Back to Journals » Drug Design, Development and Therapy » Volume 17
A Systematic Review of Systematic Reviews on the Use of Aromatase Inhibitors for the Treatment of Endometriosis: The Evidence to Date
Authors Peitsidis P , Tsikouras P, Laganà AS, Laios A, Gkegkes ID , Iavazzo C
Received 12 January 2023
Accepted for publication 27 April 2023
Published 4 May 2023 Volume 2023:17 Pages 1329—1346
DOI https://doi.org/10.2147/DDDT.S315726
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Panagiotis Peitsidis,1 Panagiotis Tsikouras,2 Antonio Simone Laganà,3 Alexandros Laios,4 Ioannis D Gkegkes,5 Christos Iavazzo6
1Department of Obstetrics and Gynecology Helena Venizelou Hospital, Athens, Greece; 2Department of Obstetrics and Gynecology.The Democritus University of Thrace, Alexandroupolis, Greece; 3Unit of Gynecologic Oncology ARNAS “Civico-Di Cristina-Benfratelli”, Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties, Palermo, Italy; 4Department of Gynecological Oncology St James Institute of Oncology, Leeds Teaching Hospitals, Leeds, Uk; 5Department of Colorectal Surgery, Royal Devon and Exeter NHS Foundation, Devon, UK; 6Gynaecological Oncology Department, Metaxa Cancer Hospital, Piraeus, Greece
Correspondence: Panagiotis Peitsidis, Department of Obstetrics and Gynecology, Helena Venizelou Hospital Athens Greece, Helena Venizelou 2 Street, P.C, Athens, 11521, Greece, Tel +306972221553 ; +302107473793, Email [email protected]
Abstract: Endometriosis is a chronic gynecologic condition that affects around 6– 10% of reproductive age women. This clinical entity is characterized with pelvic pain, dysmenorrhea, dyspareunia, and infertility which are the most often presenting symptoms. Aromatase P450 is the key enzyme for ovarian estrogen biosynthesis and there is evidence that endometriotic lesions express aromatase and are able to synthesize their own estrogens. Aromatase inhibitors (AIs) are potent drugs that suppress the estrogen synthesis via suppression of aromatase. We performed a systematic review of systematic reviews and narrative reviews on the use of aromatase inhibitors in the medical management of endometriosis. We searched: PubMed (1950– 2022), Google Scholar (2004– 2022), Cochrane Library (2010– 2022) and Researchgate (2010– 2022). The search included the following medical subject headings (MeSH) or keywords: “Aromatase Inhibitors” AND “Endometriosis” AND “Systematic reviews” OR “Systematic review” AND “Reviews” OR “Reviews” AND “Endometriosis”. The electronic database search yielded initially 12,106 studies from the different databases. Further assessment of the studies resulted in exclusion of (n = 12,015) studies due to duplicates and irrelevance; Finally, 24 studies were selected for inclusion, 5 were Systematic reviews and 19 were Narrative reviews. The 5 systematic reviews were assessed by AMSTAR-2 criteria and were found to have low quality. Narrative reviews were assessed with SANRA criteria and were found to have high-quality aromatase inhibitors are potent drugs that can manage the endometriosis-related symptoms in cases where initial medical management has failed to show positive results. However, their use is limited by the adverse effects that are linked with menopausal symptoms. aromatase inhibitors can be administered as an alternative treatment in patients. Future studies with randomized design are required to reach safer conclusions and further investigation. These studies should define the therapeutic dose, new add-back therapy modalities. Future directions should examine the most-appropriate way of administration and the duration of therapy.
Keywords: endometriosis, aromatase inhibitors, systematic review, pelvic pain, adverse effects
Introduction
Endometriosis is a chronic gynecologic condition that affects around 6–10% of reproductive-age women. This clinical entity is characterized by pelvic pain, dysmenorrhea, dyspareunia, and infertility which are the most often presenting symptoms. The disease exhibits an estrogen-dependent growth of the endometrial glands and stroma outside the endometrial cavity.1 Several risk factors of endometriosis have been reported, such as early menarche, short menstrual cycles, late menopause, low body mass index (BMI), nulliparity, increased consumption of alcohol, caffeine, and prolonged menstruation.2
The most common theory of the pathogenesis of endometriosis is the theory of retrograde menstruation; however, retrograde menstruation occurs in nearly all women and not all women are afflicted with this condition. Hence, it has been postulated that women with endometriosis are likely to contain underlying molecular abnormalities that promote the continuous growth of endometrial tissues outside the uterine cavity.3
Aromatase P450 is the key enzyme for ovarian estrogen biosynthesis. It catalyzes the conversion of androstenedione and testosterone produced in the ovarian theca cells to estrone and estradiol (E2) in the ovarian granulosa cells. Recently, there is evidence that demonstrates that endometriotic lesions express aromatase and can synthesize their own E2.4 Aromatase inhibitors (AIs) Aromatase Inhibitors were first used for the treatment of postmenopausal, estrogen receptor-positive advances.
Breast cancer: during the first decade of 2000 their use was established as alternative medical management of endometriosis-related symptoms.5
AIs are present in three generations, Aminoglutethimide, a first-generation inhibitor, suppressed the adrenals and resulted in many side effects, such as lethargy, skin rashes, and nausea, therefore its use was limited. Fadrozole and formestane are more selective second-generation inhibitors with fewer side effects; however, their administration is only intramuscular. Letrozole, anastrozole, and exemestane are the third generation of AIs. Letrozole and anastrozole are triazole derivatives characterized by being selective, reversible, and potent AIs. Figure 1. Administered orally at doses of 1–5 mg/day, they inhibit estrogen levels by 97% to more than 99%; 11–13; meanwhile, exemestane is a steroidal irreversible AI effectively working at a dose of 25 mg/day.6 Mauri et al5 in 2006 published one of the first systematic reviews and metanalyses in the international literature that compared several generations of aromatase inhibitors and inactivators with standard hormonal treatment in patients with advanced breast cancer. Similarly, since this review, several systematic and narrative reviews have been published which reported the importance of Ais in the treatment of endometriosis and the endometriosis-related symptoms in the clinical practice.
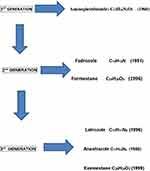 |
Figure 1 Exhibition of all generations of Aromatase inhibitors, chemical types and years of first distribution as therapeutic agents are demonstrated. |
Aim
The current study aimed to carry out a systematic review of all available systematic review studies evaluating the use of aromatase inhibitors in the clinical management of endometriosis-related symptoms. In addition, we performed a systematic review of the narrative reviews in the international literature. A methodological quality assessment of all the selected studies was performed with the use of critical appraisal tools.
Materials and Methods
Search Strategy
We searched the following electronic databases: PubMed (1950–2022), Google Scholar (2004–2022), Cochrane Library (2010–2022), and Researchgate (2010–2022). The electronic literature search was conducted from January 2021 to September 2022. The search included the following (MeSH) medical subject headings or keywords: “Aromatase Inhibitors” AND “Endometriosis” AND “Systematic reviews” OR “Systematic review” AND “Reviews” OR “Reviews” AND “Endometriosis”. The last search was performed on 08/12/2021. The systematic review and the flowchart diagram were performed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Metanalyses).http://prisma-statement.org/prismastatement/flowdiagram.aspx.7
Inclusion and Exclusion Criteria
Full-text articles published in peer-reviewed journals and written in the English language were deemed eligible to be included in the review. Studies were excluded from the review if they had the following characteristics:
- Studies other than Systematic reviews and Narrative reviews.
- Studies not written in the English language.
- Conference abstracts and studies not providing sufficient clinical data.
- Studies report aromatase administration in animals, in surgical specimens, and in an in vitro environment.
- Studies reporting administration of (AIs) in patients with adenomyosis.
- Studies reporting administration of (AIs) in patients with breast cancer.
- Studies reporting administration of (AIs) for assisted reproduction.
- Studies reporting administration of (AIs) in patients with myomas.
- Studies reporting administration of (AIs) in patients with gynecological cancers.
We included systematic reviews and narrative reviews that were related only to the use of aromatase inhibitors (AIs) in the management of endometriosis-related symptoms. No institutional board was required because there was an analysis of previously published clinical data.
Data Extraction
The extraction form included: the primary author; year of publication; country and city in which the study was accomplished; databases searched; flowchart methodology number of studies included; the population participants enrolled in the review; the mean age of the participants; the type of studies included in the review; the aim; the interventions and dosage of regiment reported in; the duration of treatment in months; the inclusion and exclusion criteria; the side effects of the treatments; the pain relief; and the outcomes. All articles were obtained in full text and scrutinized for the collection of clinical data. To reduce selection bias, the abstracts and full-text papers were assessed by masking the authors as far as possible. Discrepancies between the authors were resolved through a mutual decision after discussion. Two reviewers independently appraised the articles and extracted data (PP and PT).
Quality Assessment of the Included Studies
The methodological quality of the included systematic reviews was evaluated using the AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews) tool, https://amstar.ca/Amstar-2.php.8 AMSTAR 2 is a critical appraisal tool that consists of 16 items defining the quality criteria for the evaluation of the systematic reviews.8
The quality rating criteria are divided into four categories according to the assessment of the 16 items and are the following:
- High → Zero or one non-critical weakness: The systematic review provides an accurate and comprehensive summary of the results of the available studies that address the question of interest.
- Moderate → More than one non-critical weakness: The systematic review has more than one weakness, but no critical flaws. It may provide an accurate summary of the results of the available studies that were included in the review.
- Low → One critical flaw with or without non-critical weaknesses: The review has a critical flaw and may not provide an accurate and comprehensive summary of the available studies that address the question of interest.
- Critically low →More than one critical flaw with or without non-critical weaknesses: The review has more than one critical flaw and should not be relied on to provide an accurate and comprehensive summary of the available studies.
The methodological quality of the narrative reviews was evaluated according to SANRA (Scale for the Assessment of Narrative Review Articles) tool for assessment of narrative reviews https://www.cognibrain.com/sanra-tool-for-assessing-narrative-review-articles/.9 SANRA is a critical appraisal tool used to assess the quality of narrative reviews and research articles, it consists of a six-question questionnaire. Each question is evaluated on a scale from zero to two (ie, 0, 1, and 2) resulting in a maximum cumulative score of 12 for each review. Studies with a maximum score of five (ie, 0–5) were considered low-quality, those with a total score from five to seven (ie, 5–7) were regarded as a medium-quality, and those with a score from seven to ten (ie, 7–10) were considered as high-quality. Initial screening of titles and abstracts and exclusion of duplicate studies was performed in EndNote (Clarivate Analytics, Philadelphia, PA, USA). Rating of studies with AMSTAR-2 and SANRA tools, respectively, was performed by two reviewers independently PP and PT.
Statistical Analysis
Clinical data collected from the selected studies were entered into an Excel v160 spreadsheet (Microsoft Corporation 2018). We performed descriptive statistical analyses using SPSS version 23 (IBM Corporation) and Excel version 16.0 (Microsoft Corporation, 2018). The quantity of included publications was calculated per year and per country. We calculated the mean values and standard deviation of the age of participants, time of follow-up, and time of duration of treatment as reported in the systematic reviews.
Results
The electronic database search yielded initially 12,106 studies from the following databases PubMed (n = 155), Google Scholar (n = 11,400), Researchgate (n = 500), and Cochrane (n = 1). Further assessment of the studies resulted in exclusion of 12,015 studies due to duplicates and irrelevance; 58 studies reporting AIs for reproductive reasons; 10 for non-relevant criteria (Clinical conditions other than endometriosis: Breast cancer, Adenomyosis, Myomas, Endometrial cancer). Finally, 24 studies were selected for inclusion, 5 were Systematic reviews10–14 and 19 were Narrative reviews.15–33 The PRISMA flowchart of the process of the selection of the studies is exhibited in Figure 2 The systematic reviews dated from 2008 to 202110,14 and the narrative reviews dated from 1999 to 2018.15,33 The majority of the studies originated from the USA (n = 9)-37.5%,15–17,20,22,24,25,27,28 followed by Italy (n = 7)-29.1%12,13,19,23,29,31,33 and Belgium (n = 2)-8.3%18,26 the rest of the countries UK,10 Greece,11 China,14 Turkey,21 Egypt30 Poland32 presented with one study,(n=1)-4.1%. The most frequent year of publications was 2011 with (n = 4)-16.6% studies.11,12,26,27 The percentage of the included publications per country and per year are exhibited in Figure 3. All studies were performed in university teaching settings; the total population of patients enrolled in these 5 systematic reviews was 2650 women.
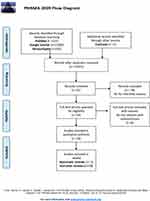 |
Figure 2 The PRISMA flowchart of the process of the selection of the studies. |
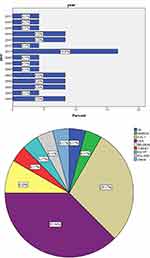 |
Figure 3 Histogram exhibiting the percentages of publications per year and pie-chart exhibiting the percentages of publications of the included studies per country. |
Overview of Systematic Reviews
In total 5 systematic reviews were included in the study.10–14 The studies originated from UK,10 Greece,11 Italy12,13 and China.14 The clinical data of the included systematic reviews are exhibited in Table 1.
 |
Table 1 With the Data of the 5 Included Systematic Reviews |
In the first study produced by Nawathe et al10 the authors performed a systematic review retrieving information from (n = 4) databases (MEDLINE, EMBASE, CINAHL, Cochrane), and flowchart methodology was used. Quality assessment of the selected studies was reported as follows: Studies with the randomized design were considered by the authors they provide a high level of evidence; the lowest level of evidence was provided by the case reports; no tool for quality assessment of these studies was reported. Inclusion criteria were women previously treated surgically and medically for endometriosis. The authors systematically reviewed (n = 7) observational studies consisting of (n = 4) case reports,34–37 (n = 3) non-randomized38–40 and (n = 1) randomized control trial (RCT),41 enrolling in a total of 137 women. Of all these women n = 135-(98.5%) were premenopausal and n = 2-(1.5%) were postmenopausal. The mean age of the enrolled participants was 31.3±4,9 range (25–57) years. The main outcomes of the studies were: Pelvic pain, Lesion size, Quality of life (QOL), and Bone density (BD). The mean treatment duration in months was 7.5±4.5 range (3–18) months and the mean duration of follow-up in months was 8.75± 6.2 months range (6–24) months. An RCT with 97 women demonstrated that AIs in combination with GnRH analogs ameliorated pain scores (P < 0.0001) combined with significant improvement in 24 months of therapy, multidimensional scores (P < 0.0001) compared with GnRH analogs alone.41 Lesion size was assessed according to ASRM (American Society of Reproductive Medicine) score of42 in a non-randomized study the authors reported a reduction of lesion size after combined treatment with letrozole and norethindrone.38 A significant reduction of the endometriotic lesion from 900 mm2 to 90 mm2 was reported after treatment with Anastrazole; this was the first report in the literature of AIs as a treatment modality in endometriosis; however, a decrease of 6.2% of the Bone Density (BD) was observed.34 Quality of life was reported as improved in a non-randomized study after vaginal administration of Anastrazole in patients with rectovaginal endometriosis.39
Another mini-systematic review consisted only of (n = 5) case reports and reported the administration of AIs as medical management of endometriosis in postmenopausal women which was the inclusion criteria.11 Polyzos et al11 did not use flowchart methodology did not report a search strategy and did not assess the quality of studies. The outcomes of the studies were: Pelvic pain, Lesion size, and Bone density (BD). The mean age of these women was 31.5±1.68 range (46–61) years; the mean duration of treatment was 9±5.6 months range (15 days-18 months). All AIs of the 3rd generation were used as a treatment regimen. The authors reported pain relief in (n = 4) studies;34,37,43,44 a reduction of the size of lesions in (n = 3) studies.34,37,44 Reduction of BD in (n = 1) study34 and hot flushes in (n = 1) study.45
Ferrero et al12 published a systematic review in 2011. The authors searched (n = 4) databases (MEDLINE, EMBASE, Scopus, Cochrane) with the use of flowchart methodology according to MOOSE (Metanalysis of Observational studies in Epidemiology) guidelines as proposed by Stroup et al.46 Inclusion criteria were: Premenopausal women with primary or recurrent endometriosis previously treated medically or surgically and women with dyspareunia, dysmenorrhea, pelvic pain, and dyschezia. The outcomes were: Changes in the intensity of endometriosis-related pelvic pain during treatment with AIs either alone or combined with other hormonal therapies but not combined with surgery (primary outcome). Efficacy of AIs either alone or combined with other hormonal therapies in preventing the recurrence of pain after surgery for endometriosis (secondary outcome). The review included (n = 4) randomized control studies41,47–49 and (n = 5) prospective non-comparative-observational studies38–40,50,51 and (n = 1) prospective patient preference trial.52 The mean age of the enrolled participants was 31.5±1.68 range (23–51) years; the mean duration of treatment was 5.6±1.2 range (2–6) months. In this study, 3 new RCTs were added.47–49 An RCT compared AIs or danazol for 6 months, a significant reduction in pain intensity was reported.47 Furthermore, Alborzi et al48 carried out an RCT that compared letrozole or triptorelin or no treatment, the authors reported that the rate of recurrence was 6.4% in the letrozole group, 5.0% in patients treated with triptorelin and 5.3% in patients receiving no treatment (not statistically significant). Ferrero et al49 compared Letrozole or norethisterone acetate or triptorelin treatment and reported a decrease in the intensity of pelvic pain. In most studies, additional treatment with Calcium and Vit D was provided.47,49–51 In a study by Remorgida et al,50 the authors administered Letrozole+Desogestrel, the authors reported that all patients (n = 12) were diagnosed with cysts and the study was discontinued. In a prospective patient preference trial,52 combining letrozole and norethisterone acetate or norethisterone acetate (NETA) various adverse effects (irregular bleeding, depression, weight gain, insomnia, migraine, and decrease of libido) were reported in (n = 23) patients.
In the fourth systematic review published by Garzon et al,13 the authors searched (n = 4) databases (MEDLINE, EMBASE, Cochrane, Web of Science), without flowchart methodology. Inclusion criteria were as follows: Patients with endometriosis (any type of diagnosis) and underwent AIs administration with or without add-back therapy, after surgery or as exclusive therapy. The authors updated the review and added 5 new studies which were not included in the previous systematic reviews.14,53–56 The mean age was 32±1.8 years, the mean duration of treatment was 6±3.28 range (3–12) months.
One study reported reduction in the volume of endometriomas and reduction in the levels of Ca-125 in patients treated with goserelin 3.6 mg sc + anastrazole 1 mg before in vitro fertility procedures (IVF)14 Improvement of gastrointestinal symptoms and improvement of quality of life after treatment with Letrozole and NETA were reported in a study by Ferrero et al.53
Combination of letrozole (5 mg/d) +norethindrone acetate (5 mg/d) add-back therapy (daily progestins or conjugated estrogens and progestins) resulted to a decrease of 50% in volume of endometriomas after transvaginal ultrasound assessment.54
A combination of Letrozole 2.5mg/d + NETA 2.5mg/d + Ca 1000mg/d + vitamin D 880, NETA, or triptorelin +tibolone, or desogestrel, or sequential oral contraceptive pill led to reduction of rectovaginal nodules in 67% of patients and improvement of quality of life as reported by Ferrero et al.55
A prospective patient preference study reported reduction of endometriomas after administration of Letrozole 2.5 mg +NETA 2.5mg or NETA 2.5 mg alone.56
Sun et al14 performed a systematic review and meta-analysis of 19 studies published in Chinese language.57–75
The meta-analysis was registered in PROSPERO website (https://www.crd.york.ac.uk/prospero). The authors searched the PubMed, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang databases and VIP Database. Randomized control trials (RCT) were included only in the systematic review if they compared Letrozole+Dydrogesterone (experiment group) vs Letrozole alone (control group) for treatment of endometriosis; flowchart methodology was used; dosage regimen was not specified; duration of treatment was 6 months. The outcome measures were the following: Total effectiveness, Vascular Endothelial Growth Factor (VEGF) level, Carbohydrate Antigen 125 (CA125) level, Follicle-Stimulating Hormone (FSH) level, Luteinizing Hormone (LH) level, estrogen (E2) level, progesterone (P) level, interleukin-6 (IL-6) level, and tumor necrosis factor-a (TNF-a) levels. Meta-analysis exhibited that total effectiveness was significantly higher in experiment group (OR 6.21, 95% CI 4.17 to 9.24; p < 0:00001); levels of VEGF, CA125, E2, P, IL-6 and TNF-a were found to be lower in experiment group (Letrozole+Dydrogesterone); whilst no changes in levels of FSH and LH were observed in both groups. The authors performed risk of bias assessment with Cochrane quality assessment tool https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials; great heterogeneity was observed between the studies in the interpretation of the results. Adverse effects of the treatment were not reported by the authors in any group.
Quality Assessment of the Systematic Reviews
The methodological quality of the included systematic reviews was evaluated with AMSTAR 2 tool8 which is online at https://amstar.ca/Amstar_Checklist.php. The results of the assessment are exhibited in Table 2. The analysis showed that one systematic review was of critically low-quality,34 three systematic reviews10,35,36 were of low quality and one systematic review14 was of high quality according to the 16 items of AMSTAR 2. Four systematic reviews10,34–36 did not meet the criteria in item 9 which questions if the authors used a satisfactory technique for assessing the risk of bias (RoB) in individual studies that were included in the review. In addition, all the studies did not meet the criteria in item 10 which questioned if the authors reported on the sources of funding for the studies included in the review. Four studies did not report in item 15, which asked If they performed the quantitative synthesis and if the authors carried out an adequate investigation of publication bias (small study bias) and discussed its likely impact on the results of the review. Finally, items 11 and items 12 were not applied in four systematic reviews,10,34–36 because it was not performed by the authors; these items addressed the question if a meta-analysis was performed with statistical methods (item 11), and if this meta-analysis which was performed included an assessment of the Risk of Bias-(RoB) (item 12). The systematic review,14 which was assessed and found to be of high quality, met the criteria of 15 items.
 |
Table 2 Table Exhibiting the Results of Quality Assessment of the Included Systematic Reviews According to AMSTAR-2 Criteria |
Narrative Reviews and Quality Assessment
In total 19 narrative reviews were included in the systematic review. Studies were assessed with SANRA tool for assessment of narrative reviews. All studies performed a detailed and well-designed narrative review on the use of AIs as a medical treatment for endometriosis. The summary of the included narrative reviews is exhibited in Table 3. The table contains the following data: Author and year of publication; the country and city of publication; conclusions of the narrative reviews; the scores; the quality of narrative reviews. Detailed aspects and information about the biological mechanisms of suppression of endometriosis by aromatase inhibitors were provided from all reviews. All the studies were assessed and showed all high quality. Only one study30 obtained the highest score of 11, this was because the authors performed and described a literature search, which is the 3rd item of all 6 items of SANRA. Nine studies obtained a score of 10, in these reviews, the authors neither performed nor described a literature search.17,24–29,31,33 Nine studies obtained a score of 9 this rating was because the aim of these studies was formulated in general and not in concrete questions according to the 2nd item of SANRA.15,16,18–23,32
 |
Table 3 Demonstration of the Narrative Reviews and Their Assessment According to SANRA (Scale for the Assessment of Narrative Review Articles) |
Strengths and Limitations
In the current study, we strived to perform an extensive and analytical systematic review of systematic reviews and narrative reviews. This systematic review is the first of its kind and has used a specific flowchart methodology according to PRISMA guidelines. The selected studies’ systematic and narrative reviews have been assessed for their quality according to AMSTAR-2 and SANRA criteria, respectively. Low quality was observed in four of the systematic reviews; one systematic review was assessed and rated of high quality. Sun et al14 performed a systematic review and meta-analysis of 19 studies; however, these studies were written in Chinese language and accessible only to native language researchers. The authors reported that there was heterogeneity between the studies; evidence which was firstly due to sample size and measuring method and secondly due to the lack of English literature studies; which may have affected the extrapolation of results.14 High-quality assessment was observed in all narrative reviews. The fact of low quality in four of the systematic reviews was due to the lack of metanalysis of randomized control studies and the inclusion of non-homogenous observational studies and case reports. These studies showed a greater risk of bias (RoB). About the narrative reviews, it was observed a high rating due to the agreement in most of the criteria of SANRA.
Discussion
In the current review, we observed that four systematic reviews10–13 were associated with low methodological quality due to lack of meta-analysis and one systematic review with high methodological quality due to performance of meta-analysis.14 Clinical data from observational studies have not been conclusive. Narrative reviews exhibited high quality; however, the level of evidence provided by these studies is significantly lower than the systematic reviews.
Aromatase inhibitors are in the first line of treatment for estrogen-receptor-positive breast cancer.76
All the trials and reviews reported data about third-generation AIs. The most investigated aromatase inhibitor, which demonstrated effectiveness, was letrozole at the dosage of 2.5 mg/day combined with norethisterone-acetate (NETA) 2.5 mg/day for six months as reported by Garzon et al.13 Sun et al14 performed a meta-analysis of 19 studies comparing the administration of Letrozole+Dydrogesterone vs Letrozole alone for 6 months. The authors did not define the dosage, furthermore adverse effects were not reported; the studies were all written in Chinese language. Meta-analysis exhibited significant heterogeneity between the studies.14 A standard dose and standard regiment of treatment were not defined. Aromatase inhibitors are administered orally and can be given vaginally, a well-established route that maintains efficacy, avoids hepatic-first-pass metabolic effects, and has a better safety profile.13,39 Hefler et al39 administered vaginally 0.25 mg Anastrazole in a 2-gr suppository as treatment of pelvic in patients with rectovaginal endometriosis with good results.
Another type of vaginal administration is the vaginal ring with silicone elastomer covered by a single continuous transparent elastomeric membrane for controlled drug release containing Anastrazole and Levonorgestrel.77–79 The first-in-human study was conducted by Schultze-Mosgau et al,77 it was a randomized open-label, multicenter, Phase 1 study with 3 parallel groups of healthy women who received a three-dose combined Anastrazole-Levonorgestrel intravaginal ring for 56 days. Pharmacokinetics, Pharmacodynamics, and clinical safety were assessed. Further investigation of this route of administration was performed by Reinecke et al78 a randomized, parallel-group, double-blind phase IIb clinical trial, and the authors have reached the conclusion that Anastrazole and Levonorgestrel combined in a vaginal ring do not cause functional cysts and ovulation is not inhibited.
A factor that limits the administration of AIs as a treatment option for endometriosis is the development of menopausal symptoms and adverse effects.48 Different studies have reported that AIs decrease bone density;34,38,40,41 hot flushes;45 irregular bleeding, depression, weight gain, insomnia, migraine, and decrease of libido.52 In all studies, patients had a bone density assessment before treatment with AIs; moreover, patients with osteopenia were excluded.13 In patients with decreased bone density in the premenopausal period, Calcium and Vit D should be administered simultaneously.
In addition, combination of AIs with desogestrel has resulted in the formation of cysts in all patients and the discontinuation of a trial.50
Adverse effects due to the suppression of estrogens can be managed with combined treatment with norethisterone acetate.51 Therefore, the application of monotherapy of AIs can increase the risk of adverse effects. We should also consider the addition of add back therapy-daily small doses of progestins or conjugated estrogens with progestins given daily to reduce the effects of antiestrogenic treatment. Di Vasta et al80 administered add back therapy in patients with endometriosis treated initially with GnRH analogs, and they reported that hormonal add-back successfully preserved bone health and improved quality of life of the randomized participants.
What is significant to be reported despite the adverse effects all studies did not report significant alterations in the hematological, cardiological, and hepatological status of the patients.13 This finding is of cardinal importance in minimizing the risk of subsequent mortality after AIs administration.
Dunselman et al81 in 2014 reported that the publication of only 4 randomized control trials does not support the fact that AIs can be used as first-line treatment of Endometriosis. However, it may act as an alternative treatment for endometriosis in cases where progestins, contraceptives, and GnRH (Gonadotropin releasing hormone) analogs do not provide therapeutic benefits.81 We must not forget that AIs have protective action in breast malignant and premalignant diseases, opposite progestins and contraceptives do not exhibit these actions and may increase the risk of developing premalignant and malignant breast lesions.
Conclusion
This review provides an overview of aromatase inhibitors for the treatment of endometriosis and it is the first of its kind. Analytical and extensive systematic review of previous systematic reviews and narrative reviews was performed. Endometriosis is a frequent disease that leads to socioeconomic problems, lack of cost-effectiveness in treatment. Currently, 3rd generation Aromatase inhibitors are used in clinical practice and may be used as alternative treatment in cases where first-line treatment has not been beneficial. The combination of letrozole at the dosage of 2.5 mg/day with norethisterone-acetate (NETA) 2.5 mg/day for six months could be used in the future as treatment option. Additional studies with randomized design should be implemented in the future. These studies should define the therapeutic dose, the combination therapy which will decrease adverse effects and new add-back therapy modalities. Future directions should examine the most-appropriate way of administration and the duration of therapy.
Abbreviations
Ais, Aromatase inhibitors; RCT, Randomized control trial; NoR, Non randomized; PNoR, Prospective non randomized; CR, Case report; BD, Bone density; Mts, Months; Nco, Non comparative prospective; PPP, Prospective patient preference trial; NETA, Norethisterone acetate; PP, Prospective pilot study; Sc, Subcutaneous; IVF, In vitro fertility; QOL, Quality of life; RoB, Risk of Bias; PV; Per vaginam; GnRH, Gonadotropin releasing hormone; AMSTAR 2, A Measurement Tool to Assess Systematic Reviews; SANRA; Scale for the Assessment of Narrative Review Articles; MOOSE, Metanalysis of Observational studies in Epidemiology; ASRM, American Society of Reproductive Medicine; PRISMA, Preferred Reporting Items for Systematic Reviews and Metanalyses; MeSH, medical subject headings; CINAHL, Cumulative Index to Nursing and Allied Health Literature; BMI, Body mass index; CNKI, China National Knowledge Infrastructure.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Missmer SA, Hankinson SE, Spiegelman D, et al. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784–796.
2. Ashrafi M, Sadatmahalleh SJ, Akhoond MR, Talebi M. Evaluation of risk factors associated with endometriosis in infertile women. Int J Fertil Steril. 2016;10(1):11–21. doi:10.22074/ijfs.2016.4763
3. Lucidi RS, Witz CA, Chrisco M, Binkley PA, Shain SA, Schenken RS. A novel in vitro model of the early endometriotic lesion demonstrates that attachment of endometrial cells to mesothelial cells is dependent on the source of endometrial cells. Fertil Steril. 2005;84:16–21. doi:10.1016/j.fertnstert.2004.10.058
4. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–519. doi:10.1016/j.fertnstert.2012.06.029
5. Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP. Survival with aromataseinhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: a meta-analysis. J Natl Cancer Inst. 2006;98:1285–1291. doi:10.1093/jnci/djj357
6. Geisler J, King N, Anker G, et al. In vivo inhibition of aromatization by exemestane, a novel irreversible aromatase inhibitor, in postmenopausal breast cancer patients. Clin Cancer Res. 1998;4(9):2089–2093.
7. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;21(339):b2700. doi:10.1136/bmj.b2700
8. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi:10.1136/bmj.j4008
9. Baethge C, Goldbeck-Wood S, Mertens S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;26(4):5. doi:10.1186/s41073-019-0064-8
10. Nawathe A, Patwardhan S, Yates D, Harrison GR, Khan KS. Systematic review of the effects of aromatase inhibitors on pain associated with endometriosis. BJOG. 2008;115(7):818–822. doi:10.1111/j.1471-0528.2008.01740.x
11. Polyzos NP, Fatemi HM, Zavos A, et al. Aromatase inhibitors in post-menopausal endometriosis. Reprod Biol Endocrinol. 2011;21(9):90. doi:10.1186/1477-7827-9-90
12. Ferrero S, Gillott DJ, Venturini PL, Remorgida V. Use of aromatase inhibitors to treat endometriosis-related pain symptoms: a systematic review. Reprod Biol Endocrinol. 2011;21(9):89. doi:10.1186/1477-7827-9-89
13. Garzon S, Laganà AS, Barra F, et al. Aromatase inhibitors for the treatment of endometriosis: a systematic review about efficacy, safety and early clinical development. Expert Opin Investig Drugs. 2020;29(12):1377–1388. doi:10.1080/13543784.2020.1842356
14. Sun S, Zhang H, Zhong P, Xu Z. The effect of letrozole combined with dydrogesterone for endometriosis in China: a meta-analysis. Biomed Res Int. 2021;2(2021):9946060.
15. Zeitoun KM, Bulun SE. Aromatase: a key molecule in the pathophysiology of endometriosis and a therapeutic target. Fertil Steril. 1999;72(6):961–969. doi:10.1016/S0015-0282(99)00393-3
16. Bulun SE, Zeitoun K, Takayama K, et al. Estrogen production in endometriosis and use of aromatase inhibitors to treat endometriosis. Endocr Relat Cancer. 1999;6(2):293–301. doi:10.1677/erc.0.0060293
17. Bulun S, Zeitoun K, Takayama K, Sasano H. Molecular basis for treating endometriosis with aromatase inhibitors. Hum Reprod Update. 2000;6:413–418. doi:10.1093/humupd/6.5.413
18. D’Hooghe TM. Immunomodulators and aromatase inhibitors: are they the next generation of treatment for endometriosis? Curr Opin Obstet Gynecol. 2003;15(3):243–249. doi:10.1097/00001703-200306000-00006
19. Viganò P, Mangioni S, Odorizzi MP, Chiodini A, Rocca S, Chiodo I. Use of estrogen antagonists and aromatase inhibitors in endometriosis. Curr Opin Investig Drugs. 2003;4(10):1209–1212.
20. Bulun SE, Fang Z, Imir G, et al. Aromatase and endometriosis. Semin Reprod Med. 2004;22(1):45–50.
21. Karaer O, Oruç S, Koyuncu FM. Aromatase inhibitors: possible future applications. Acta Obstet Gynecol Scand. 2004;83(8):699–706. doi:10.1111/j.0001-6349.2004.00562.x
22. Bulun SE, Imir G, Utsunomiya H, et al. Aromatase in endometriosis and uterine leiomyomata. J Steroid Biochem Mol Biol. 2005;95(1–5):57–62. doi:10.1016/j.jsbmb.2005.04.012
23. Ferrero S, Abbamonte LH, Anserini P, Remorgida V, Ragni N. Future perspectives in the medical treatment of endometriosis. Obstet Gynecol Surv. 2005;60(12):817–826. doi:10.1097/01.ogx.0000189153.87365.dc
24. Attar E, Bulun SE. Aromatase inhibitors: the next generation of therapeutics for endometriosis? Fertil Steril. 2006;85(5):1307–1318. doi:10.1016/j.fertnstert.2005.09.064
25. Ferrero S, Venturini PL, Ragni N, Camerini G, Remorgida V. Pharmacological treatment of endometriosis: experience with aromatase inhibitors. Drugs. 2009;69(8):943–952. doi:10.2165/00003495-200969080-00001
26. Colette S, Donnez J. Are aromatase inhibitors effective in endometriosis treatment? Expert Opin Investig Drugs. 2011;20(7):917–931. doi:10.1517/13543784.2011.581226
27. Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod Biol Endocrinol. 2011;21(9):87. doi:10.1186/1477-7827-9-87
28. Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis. Fertil Steril. 2012;98(6):1370–1379. doi:10.1016/j.fertnstert.2012.08.053
29. Ferrero S, Remorgida V, Maganza C, et al. Aromatase and endometriosis: estrogens play a role. Ann N Y Acad Sci. 2014;1317:17–23. doi:10.1111/nyas.12411
30. Abu Hashim H. Potential role of aromatase inhibitors in the treatment of endometriosis. Int J Womens Health. 2014;21(6):671–680. doi:10.2147/IJWH.S34684
31. Benagiano G, Petraglia F, Gordts S, Brosens I. A new approach to the management of ovarian endometrioma to prevent tissue damage and recurrence. Reprod Biomed Online. 2016;32(6):556–562. doi:10.1016/j.rbmo.2016.03.001
32. Słopień R, Męczekalski B. Aromatase inhibitors in the treatment of endometriosis. Prz Menopauzalny. 2016;15(1):43–47. doi:10.5114/pm.2016.58773
33. Ferrero S, Barra F, Leone Roberti Maggiore U. Current and emerging therapeutics for the management of endometriosis. Drugs. 2018;78(10):995–1012. doi:10.1007/s40265-018-0928-0
34. Takayama K, Zeitun K, Gunby RT, Sasano H, Carr BR, Bulun SE. Treatment of severe postmenopausal endometriosis with an aromatase inhibitor. Fertil Steril. 1998;69:709–713. doi:10.1016/S0015-0282(98)00022-3
35. Shippen ER, West WJ
36. Razzi S, Fava A. Treatment of severe recurrent endometriosis with an aromatase inhibitor in a young ovariectomized woman. BJOG. 2004;81:290–296.
37. Fatemi HM, Al-Turki HA, Papanikolaou EG, Kosmas L, De Sutter P, Devroey P. Successful treatment of an aggressive recurrent postmenopausal endometriosis with an aromatase inhibitor. Reprod Biomed Online. 2005;11:455–457. doi:10.1016/S1472-6483(10)61140-6
38. Ailawadi R, Jobanputra S, Kataria M, Gurates B, Bulun SE. Treatment of endometriosis and chronic pelvic pain with letrozole and norethindrone acetate: a pilot study. Fertil Steril. 2004;81:290–296. doi:10.1016/j.fertnstert.2003.09.029
39. Hefler L, Grimm C, van Trotsenburg M, Nagele F. Role of the vaginally administered aromatase inhibitor anastrozole in women with rectovaginal endometriosis: a pilot study. Fertil Steril. 2005;84:1033–1036. doi:10.1016/j.fertnstert.2005.04.059
40. Amsterdam LL, Gentry W, Jobanputra S, Wolf M, Rubin SD, Bulun SE. Anastrazole and oral contraceptives: a novel treatment for endometriosis. Fertil Steril. 2005;84:300–304. doi:10.1016/j.fertnstert.2005.02.018
41. Soysal S, Soysal M, Ozer S, Gul N, Gezgin T. The effects of post-surgical administration of goserelin plus anastrozole compared to goserelin alone in patients with severe endometriosis: a prospective randomized trial. Hum Reprod. 2004;19:160–167. doi:10.1093/humrep/deh035
42. American Society for Reproductive Medicine. Revised American society for reproductive medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. doi:10.1016/S0015-0282(97)81391-X
43. Bohrer J, Chen CC, Falcone T. Persistent bilateral ureteral obstruction secondary to endometriosis despite treatment with an aromatase inhibitor. Fertil Steril. 2008;90:e2007. doi:10.1016/j.fertnstert.2008.03.040
44. Sasson IE, Taylor HS. Aromatase inhibitor for treatment of a recurrent abdominal wall endometrioma in a postmenopausal woman. Fertil Steril. 2009;92:e1171–e1174. doi:10.1016/j.fertnstert.2009.05.071
45. Mousa NA, Bedaiwy MA, Casper RF. Aromatase inhibitors in the treatment of severe endometriosis. Obstet Gynecol. 2007;109:1421–1423. doi:10.1097/01.AOG.0000265807.19397.6d
46. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi:10.1001/jama.283.15.2008
47. Rogaei MA, Tehrani HG, Taherian A, Koleini N. Evaluation of effects of letrozole compared to danazole in patients confirmed endometriosis: a randomized clinical trial. J Isfahan Med Sch. 2010;28:416–424.
48. Alborzi S, Hamedi B, Omidvar A, Dehbashi S, Alborzi S, Alborzi M. A comparison of the effect of short-term aromatase inhibitor (letrozole) and GnRH agonist (triptorelin) versus case control on pregnancy rate and symptom and sign recurrence after laparoscopic treatment of endometriosis. Arch Gynecol Obstet. 2011;284(1):105–110. doi:10.1007/s00404-010-1599-6
49. Ferrero S, Venturini PL, Gillott DJ, Remorgida V. Letrozole and norethisterone acetate versus letrozole and triptorelin in the treatment of endometriosis related pain symptoms: a randomized controlled trial. Reprod Biol Endocrinol. 2011;21(9):88. doi:10.1186/1477-7827-9-88
50. Remorgida V, Abbamonte LH, Ragni N, Fulcheri E, Ferrero S. Letrozole and desogestrel-only contraceptive pill for the treatment of stage IV endometriosis. Aust N Z J Obstet Gynaecol. 2007;47:222–225. doi:10.1111/j.1479-828X.2007.00722.x
51. Remorgida V, Abbamonte HL, Ragni N, Fulcheri E, Ferrero S. Letrozole and norethisterone acetate in rectovaginal endometriosis. Fertil Steril. 2007;88:724–726. doi:10.1016/j.fertnstert.2006.12.027
52. Ferrero S, Camerini G, Seracchioli R, Ragni N, Venturini PL, Remorgida V. Letrozole combined with norethisterone acetate compared with norethisterone acetate alone in the treatment of pain symptoms caused by endometriosis. Hum Reprod. 2009;24:3033–3041. doi:10.1093/humrep/dep302
53. Lossl K, Loft A, Freiesleben NLC, et al. Combined down-regulation by aromatase inhibitor and GnRH-agonist in IVF patients with endometriomas-A pilot study. Eur J Obstet Gynecol Reprod Biol. 2009;144:48–53. doi:10.1016/j.ejogrb.2009.02.001
54. Ferrero S, Camerini G, Ragni N, et al. Letrozole and norethisterone acetate in colorectal endometriosis. Eur J Obstet Gynecol Reprod Biol. 2010;150:199–202. doi:10.1016/j.ejogrb.2010.02.023
55. Agarwal SK, Foster WG. Reduction in endometrioma size with three months of aromatase inhibition and progestin add-back. BioMed Res Int. 2015;2015:878517. doi:10.1155/2015/878517
56. Ferrero S, Leone Roberti Maggiore U, Scala C, et al. Changes in the size of rectovaginal endometriotic nodules infiltrating the rectum during hormonal therapies. Arch Gynecol Obstet. 2013;287:447–453. doi:10.1007/s00404-012-2581-2
57. Lian JM. Effects of didrogesterone combined with letrozole on Kupperman score, ALDH1 level and ovarian reserve function in patients with endometriosis (in Chinese). China Licensed Pharmacist. 2020;17:35–39.
58. Wang J. Effects of dydrogesterone combined with letrozole on hormone levels and serum VEGF levels in patients with endometriosis (in Chinese). Henan Med Res. 2020;29:297–298.
59. Zhang J, Liu Y, Liu FX. Effect of desdrogesterone combined with letrozole on serum carbohydrate antigen 125 level and recurrence rate in patients with endometriosis (in Chinese). Chin J Pharmaceutl Eco. 2019;14:108–110.
60. Xie AL. Effects of dydrogesterone combined with letrozole on estrogen and progesterone and VEGF levels in patients with endometriosis (in Chinese). Henan Med Res. 2019;28:3726–3728.
61. Li XZ, Liu XM. Study on the value of dydrogesterone combined with letrozole in the treatment of endometriosis (in Chinese). Dep Oral Med Elect Magazine. 2019;6:177–178.
62. Liu ZJ. Analysis of the effect of desorgestrel combined with letrozole in the treatment of endometriosis (in Chinese). Inner Mongol Medl J. 2019;51:914–916.
63. Zhao YZ. Effects of dydrogesterone combined with letrozole on sex hormones and menstruation in patients with endometriosis (in Chinese). Clin Res. 2019;27:88–89.
64. Zhang W. Clinical efficacy of treatment with triproterone combined with letrozole in the treatment of endometriosis (in Chinese). J Pract Gynaecol Endocrinol. 2019;6:142–145.
65. Wang LN. Clinical efficacy of treatment with triproterone combined with letrozole in the treatment of endometriosis (in Chinese). Contemp Med. 2019;25:56–58.
66. Bai YX. Clinical effect of dydrogesterone combined with letrozole in the treatment of endometriosis (in Chinese). Henan Med Res. 2019;28:1275–1277.
67. Tan XF, Li HF, Wang YF, Gu YF. Effect of dydrogesterone combined with letrozole on sex hormones, inflammatory factors, CA125 and VEGF in patients with endometriosis (in Chinese). Bas Clin Med. 2019;39:392–395.
68. Cao N. Observation on the effect of dydrogesterone combined with letrozole in the treatment of endometriosis (in Chinese). Med J Chin People’s Health. 2018;30:35–37.
69. Lian JM. Effects of dydrogesterone combined with letrozole on serum sex hormones in patients with endometriosis (in Chinese). Inner Mongol Medl J. 2018;50:311–312.
70. Chen WL. Effects of dydrogesterone combined with letrozole on serum sex hormone levels in patients with endometriosis (in Chinese). Clin Med (Northfield Il). 2018;38:92–94.
71. Hu YR. Efficacy of dydrogesterone and combined with letrozole on patients with endometriosis: the levels of E2, VEGF, and CA125 (in Chinese). Tibet J Med. 2017;38:39–41.
72. Chen XH. Clinical efficacy of treatment with triproterone combined with letrozole in the treatment of endometriosis (in Chinese). Healthmust-Readmagazine. 2019;46:1.
73. Yue HL. To explore the therapeutic effect of dydrogesterone combined with letrozole in patients with endometriosis (in Chinese). Spec Health. 2020;15:55.
74. Liang J. Effects of dydrogesterone combined with letrozole on VEGF and CA125 levels in patients with endometriosis (in Chinese). Chin Health Care Nutrit. 2017;27:366.
75. Chen JL. Effects of dydrogesterone combined with letrozole on serum sex hormone, VEGF and CA125 levels in patients with endometriosis (in Chinese). Heilong Med J. 2020;44:1235–1236.
76. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomized trials. Lancet Lond Engl. 2015;386:1341–1352. doi:10.1016/S0140-6736(15)61074-1
77. Schultze-Mosgau M-H, Waellnitz K, Nave R, et al. Pharmacokinetics, pharmacodynamics, safety and tolerability of an intravaginal ring releasing anastrozole and levonorgestrel in healthy premenopausal women: a Phase 1 randomized controlled trial. Hum Reprod Oxf Engl. 2016;31:1713–1722. doi:10.1093/humrep/dew145
78. Reinecke I, Schultze-Mosgau M-H, Nave R, et al. Model-based dose selection for intravaginal ring formulations releasing anastrozole and levonorgestrel intended for the treatment of endometriosis symptoms. J Clin Pharmacol. 2017;57:640–651. doi:10.1002/jcph.846
79. Nave R. Development of an intravaginal ring delivering simultaneously anastrazole and levonorgestrel: apharmacokinetic prospective. Drug Deliv. 2019;26(1):586–594. doi:10.1080/10717544.2019.1622609
80. DiVasta AD, Feldman HA, Sadler Gallagher J, et al. Hormonal add-back therapy for females treated with gonadotropin-releasing hormone agonist for endometriosis: a randomized controlled trial. Obstet Gynecol. 2015;126(3):617–627. doi:10.1097/AOG.0000000000000964
81. Dunselman GAJ, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29:400–412. doi:10.1093/humrep/det457
82. Ferrero S, Remorgida V, Venturini PL, et al. Norethisterone acetate versus norethisterone acetate combined with letrozole for the treatment of ovarian endometriotic cysts: a patient preference study. Eur J Obstet Gynecol Reprod Biol. 2014;174:117–122. doi:10.1016/j.ejogrb.2013.11.030
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
