Back to Journals » Adolescent Health, Medicine and Therapeutics » Volume 13
A Systematic Review of the Scope of Study of mHealth Interventions for Wellness and Related Challenges in Pediatric and Young Adult Populations
Authors Bond SJ, Parikh N, Majmudar S, Pin S, Wang C , Willis L, Haga SB
Received 5 October 2021
Accepted for publication 25 January 2022
Published 7 February 2022 Volume 2022:13 Pages 23—38
DOI https://doi.org/10.2147/AHMT.S342811
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Alastair Sutcliffe
Sarah J Bond,1 Nathan Parikh,1 Shrey Majmudar,1 Sabrina Pin,1 Christine Wang,1 Lauren Willis,1 Susanne B Haga1,2
1Duke University, Durham, NC, 27708, USA; 2Duke University School of Medicine, Durham, NC, 27708, USA
Correspondence: Susanne B Haga
Duke University, 101 Science Drive, Box 3382, Durham, NC, 27708, USA
, Tel +1 919 684 0325
, Fax +1 919 681 8973
, Email [email protected]
Background: Despite the purported advantages and potential efficacy of mHealth interventions to promote wellness in children, adolescents, and young adults, it is not clear what areas have been explored and the challenges reported in the biomedical literature.
Methods: We conducted a scoping review of publications between 2015 and 2019.
Results: We identified 54 papers that met our inclusion criteria. Studies were conducted in 21 countries and ranged in size from six to 9851 participants (median: 184). A total of 41% of studies enrolled adolescents only (n = 19). Of the seven types of mHealth interventions identified, apps were the most common intervention (59%; n = 32) evaluated and 44% of the studies evaluated two or more interventions. The most common topic of the studies reviewed was sexual and reproductive health (24%; n = 13).
Conclusion: Most pediatric mHealth intervention studies are conducted in adolescents in large part, and sexual and reproductive health is the most commonly studied topic. With the easy and widespread accessibility to smartphone technology, the use of mobile apps for wellness interventions will likely continue to expand to other wellness topics.
Keywords: mHealth, wellness, children, adolescents, young adults
Introduction
The explosion of mobile health (mHealth) devices, including smartphones, smartwatches and apps, has enabled widespread usage by a greater segment of the population for a variety of health-related purposes.1 Each new release and device yields more options and features for monitoring and tracking, reminders, bands or styles, partnerships with other brands (eg, luxury designers or sporting goods), and decreasing costs. mHealth technologies have ushered in the generation of “digital” phenotypes for disease monitoring and early detection.2–4 These technologies are being evaluated for a range of clinical purposes, such as disease screening and monitoring as well as health behaviors associated with, recovery, disease management, and compliance with treatment regimens.5–8 In parallel, mHealth interventions are being evaluated to promote and sustain healthy behaviors through approaches like daily monitoring, reminders and education to maintain well-being and wellness.9–12 Some wearables are approved by the US Food and Drug Administration (FDA) as clinical devices, though accessible through retail stores without a prescription.13
Children, adolescents, and young adults have widespread access to digital technologies. A 2018 survey reported that 95% of US teens have access to a smartphone,14 up from 75% in 2015.15 A 2019 survey reported that almost half of children by age 11 own or have access to a smartphone.16 Thus, the introduction of mHealth interventions as educational and self-tracking tools to promote healthy behaviors and choices has garnered much attention, though to a lesser extent than that of adult populations.11 For wellness-related activities, the ability to monitor and track personal activities is hoped to initiate and sustain healthy behaviors to promote well-being and reduce disease risks. In particular, children and adolescents may greatly benefit from mHealth technologies by learning and adopting healthy behaviors to reduce the onset of health risks, such as obesity and smoking. Establishing healthy behaviors in children and adolescents can build the foundation for lifelong healthy behaviors with substantive returns.17
However, there remain many questions to address, including which behaviors or topics would benefit from mHealth technologies, which age groups would be most appropriate, and how to sustain long-term use in children and adolescents. While there have been several reviews published on the use of mHealth interventions and children, particularly on physical activity,18–20 there has not been a more comprehensive review to date. To identify the range of wellness topics and age groups in which mHealth technologies have been evaluated thus far, we conducted a scoping literature review and present our findings here. We are particularly interested in identifying what types of populations have been studied (concerns about access and diversity) and the challenges encountered by researchers. We anticipate that the findings will provide a better understanding of the topics, age groups, and challenges faced by these studies, and can help identify both gaps in research as well as areas that may benefit from expansion to other age groups or related topics.
Methods
We conducted a scoping review to explore three areas: 1) the scope of mHealth interventions used to improve wellness among healthy children, adolescents, and young adults; 2) the range of wellness topics and corresponding interventions/technologies and age group; and 3) reported challenges with the use of mHealth technologies for wellness in children, adolescents, and young adults. In addition to the absence of a disease state, wellness has been defined as a multi-faceted state of health including emotional, physical, occupational, intellectual, financial, social, environmental, and spiritual parts.21–23
Search Strategy and Selection Criteria
We used the following databases to identify publications on mHealth, wellness, and children, adolescents and young adults: PubMed, Cochrane Central Register of Controlled Trials (CENTRAL) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) databases. For the PubMed search, we used the following search string with restrictions for age group (all children) and language (English): Wearable[Title/Abstract] OR mobile applications[Title/Abstract] OR mobile phone apps[Title/Abstract] OR text messaging[Title/Abstract] OR apps[Title/Abstract] OR “fitness trackers”[MeSH Terms] OR (“fitness”[All Fields] AND “trackers”[All Fields]) OR “fitness trackers”[All Fields] OR “mobile applications”[MeSH Terms] OR (“mobile”[All Fields] AND “applications”[All Fields]) OR “mobile applications”[All Fields] AND (Clinical Trial[ptyp] AND (“2015/01/01”[PDAT]: “2019/12/31”[PDAT]) AND “humans”[MeSH Terms] AND English[lang]). For CINAHL, we conducted a search with the following search string ((MM “Cellular Phone”) OR (MM “Mobile Applications”) OR (MM “Fitness Trackers”)) and limited it to original research articles, age group (all children), publication date (01/01/2015–12/31/2019), and language (English).
We reviewed the publication titles and abstracts to screen publications that met our search criteria for mHealth interventions, wellness, and children and young adults. Papers were excluded if they did not report empirical results, such as protocol or design papers, if the study did not focus on healthy children and young people, and if no health technology intervention was used (no technology used at all or the technology used was not a form of mHealth, social media, website, or wearable device). Specifically, studies were excluded if they focused on an affected population (eg, eligibility to participate included disease diagnosis), a population over the age of 25 years of age, a population of caregivers (the intervention was applied to parents or caregivers of children rather than children themselves), or a population of healthcare workers (eg, the technology was used as an intervention for health professionals, not for patients). In addition, qualitative research publications that did not involve an evaluation of a specific mHealth intervention were excluded as we were focused on the scope and challenges encountered in evaluations of mHealth interventions in pediatric populations. Studies were also excluded if their abstracts or full-text versions were not available online, open to all or accessible through the Duke University Library. Two reviewers screened all publications based on these exclusion criteria to identify the set of publications that would be analyzed in-depth.
Data Extraction and Analysis
After finalizing the set of publications to be analyzed, each publication was reviewed by two authors in its entirety and the following study details extracted: country where study was conducted, study purpose/focus, age range of study population, study population size, study design, study population make-up (gender, race/ethnicity), and type of intervention(s). In addition, we extracted reported challenges and limitations related to either the mHealth intervention or study protocol or dataset. Analysis of race/ethnicity make-up was restricted to US-based studies due to differences in categories or non-reporting in non-US studies. Categories and themes were then defined and papers were re-coded accordingly. Age was categorized into one of the three distinct age categories as defined by PubMed’s online search filters: children (6–12 years), adolescents (13–18 years), and young adults (19–25 years) or a combination thereof. Three additional categories were created to allow coding of publications with more than one age group: CA (children + adolescents), AY (adolescents + young adults), CAY (children + adolescents + young adults). Each publication was reviewed by a minimum of two authors; discrepancies in coding were resolved through discussion with the entire team. Summary statistics were generated in Excel.
Results
Overview
Our search results are displayed using the Preferred Reporting Items for Systematic Review and MetaAnalyses (PRISMA) flow diagram (Figure 1).24 After duplicates were removed, our search yielded a total of 846 unique papers: 371 from PubMed, 384 from CINAHL, and 91 from the Cochrane database. From these results, we excluded 692 during the title/abstract screening because they did not fit our criteria. The five most common reasons for exclusion were that the study focused on an affected patient population (n = 303), no health technology intervention was used in the study (n = 167), the publication was the study protocol, and no data had been collected (n = 66), the participants were caregivers of children and young people (n = 66), and the participants were older than 25 years (n = 59). After reviewing the title and abstract, we accessed the full text of the remaining 154 studies to confirm that they met our study criteria. During this step, we excluded 100 additional studies primarily because the full text was unavailable (n = 36), no health technology intervention was used in the study (n = 29), and participants were older than 25 years (n = 27). A total of 54 studies were included in our final analysis (Table 1). The major datapoints abstracted from the publications are shown in Figure 2.
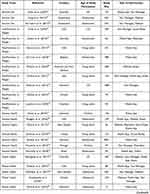 | 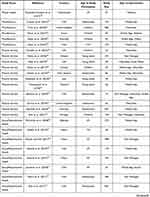 |  |
Table 1 List of 54 Publications Identified in the Search That Met the Criteria for Analysis and Key Datapoints |
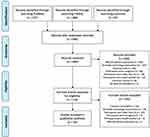 |
Figure 1 PRISMA flow chart of publication search and screening. Notes: Adapted from Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine. 2009;6(7):e1000100. doi:10.1371/journal.pmed.1000100.24 © 2009 Liberati et al. Creative Commons Attribution License. |
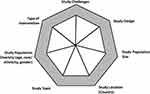 |
Figure 2 The seven key datapoints abstracted from the 54 publications reviewed. |
Study Design
Fifty-seven percent (n = 31) of studies conducted randomized controlled trials and 22% (n = 12) conducted cluster-randomized controlled trials (eg, schools, classes, or geographic regions/neighborhoods). Fifteen percent (n = 8) were observational or cohort studies, and the remaining papers were longitudinal studies, cross-over studies, and cluster (non-randomized) controlled trials.
Study Location
We reviewed where (by country) the studies were conducted and identified a total of 21 countries. The largest proportion of studies were conducted in the United States, accounting for 32% (n = 18) of total studies. The remainder of the studies were conducted in Europe (14), Australia and New Zealand (10), Africa (3), Canada (2), the United Kingdom (2), and one study each in Korea, Thailand, Tajikistan, Brazil, and Turkey.
Study Population Characteristics
We analyzed the study population size and diversity with respect to gender, race/ethnicity, and age group. Of the 54 total papers analyzed, there were a total of 26,817 participants enrolled. Study population sizes ranged from 6 participants to 9851 participants, with a median of 184 participants (mean: 497; SD: 1342). Almost all studies reported their gender breakdown (93%, n = 50) with the plurality of studies having a near 50–50 male-female ratio (38%, n = 19). Fourteen studies had >60% female enrollment, while eight studies had <40% female enrollment.
Overall, 48% (n = 26) of publications reported racial/ethnic diversity of their study populations, most predominantly by the U.S.-based studies (83%; n = 15). Among the U.S.-based studies reporting race, 40% (n = 6) of studies enrolled exclusively or predominantly a non-White population (defined as <20% White participants), while seven studies had >50% White participants.
We assigned publications into one of the three distinct age categories: children (6–12 years), adolescents (13–18 years), and young adults (19–25 years), or a combination thereof. Of the 54 studies, 15% (n = 8) enrolled solely children, 35% enrolled adolescents (n = 19), and 24% (n = 13) enrolled young adults (Figure 3). Twenty-six percent of studies (n = 14) enrolled multiple age groups with the largest combined age groups being adolescents and young adults (AY) (18%; n = 10). One study enrolled all three age groups (CAY).
Types of mHealth Interventions
We categorized the mHealth interventions into eight categories: (1) mobile apps, (2) text messages, (3) wearables, (4) website, (5) emails, (6) social media, (7) videos, and (8) virtual reality (Figure 4). Mobile apps were the most common intervention, evaluated in 59% (n = 32) of studies, followed by text message intervention studies (41%; n = 22). Virtual reality was the least common intervention with only one study assessing the use of virtual reality images as a distractive pain intervention. Fifty-six percent (n = 30) of the studies included only one intervention and 44% (n = 24) included two or more interventions. For the US-based studies, five of the 18 studies provided a device for participants to use to complete the study – two wearable studies and 3 app-based studies. Only one study specifically stated that provision of the device (smartphone) was part of the remuneration and thus, could be kept by the participants;50 it was unclear if participants of other studies were able to keep the device.
 |
Figure 4 Breakdown of types of interventions. |
We examined a specific type of mHealth intervention evaluated by age group (Table 2). Adolescents were the most common age group to be enrolled in text message intervention studies, participating in 59% of all studies evaluating text messaging. Mobile apps were the most common intervention evaluated in children. Adolescents and young adults were most often enrolled in studies including a mobile app intervention.
 |
Table 2 Use of Interventions by Age Category |
Study Purpose/Focus
We identified seven categories for the study focus or purpose (eg, behavior and health measure): 1) sexual/reproductive health; 2) diet/nutrition or weight; 3) activity; 4) general health; 5) mental health; 6) alcohol use, and 7) miscellaneous (Figure 5). A few studies evaluated mHealth interventions relevant to multiple purposes; for those publications, we categorized them with the topic that aligned best with the primary purpose of the study. Out of the 54 total studies reviewed, the most common purpose was sexual/reproductive health (24%; n = 13). Twenty percent of the studies focused on physical activity (n = 11) and seventeen percent focused on diet/nutrition or weight (n = 9). Thirteen percent (n = 7) of studies focused on general health. The remainder of the studies were coded as “miscellaneous” (n = 6) as they did not focus on any particular wellness/disease type or those that assessed specific features (eg, gaming) but were not associated with a wellness/health focus (topics such as novice teen driving, self-reported compliance of daily diary-keeping, and effects of screen media on children’s executive function).
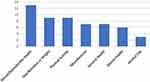 |
Figure 5 Breakdown by study focus. |
With respect to type of mHealth intervention, Table 3 shows the interventions evaluated by study focus. Further analysis of each study topic is described in the sections below (excluding the miscellaneous category for a total of 48 studies).
 |
Table 3 Number of Studies by Topic and mHealth Intervention (Top Table) and by Topic and Age Category (Bottom Table) |
Sexual/Reproductive Health
The most common purpose among the publications reviewed was sexual and reproductive health (n = 13). Specifically, studies focused on reducing the incidence of sexually transmitted infections and increasing contraception use. Two-thirds (n = 9) of the sexual/reproductive health studies were conducted in the US Five of the nine U.S.-based studies enrolled a majority of non-White participants with one study focused exclusively on African-American and Latina girls. In addition to the bias towards minority participants, most were single-sex studies (six were female-only and two male-only).
The majority of studies (n = 7) enrolled both adolescents and young adults, with five studies exclusively targeting adolescents, and one exclusively for young adults. The median study population size was 283 (range: 25–852). Four types of interventions were used, with text messages being the most common intervention (61%; n = 8), followed by mobile apps (38%; n = 5), email combined with mobile apps (8%; n = 1), and wearables (8%; n = 1).
Diet/Nutrition and Weight
The second most prevalent study purpose was diet/nutrition or weight, comprising 17% (n = 9) of the publications. Three studies were conducted in Australia, two in the US, and one each in New Zealand, Finland, Denmark, Belgium, and Italy. Of the U.S.-based studies, one focused primarily on African Americans. With respect to age, four studies enrolled only adolescents and five enrolled only young adults. The median study population size was 401 (range: 79–1488). Studies only enrolled young adults and adolescents. With respect to gender, five of the nine publications reported a majority of female participants (two studies did not report gender breakdown). One paper focused exclusively on females and another exclusively on adolescent boys. The most common intervention used in the nine studies was mobile apps (56%; n = 5), followed by text messages (44%; n = 4). Two studies used wearables and two other studies used email. Four studies evaluated two or more mHealth interventions.
Physical Activity
Tied with diet/nutrition and weight, there were 11 studies on physical activity, focused primarily on increasing physical activity. The studies enrolled participants across the three age groups, with three studies focused on children, four focused each on adolescents and young adults. There were no single-sex studies, though females comprised the majority of the study population in six studies. The median study population size was 62 (range: 10–607). With respect to race/ethnicity, only one study was conducted in the US and reported a 90% White enrollment. Other studies were conducted in Canada, Australia, New Zealand, Italy, Portugal, the United Kingdom, and Thailand. Six studies used mobile apps and six studies evaluated a combination of interventions (eg, mobile app plus wearable and text messages plus wearable). Wearables were evaluated in seven (64%) studies and apps were evaluated in six (54%) studies on physical activity; in comparison, wearables were only evaluated in 20% of the studies overall.
General Health
Thirteen percent (n = 7) of studies reviewed focused on general health. Each of these studies was conducted in a different country: US, Brazil, Germany, Australia, Portugal, Indonesia, New Zealand, and Turkey. Half of the studies (n = 4) focused on young adults exclusively, while the remainder focused on adolescents (n = 2) or children (n = 2). For the interventions, six of the seven studies evaluated a mobile app (3 studies in combination with other interventions including wearables, a website, text messages, social media, emails, and/or virtual reality).
The median study population size was 172 participants (range: 49–9851). With respect to race/ethnicity for the U.S.-based study, 56% of participants were White.
Mental Health
Six (11%) of the reviewed studies focused on interventions for mental health. Specifically, these studies assessed the use of mHealth applications to improve mindfulness and wellness, and reduce the risk of depression. Each of these studies was conducted in a different country: US, Australia, New Zealand, the Netherlands, Finland, and Denmark. With respect to age, three studies enrolled solely adolescents, one study enrolled solely young adults, and two enrolled both adolescents and young adults. The median study population size was 171 participants (range: 6–855). There was no predominant mHealth intervention evaluated – equally divided between text messaging (n = 4), mobile apps (n = 4), and websites (n = 4). Four studies evaluated more than one mHealth intervention, one study evaluated only a mobile app and the other a website. A minority of participants (28%) were White and 15% Hispanic in the only U.S.-based study.
Alcohol Use
The remaining three publications of the 54 reviewed focused on alcohol use. Two of the three studies were conducted in Switzerland and one in Australia. With respect to age, two studies only enrolled adolescents and the other study enrolled both young adults and adolescents. For all studies, text messaging was evaluated in combination with a second intervention (a website in two studies and a mobile app in the other study). The median study population size was 1041 participants (range: 197–1355). One study enrolled predominantly female participants (78%), while the other studies were gender-balanced.
Challenges with mHealth Interventions and Study Limitations
Studies reported multiple challenges or limitations related to the mHealth intervention. We categorized the reported challenges related to the mHealth intervention into three categories: (1) self-monitoring and/or self-reporting; 2) device-related; and 3) application-related bugs, issues, or insufficiencies. Self-monitoring and/or self-reporting results was the most common challenge, reported in 30% (n = 16) of articles. Of the 16 studies that reported this challenge, 42% (n = 10) involved studies evaluating a mobile app. A total of 31% (n = 5) reported this challenge in studies conducted on young adults only and 44% (n = 4) on adolescents only. Device-related issues and application-related bugs, issues, or insufficiencies were reported in six and eight studies, respectively.
Limitations related to the study design or implementation were divided into nine categories: 1) lack of diversity in the study population; 2) insufficient sample size; 3) experimental versus control limitations; 4) short follow-up or intervention period; 5) randomization and stratification issues; 6) participant attrition and/or lack of compliance; 7) survey/questionnaire limitations; 8) missing or insufficient data; 9) could not isolate intervention. The most common limitation was the lack of diversity in study populations, which was reported in 28% (n = 15) of publications. This limitation was particularly common in studies regarding sexual/reproductive health wherein 54% (n = 7) of publications reported this challenge. Participant attrition and/or lack of compliance was reported in 13 studies, followed by small study sample size (n = 11), and experimental vs control limitations (n = 10).
Overall, the highest number of issues was reported by diet/nutrition and weight studies (2.8 issues/study), closely followed by physical activity studies (2.4 issues/study). Further analysis demonstrated differences in reported study challenges by study purpose. In the mental health studies, the most frequently mentioned challenge was a participant attrition and/or lack of compliance, reported in five of the six studies. In physical activity studies, the major reported issue was limited sample size (64%; n = 7), followed by participant attrition (36%; n = 4). In diet and nutrition-related studies, the major challenges reported were self-monitoring/reporting and participant attrition and/or lack of compliance (44%; n = 4 each). For studies on sexual health and reproduction, the most commonly reported challenge and lack of diversity (54%; n = 7), followed by self-monitoring and/or self-reporting challenges (31%; n = 4).
In human subject studies for non-adult participants (defined as under the age of 18 years in the U.S.), obtaining consent may present another challenge. We did not abstract information about consent from all studies given the mix of US and non-US studies and the differing policies regarding human subjects protection that may impact the conduct of research. In the U.S.-based studies, a total of 16 of the 18 studies conducted disclosed that they had obtained approval by an institutional review board. Of the 12 studies that included a description of the consent process, three approaches were used: child assent and parental consent; parental consent; and parental consent waived.
Discussion
mHealth technologies may be an effective tool in the promotion, initiation and sustainability of healthy behaviors in children, adolescents, and young adults. Although each age group may present different challenges and health concerns, the growing widespread use of digital technologies at early ages presents an opportunity to introduce mHealth as both an educational and monitoring tool to motivate healthy behaviors. Furthermore, the opportunity to embed gamification and rewards, connecting and sharing with peers, and other age-appropriate features that can be personalized further in mHealth technologies may be quite advantageous compared to traditional approaches for improving healthy behaviors, health education and monitoring.
In our review, we identified a global group of researchers evaluating mHealth technologies spanning 21 countries across all three age groups, with an understandable bias towards adolescents and young adults due to their maturity and ability to use the devices with minimal adult supervision and support. The US had the highest proportion of publications, followed by Europe. Notably, there were no publications from many low- and middle-income countries, a trend also documented by other reviewers,79 although others have found the use of mHealth interventions to be increased in low and middle-income countries.80 However, the exploration of mHealth-based interventions has been increasing for adult conditions in some countries such as Ethiopia.81 Cost, access to devices, and connectivity may limit the widespread use of mHealth applications for children and young adults.82 Availability of apps across common smartphone platforms (eg Apple, Android, and both) may improve accessibility and equity of access for owners of different phone types, particularly given the cost differences between platforms and some developers advocate for cross-platforms to improve access.83 However, some data suggest that cross-platform availability does not exist for some health apps; one study reported only a third of apps analyzed were available as Apple and Android versions, but half were only available through Apple.84
The most common focus of mHealth studies was on sexual and reproductive health – most of which centered around youth contraception or HIV. L’Engle et al79 identified even more studies (35) in their review of mobile phone-based interventions and sexual and reproductive health but also included interventions such as vaccine reminders, which we did not. Among the diet/nutrition and weight or (physical) activity studies, many specifically focused either on food/caloric intake (eg, snacking) or obesity. Schoeppe et al reported 25 apps that had been developed and evaluated for diet and physical activity in children and adolescents.85
Matching the appropriate mHealth technology with the health goal and age group is critical to the success of the intervention. Of the seven mHealth interventions identified in the 54 studies, apps were the most common intervention evaluated, appearing in 61% of studies, though biased to young adults and adolescent study populations. For some topics, like physical activity, apps were the predominant type of intervention evaluated. Apps are an appealing intervention as they have widespread outreach, do not require in-person interaction, and can function as educational, motivational or a combination thereof;86 gamification may be a particularly attractive feature in apps for young populations.87 Despite their popularity with adults, wearables were evaluated in only 20% of studies overall though evidence has been reported that use of wearables is feasible in younger children.88 Wearables were least studied in children (1/11) and most studied in adolescents (9/11 studies). In addition, for children and adolescents, a single intervention may be insufficient to achieve the desired wellness goals with 44% of studies evaluating two or more interventions. Compared to wearables, mobile apps can be customized to their targeted age group and include features such as gamification to incentivize sustained use.87 However, the use of combined interventions is often reported to present challenges in determining the predominant intervention.
One-third of the U.S.-based studies reviewed exclusively or almost exclusively enrolled non-White populations (<10%) and all but one of the remaining had diverse participation (one study had enrollment of >90% White participants). In some cases, a mHealth intervention was designed for a specific target population such as the African American and Latina females.69 With respect to gender, about 40% of studies reported almost equal enrollment of males/females and in a quarter of the studies, more than 60% of participants were females. Female enrollment was particularly high in studies focused on mental health with all but one study enrolling more than 60% female participants.
We found that many studies listed self-reporting as a major study challenge, as anticipated for studies with young populations. This challenge was noted in 71% of young adult populations, likely due to the higher use of data collection methods, such as surveys in this age group compared to younger children. In addition to low completion rates, recall bias has also been reported as another challenge in young study populations.89 Compliance is another issue often reported, consistent with older reports of inconsistent or low use of wearables in children.19,20,90 Reminders may be helpful to increase regular use of devices.91–93 The racial make-up of the study populations was inconsistent, with five US-based studies enrolling a majority non-White population, and seven studies recruiting 50% or more White participants (three studies enrolled >70% White participants).
Some limitations of our findings should be noted. We opted to exclude publications that gathered data from caregivers or affected populations, which may have skewed the number of articles that focused on younger children. In addition, the exclusion of non-English language may have resulted in a lower count of publications in other countries. Long-term longitudinal studies may have followed participants enrolled as children or adolescents into adulthood and potentially biased findings reported here. The review is limited to 2015–2019 and thus does not reflect more recent work; an analysis of the next 5-year period could characterize the growth of the field and direction of the field for mHealth interventions in children and young adults.
In summary, during this early stage of mHealth intervention research, we found that a wide range of studies have been conducted around the world, though mostly concentrated in the US, in children, adolescents, and young adults. Of the seven areas of wellness identified, mental health had the highest number of studies. With the higher maturity level and age-specific issues, adolescents and young adults were the primary focus on mHealth interventions, and mobile apps were the most common type of intervention evaluated to date. While cost and access to devices may be limiting factors in expanding research in children and young adults, the more specific challenges regarding the utilization of mHealth reflect user interest, appeal and sustainability. We anticipate that the scope of topics and number of studies will expand as the impact of these interventions is demonstrated and more features and tools are developed to engage younger users for longer periods of time and potentially habituate regular use.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhao G, Wei D. Mobile health: new technologies, new modes and new era. J Biomed Res. 2016;30(4):251–252. doi:10.7555/jbr.30.20160002
2. Insel TR. Digital phenotyping: technology for a new science of behavior. JAMA. 2017;318(13):1215–1216. doi:10.1001/jama.2017.11295
3. Blom JMC, Colliva C, Benatti C, Tascedda F, Pani L. Digital phenotyping and dynamic monitoring of adolescents treated for cancer to guide intervention: embracing a new era. Front Oncol. 2021;11:673581. doi:10.3389/fonc.2021.673581
4. Bruno E, Böttcher S, Viana PF, et al. Wearable devices for seizure detection: practical experiences and recommendations from the Wearables for Epilepsy And Research (WEAR) International Study Group. Epilepsia. 2021;62(10):2307–2321. doi:10.1111/epi.17044
5. Lopez AR, Wiskow KM. Teaching children with autism to initiate social interactions using textual prompts delivered via apple watches®. Behav Anal Pract. 2020;13(3):641–647. doi:10.1007/s40617-019-00385-y
6. Tully L, Burls A, Sorensen J, El-Moslemany R, O’Malley G. Mobile health for pediatric weight management: systematic scoping review. JMIR mHealth and uHealth. 2020;8(6):e16214. doi:10.2196/16214
7. O’Connor A, Tai A, Carson-Chahhoud K. Isn’t there an app for that? The role of smartphone and tablet applications for asthma education and self-management in adolescents. Children. 2021;8(9). doi:10.3390/children8090786
8. Dirzu N, Hotea I, Jitaru C, et al. Mobile health technology for the personalized therapy of hemophilia. Front Med. 2021;8:711973. doi:10.3389/fmed.2021.711973
9. Radovic A, Badawy SM. Technology use for adolescent health and wellness. Pediatrics. 2020;145(Supplement_2):S186–s194. doi:10.1542/peds.2019-2056G
10. Reeder B, David A. Health at hand: a systematic review of smart watch uses for health and wellness. J Biomed Inform. 2016;63:269–276. doi:10.1016/j.jbi.2016.09.001
11. Domin A, Spruijt-Metz D, Theisen D, Ouzzahra Y, Vögele C. Smartphone-based interventions for physical activity promotion: scoping review of the evidence over the last 10 years. JMIR Mhealth Uhealth. 2021;9(7):e24308. doi:10.2196/24308
12. Conger SA, Toth LP, Cretsinger C, et al. Time trends in physical activity using wearable devices: a systematic review and meta-analysis of studies from 1995 to 2017. Med Sci Sports Exerc. 2021. doi:10.1249/mss.0000000000002794
13. Piwek L, Ellis DA, Andrews S, Joinson A. The rise of consumer health wearables: promises and barriers. PLoS Med. 2016;13(2):e1001953. doi:10.1371/journal.pmed.1001953
14. Pew Research Center. Teens, social media and technology 2018; 2018.
15. Pew Research Center. Teens, social media and technology overview 2015; 2015.
16. Common Sense Media. The common sense census: media use by tweens and teens, 2019; 2019.
17. OECD. Educating 21st Century Children. OECD Publishing; 2019.
18. Lee AM, Chavez S, Bian J, et al. Efficacy and effectiveness of mobile health technologies for facilitating physical activity in adolescents: scoping review. JMIR Mhealth Uhealth. 2019;7(2):e11847. doi:10.2196/11847
19. Creaser AV, Clemes SA, Costa S, et al. The acceptability, feasibility, and effectiveness of wearable activity trackers for increasing physical activity in children and adolescents: a systematic review. Int J Environ Res Public Health. 2021;18(12):6211. doi:10.3390/ijerph18126211
20. Bohm B, Karwiese SD, Bohm H, Oberhoffer R. Effects of mobile health including wearable activity trackers to increase physical activity outcomes among healthy children and adolescents: systematic review. JMIR Mhealth Uhealth. 2019;7(4):e8298. doi:10.2196/mhealth.8298
21. Bart R, Ishak WW, Ganjian S, et al. The assessment and measurement of wellness in the clinical medical setting: a systematic review. Innov Clin Neurosci. 2018;15(9–10):14–23.
22. Substance Abuse Mental Health Services Administration. Creating a healthier life: a step-by-step guide to wellness; 2016.
23. Swarbrick M. A wellness approach. Psychiatr Rehabil J. 2006;29(4):311–314. doi:10.2975/29.2006.311.314
24. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi:10.1371/journal.pmed.1000100
25. Hides L, Quinn C, Cockshaw W, et al. Efficacy and outcomes of a mobile app targeting alcohol use in young people. Addict Behav. 2018;77:89–95. doi:10.1016/j.addbeh.2017.09.020
26. Haug S, Paz Castro R, Kowatsch T, et al. Efficacy of a web- and text messaging-based intervention to reduce problem drinking in adolescents: results of a cluster-randomized controlled trial. J Consult Clin Psychol. 2017;85(2):147–159. doi:10.1037/ccp0000138
27. Paz Castro R, Haug S, Kowatsch T, Filler A, Schaub MP. Moderators of outcome in a technology-based intervention to prevent and reduce problem drinking among adolescents. Addict Behav. 2017;72:64–71. doi:10.1016/j.addbeh.2017.03.013
28. Trude ACB, Surkan PJ, Cheskin LJ, Gittelsohn J. A multilevel, multicomponent childhood obesity prevention group-randomized controlled trial improves healthier food purchasing and reduces sweet-snack consumption among low-income African-American youth. Nutr J. 2018;17(1):96. doi:10.1186/s12937-018-0406-2
29. Lubans DR, Smith JJ, Plotnikoff RC, et al. Assessing the sustained impact of a school-based obesity prevention program for adolescent boys: the ATLAS cluster randomized controlled trial. Int J Behav Nutr Phys Act. 2016;13(1):92. doi:10.1186/s12966-016-0420-8
30. Sarcona A, Kovacs L, Wright J, Williams C. Differences in eating behavior, physical activity, and health-related lifestyle choices between users and nonusers of mobile health apps. Am J Health Educ. 2017;48(5):1–8. doi:10.1080/19325037.2017.1335630
31. De Cock N, Van W, Vangeel J, et al. Feasibility and impact study of a reward-based mobile application to improve adolescents’ snacking habits. Public Health Nutr. 2018;21(12):2329–2344. doi:10.1017/s1368980018000678
32. Wilksch SM, O’Shea A, Taylor CB, et al. Online prevention of disordered eating in at-risk young-adult women: a two-country pragmatic randomized controlled trial. Psychol Med. 2018;48(12):2034–2044. doi:10.1017/s0033291717003567
33. Carfora V, Caso D, Conner M. Randomised controlled trial of a text messaging intervention for reducing processed meat consumption: the mediating roles of anticipated regret and intention. Appetite. 2017;117:152–160. doi:10.1016/j.appet.2017.06.025
34. Pedersen S, Grønhøj A, Thøgersen J. Texting your way to healthier eating? Effects of participating in a feedback intervention using text messaging on adolescents’ fruit and vegetable intake. Health Education Research. 2016; 31(2):171-184.doi:10.1093/her/cyv104.
35. Heikkilä M, Lehtovirta M, Autio O, Fogelholm M, Valve R. The impact of nutrition education intervention with and without a mobile phone application on nutrition knowledge among young endurance athletes. Nutrients. 2019;11(9):2249. doi:10.3390/nu11092249
36. Lyzwinski LN, Caffery L, Bambling M, Edirippulige S. The mindfulness app trial for weight, weight-related behaviors, and stress in university students: randomized controlled trial. JMIR Mhealth Uhealth. 2019;7(4):e12210. doi:10.2196/12210
37. Utario Y, Rustina Y, Krianto T, Ayubi D. Arbi Care application increases preschool children’s hand-washing self-efficacy among preschool children. Enferm Clin. 2018;28(Suppl 1):27–30. doi:10.1016/s1130-8621(18)30031-7
38. Rodgers RF, Donovan E, Cousineau T, et al. BodiMojo: efficacy of a mobile-based intervention in improving body image and self-compassion among adolescents. J Youth Adolesc. 2018;47(7):1363–1372. doi:10.1007/s10964-017-0804-3
39. Ashton LM, Morgan PJ, Hutchesson MJ, Rollo ME, Collins CE. Feasibility and preliminary efficacy of the ‘HEYMAN’ healthy lifestyle program for young men: a pilot randomised controlled trial. Nutr J. 2017;16(1):2. doi:10.1186/s12937-017-0227-8
40. Karaman D, Erol F, Yılmaz D, Dikmen Y. Investigation of the effect of the virtual reality application on experimental pain severity in healthy. Rev Assoc Med Bras (1992). 2019;65(3):446–451. doi:10.1590/1806-9282.65.3.446
41. Brinker TJ, Holzapfel J, Baudson TG, et al. Photoaging smartphone app promoting poster campaign to reduce smoking prevalence in secondary schools: the Smokerface Randomized Trial: design and baseline characteristics. BMJ Open. 2016;6(11):e014288. doi:10.1136/bmjopen-2016-014288
42. Fassnacht DB, Ali K, Silva C, Gonçalves S, Machado PP. Use of text messaging services to promote health behaviors in children. J Nutr Educ Behav. 2015;47(1):75–80. doi:10.1016/j.jneb.2014.08.006
43. Marchetti G, Fraiz FC, Nascimento WMD, Soares GMS, Assunção L. Improving adolescents’ periodontal health: evaluation of a mobile oral health App associated with conventional educational methods: a cluster randomized trial. Int J Paediatr Dent. 2018;28(4):410–419. doi:10.1111/ipd.12371
44. Bidargaddi N, Musiat P, Winsall M, et al. Efficacy of a web-based guided recommendation service for a curated list of readily available mental health and well-being mobile apps for young people: randomized controlled trial. J Med Internet Res. 2017;19(5):e141. doi:10.2196/jmir.6775
45. Huberty J, Green J, Glissmann C, et al. Efficacy of the mindfulness meditation mobile app “Calm” to reduce stress among college students: randomized controlled trial. JMIR Mhealth Uhealth. 2019;7(6):e14273. doi:10.2196/14273
46. Whittaker R, Stasiak K, McDowell H, et al. MEMO: an mHealth intervention to prevent the onset of depression in adolescents: a double-blind, randomised, placebo-controlled trial. J Child Psychol Psychiatry. 2017;58(9):1014–1022. doi:10.1111/jcpp.12753
47. Puolakanaho A, Lappalainen R, Lappalainen P, et al. Reducing stress and enhancing academic buoyancy among adolescents using a brief web-based program based on acceptance and commitment therapy: a randomized controlled trial. J Youth Adolesc. 2019;48(2):287–305. doi:10.1007/s10964-018-0973-8
48. Mackrill T, Ørnbøll JK. The MySocialworker app system – a pilot interview study. Eur J Soc Work. 2019;22(1):134–144. doi:10.1080/13691457.2018.1469471
49. van Rosmalen-nooijens K, Lo Fo Wong S, Prins J, Lagro-Janssen T. Young people, adult worries: randomized controlled trial and feasibility study of the internet-based self-support method “Feel the ViBe” for adolescents and young adults exposed to family violence. J Med Internet Res. 2017;19(6):e204. doi:10.2196/jmir.6004
50. Creaser JI, Edwards CJ, Morris NL, Donath M. Are cellular phone blocking applications effective for novice teen drivers? J Safety Res. 2015;54:
51. Price L, Wyatt K, Lloyd J, et al. Children’s compliance with wrist-worn accelerometry within a cluster-randomized controlled trial: findings from the healthy lifestyles programme. Pediatr Exerc Sci. 2018;30(2):281–287. doi:10.1123/pes.2017-0179
52. Moon KJ, Park KM, Sung Y. Sexual Abuse Prevention Mobile Application (SAP_MobAPP) for primary school children in Korea. J Child Sex Abus. 2017;26(5):573–589. doi:10.1080/10538712.2017.1313350
53. Heino MTJ, Knittle K, Haukkala A, Vasankari T, Hankonen N. Simple and rationale-providing SMS reminders to promote accelerometer use: a within-trial randomised trial comparing persuasive messages. BMC Public Health. 2018;18(1):1352. doi:10.1186/s12889-018-6121-2
54. Huber B, Yeates M, Meyer D, Fleckhammer L, Kaufman J. The effects of screen media content on young children’s executive functioning. J Exp Child Psychol. 2018;170:72–85. doi:10.1016/j.jecp.2018.01.006
55. Taylor S, Ferguson C, Peng F, Schoeneich M, Picard RW. Use of in-game rewards to motivate daily self-report compliance: randomized controlled trial. J Med Internet Res. 2019;21(1):e11683. doi:10.2196/11683
56. Schaefer SE, Ching CC, Breen H, German JB. Wearing, thinking, and moving: testing the feasibility of fitness tracking with urban youth. Am J Health Educ. 2016;47(1):8–16. doi:10.1080/19325037.2015.1111174
57. Pope ZC, Barr-Anderson DJ, Lewis BA, Pereira MA, Gao Z. Use of wearable technology and social media to improve physical activity and dietary behaviors among college students: a 12-week randomized pilot study. Int J Environ Res Public Health. 2019;16(19):3579. doi:10.3390/ijerph16193579
58. Patten JW, Iarocci G, Bojin N. A pilot study of children’s physical activity levels during imagination-based mobile games. J Child Health Care. 2017;21(3):292–300. doi:10.1177/1367493517708477
59. Direito A, Jiang Y, Whittaker R, Maddison R. Apps for IMproving FITness and increasing physical activity among young people: the AIMFIT pragmatic randomized controlled trial. J Med Internet Res. 2015;17(8):e210. doi:10.2196/jmir.4568
60. Gabbiadini A, Greitemeyer T. Fitness mobile apps positively affect attitudes, perceived behavioral control and physical activities. J Sports Med Phys Fitness. 2018;59. doi:10.23736/S0022-4707.18.08260-9
61. Wattanapisit A, Saengow U, Ng C, Wattanapisit S, Kaewruang N. Gaming behaviour with Pokémon GO and physical activity: a preliminary study with medical students in Thailand. PLoS One. 2018;13(6):e0199813. doi:10.1371/journal.pone.0199813
62. Brannon EE, Cushing CC, Walters RW, et al. Goal feedback from whom? A physical activity intervention using an N-of-1 RCT. Psychol Health. 2018;33(6):701–712. doi:10.1080/08870446.2017.1385783
63. Kerner C, Burrows A, McGrane B. Health wearables in adolescents: implications for body satisfaction, motivation and physical activity. Int J Health Promot Educ. 2019;57(4):191–202. doi:10.1080/14635240.2019.1581641
64. Kennedy SG, Smith JJ, Morgan PJ, et al. Implementing resistance training in secondary schools: a cluster randomized controlled trial. Med Sci Sports Exerc. 2018;50(1):62–72. doi:10.1249/mss.0000000000001410
65. Silva C, Fassnacht DB, Ali K, et al. Promoting health behaviour in Portuguese children via short message service: the efficacy of a text-messaging programme. J Health Psychol. 2015;20(6):806–815. doi:10.1177/1359105315577301
66. McCarthy O, Ahamed I, Kulaeva F, et al. A randomized controlled trial of an intervention delivered by mobile phone app instant messaging to increase the acceptability of effective contraception among young people in Tajikistan. Reprod Health. 2018;15(1):28. doi:10.1186/s12978-018-0473-z
67. Sabben G, Mudhune V, Ondeng’e K, et al. A smartphone game to prevent HIV among young Africans (Tumaini): assessing intervention and study acceptability among adolescents and their parents in a randomized controlled trial. JMIR Mhealth Uhealth. 2019;7(5):e13049. doi:10.2196/13049
68. Rokicki S, Fink G. Assessing the reach and effectiveness of mHealth: evidence from a reproductive health program for adolescent girls in Ghana. BMC Public Health. 2017;17(1):969. doi:10.1186/s12889-017-4939-7
69. Akinola M, Hebert LE, Hill BJ, et al. Development of a mobile app on contraceptive options for young African American and Latina Women. Health Educ Behav. 2019;46(1):89–96. doi:10.1177/1090198118775476
70. Rokicki S, Cohen J, Salomon JA, Fink G. Impact of a text-messaging program on adolescent reproductive health: a cluster-randomized trial in Ghana. Am J Public Health. 2017;107(2):298–305. doi:10.2105/ajph.2016.303562
71. Jacobson AE, Vesely SK, Haamid F, Christian-Rancy M, O’Brien SH. Mobile application vs paper pictorial blood assessment chart to track menses in young women: a randomized cross-over design. J Pediatr Adolesc Gynecol. 2018;31(2):84–88. doi:10.1016/j.jpag.2017.09.009
72. Ybarra ML, Prescott TL, Phillips GL, et al. Pilot RCT results of an mHealth HIV prevention program for sexual minority male adolescents. Pediatrics. 2017;140(1):e20162999. doi:10.1542/peds.2016-2999
73. Bull S, Devine S, Schmiege SJ, et al. Text messaging and teen sexual health behavior: long-term follow-up of a cluster randomized trial. CIN. 2017;35:549–553. doi:10.1097/CIN.0000000000000404
74. Trent M, Thompson C, Tomaszewski K. Text messaging support for urban adolescents and young adults using injectable contraception: outcomes of the Depotext pilot trial. J Adolesc Health. 2015;57(1):100–106. doi:10.1016/j.jadohealth.2015.03.008
75. Bull S, Devine S, Schmiege SJ, et al. Text messaging, teen outreach program, and sexual health behavior: a cluster randomized trial. Am J Public Health. 2016;106(S1):S117–s124. doi:10.2105/ajph.2016.303363
76. Ybarra ML, Liu W, Prescott TL, Phillips G
77. Buchanan CRM, Tomaszewski K, Chung S-E, et al. Why didn’t you text me? Poststudy trends from the DepoText trial. Clin Pediatr. 2018;57(1):82–88. doi:10.1177/0009922816689674
78. Cordova D, Alers-Rojas F, Lua FM, et al. The usability and acceptability of an adolescent mHealth HIV/STI and drug abuse preventive intervention in primary care. Behav Med. 2018;44(1):36–47. doi:10.1080/08964289.2016.1189396
79. L’Engle KL, Mangone ER, Parcesepe AM, Agarwal S, Ippoliti NB. Mobile phone interventions for adolescent sexual and reproductive health: a systematic review. Pediatrics. 2016;138(3). doi:10.1542/peds.2016-0884
80. Hall CS, Fottrell E, Wilkinson S, Byass P. Assessing the impact of mHealth interventions in low- and middle-income countries – what has been shown to work? Glob Health Action. 2014;7(1):25606. doi:10.3402/gha.v7.25606
81. Manyazewal T, Woldeamanuel Y, Blumberg HM, Fekadu A, Marconi VC. The potential use of digital health technologies in the African context: a systematic review of evidence from Ethiopia. NPJ Digit Med. 2021;4(1):125. doi:10.1038/s41746-021-00487-4
82. Gurupur VP, Wan TTH. Challenges in implementing mHealth interventions: a technical perspective. Mhealth. 2017;3:32. doi:10.21037/mhealth.2017.07.05
83. Inupakutika D, Kaghyan S, Akopian D, Chalela P, Ramirez AG. Facilitating the development of cross-platform mHealth applications for chronic supportive care and a case study. J Biomed Inform. 2020;105:103420. doi:10.1016/j.jbi.2020.103420
84. Baxter C, Carroll JA, Keogh B, Vandelanotte C. Assessment of mobile health apps using built-in smartphone sensors for diagnosis and treatment: systematic survey of apps listed in international curated health app libraries. JMIR Mhealth Uhealth. 2020;8(2):e16741. doi:10.2196/16741
85. Schoeppe S, Alley S, Rebar AL, et al. Apps to improve diet, physical activity and sedentary behaviour in children and adolescents: a review of quality, features and behaviour change techniques. Int J Behav Nutr Phys Act. 2017;14(1):83. doi:10.1186/s12966-017-0538-3
86. Conroy DE, Yang CH, Maher JP. Behavior change techniques in top-ranked mobile apps for physical activity. Am J Prev Med. 2014;46(6):649–652. doi:10.1016/j.amepre.2014.01.010
87. Edwards EA, Lumsden J, Rivas C, et al. Gamification for health promotion: systematic review of behaviour change techniques in smartphone apps. BMJ Open. 2016;6(10):e012447. doi:10.1136/bmjopen-2016-012447
88. Schaefer SE, Van Loan M, German JB. A feasibility study of wearable activity monitors for pre-adolescent school-age children. Prev Chronic Dis. 2014;11:E85. doi:10.5888/pcd11.130262
89. Voss C, Harris KC. Physical activity evaluation in children with congenital heart disease. Heart. 2017;103(18):1408–1412. doi:10.1136/heartjnl-2017-311340
90. Rich C, Cortina-Borja M, Dezateux C, et al. Predictors of non-response in a UK-wide cohort study of children’s accelerometer-determined physical activity using postal methods. BMJ Open. 2013;3(3):e002290. doi:10.1136/bmjopen-2012-002290
91. Belton S, O’Brien W, Wickel EE, Issartel J. Patterns of noncompliance in adolescent field-based accelerometer research. J Phys Act Health. 2013;10(8):1181–1185. doi:10.1123/jpah.10.8.1181
92. Audrey S, Bell S, Hughes R, Campbell R. Adolescent perspectives on wearing accelerometers to measure physical activity in population-based trials. Eur J Public Health. 2013;23(3):475–480. doi:10.1093/eurpub/cks081
93. Ridgers ND, McNarry MA, Mackintosh KA. Feasibility and effectiveness of using wearable activity trackers in youth: a systematic review. JMIR Mhealth Uhealth. 2016;4(4):e129. doi:10.2196/mhealth.6540
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

