.23736/S0391-1977.20.03207-1
8. Salam AP, Horby PW. The breadth of viruses in human semen. Emerg Infect Dis. 2017;23(11):1922–1924. doi:10.3201/eid2311.171049
9. Xu J, Qi L, Chi X, et al. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod. 2006;74(2):410–416. doi:10.1095/biolreprod.105.044776
10. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi:10.1038/s41586-020-2012-7
11. Kayaaslan B, Korukluoglu G, Hasanoglu I, et al. Investigation of SARS-CoV-2 in semen of patients in the acute stage of COVID-19 infection. Urol Int. 2020;1–6. doi:10.1159/000511618
12. Holtmann N, Edimiris P, Andree M, et al. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril. 2020;114(2):233–238. doi:10.1016/j.fertnstert.2020.05.028
13. Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with Coronavirus disease 2019. JAMA Netw Open. 2020;3(5):e208292. doi:10.1001/jamanetworkopen.2020.8292
14. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi:10.1186/2046-4053-4-1
15. Song C, Wang Y, Li W, et al. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patientsdagger. Biol Reprod. 2020;103(1):4–6. doi:10.1093/biolre/ioaa050
16. Guo L, Zhao S, Li W, et al. Absence of SARS-CoV-2 in semen of a COVID-19 patient cohort. Andrology. 2020. doi:10.1111/andr.12848
17. Pan F, Xiao X, Guo J, et al. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113(6):1135–1139. doi:10.1016/j.fertnstert.2020.04.024
18. Rawlings SA, Ignacio C, Porrachia M, Du P, Smith DM, Chaillon A. No evidence of SARS-CoV-2 seminal shedding despite SARS-CoV-2 persistence in the upper respiratory tract. Open Forum Infect Dis. 2020;7(8). doi:10.1093/ofid/ofaa325
19. Ma L, Xie W, Li D, et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol. 2020. doi:10.1002/jmv.26259
20. Paoli D, Pallotti F, Colangelo S, et al. Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J Endocrinol Invest. 2020;43(12):1819–1822. doi:10.1007/s40618-020-01261-1
21. Chen W, Lan Y, Yuan X, et al. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect. 2020;9(1):469–473. doi:10.1080/22221751.2020.1732837
22. World Health Organization. Department of Reproductive Health and Research. WHO laboratory manual for the examination and processing of human semen Fifth edition; 2010. Available from: https://www.who.int/reproductivehealth/publications/infertility/9789241547789/en/.
23. Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;202(5):756–759. doi:10.1164/rccm.202001-0179LE
24. Arav A. A recommendation for IVF lab practice in light of the current COVID-19 pandemic. J Assist Reprod Genet. 2020;37(7):1543. doi:10.1007/s10815-020-01841-3
25. Yan L, Yang M, Guo H, et al. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat Struct Mol Biol. 2013;20(9):1131–1139. doi:10.1038/nsmb.2660
26. Kondoh G, Tojo H, Nakatani Y, et al. Angiotensin-converting enzyme is a GPI-anchored protein releasing factor crucial for fertilization. Nat Med. 2005;11(2):160–166. doi:10.1038/nm1179
27. Arav A, Natan Y, Levi-Setti PE, Menduni F, Patrizio P. New methods for cooling and storing oocytes and embryos in a clean environment of −196 degrees C. Reprod Biomed Online. 2016;33(1):71–78.
28. Anifandis G, Messini CI, Daponte A, Messinis IE. COVID-19 and fertility: a virtual reality. Reprod Biomed. 2020;114(2):157–159. doi:10.1016/j.rbmo.2020.05.001
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
