Back to Journals » Infection and Drug Resistance » Volume 11
A study on biofilm production and antifungal drug resistance among Candida species from vulvovaginal and bloodstream infections
Authors Tulasidas S , Rao P , Bhat S , Manipura R
Received 7 July 2018
Accepted for publication 31 October 2018
Published 23 November 2018 Volume 2018:11 Pages 2443—2448
DOI https://doi.org/10.2147/IDR.S179462
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Joachim Wink
Sanyuktha Tulasidas,1 Pooja Rao,2 Sevitha Bhat,2 Radhakrishna Manipura1
1Department of Microbiology, Kasturba Medical College, Manipal Academy of Higher Education, Mangalore, Manipal, India; 2Department of Microbiology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal McGill Center for Infectious Diseases, Mangalore, Manipal, India
Introduction: Candida species, one among the opportunistic fungi, has become a common pathogen causing vaginal thrush and nosocomial bloodstream infections (BSIs). This study aims to evaluate the prevalence and antifungal susceptibility of various Candida species and slime production by Candida species in BSIs and vulvovaginal candidiasis (VVC).
Materials and methods: A total of 176 samples were collected for a period of 1 year. Antifungal susceptibility testing and biofilm production testing were performed by the Kirby-Bauer method and crystal violet assay, respectively.
Results: Out of 176 samples, 74 (42%) were from BSIs and 102 (58%) were from VVC. The biofilm production was comparatively high in blood isolates, 55 (74%), than cervical isolates, 45 (44%). Increase in the trends of non-albicans Candida (NAC) species was seen in our setup. Good susceptibility rates were seen among Candida species, 82.38% to voriconazole and an increasing resistance pattern of 26.13% to fluconazole.
Conclusion: Speciation of Candida becomes important as the prevalence of NAC is increasing. Antifungal susceptibility testing by the disk diffusion method is cost effective and should be adopted in routine testing as there is an increasing azole resistance, especially in invasive NAC infections. In this study, there was no correlation of antifungal drugs with the biofilm production.
Keywords: biofilm, Candida, azoles, vulvovaginal, bloodstream infections
Introduction
Candida species are part of the normal flora of healthy individuals and are considered opportunistic pathogens as they colonize different tissues and cause systemic mycosis when the immune system of the host is depressed.1 Over the last few years, the incidence of fungal infections has progressively raised and it has been a primary cause of morbidity and mortality in immune-compromised and severely ill patients and hence is rightly called the “disease of diseased”.2 Vulvovaginal candidiasis (VVC) affects 75% of all women at least once in their lifetime, most commonly during childbearing age.3 Candida species is the fourth most common cause of nosocomial bloodstream infections (BSIs), with a significantly high attributable mortality (49%–70%). The use of central venous catheters has been found to be responsible for >70% of bloodstream and deep-tissue infections.4 Candida albicans is the most common fungal isolate recovered from blood in the last few decades.5 Infections caused by Candida spp. depend on expression of virulence factors such as germ tube formation, adhesins, phenotypic switching, biofilm formation, and the production of hydrolytic enzymes.6
In recent studies, it was found that 80% of human infections result from pathogenic biofilms. Biofilms are group of microorganisms that are embedded in an extracellular matrix (ECM), forming a complex three-dimensional architecture on biotic and abiotic surfaces.3 Depending on different sites of infection and environmental cues, quantity and composition of the biofilm vary from one species to another, but C. albicans has unique developmental characteristics because of dimorphic switching from a yeast form to a filamentous form.7,8 The presence of biofilm provides structural heterogeneity, decreases susceptibility to antimicrobials and biocides, and protects the fungus from host defenses.9 Candida shows resistance to azoles due to widespread and long term use of it, so it is necessary to identify Candida species during early infection and monitor their antifungal susceptibility for providing appropriate treatment.10 Hence, our study evaluates the prevalence, antifungal susceptibility in VVC and bloodstream candidemia (BSC), and also compares the biofilm formation in various Candida species.
Materials and methods
A 12-month cross-sectional study was conducted in the Department of Microbiology, Kasturba Medical College, Mangalore, India. Patients of all age groups clinically suspected to have candidemia or candida vaginitis have been included in the study. A total of 176 clinical isolates of Candida species was obtained. Presumptive identification of Candida isolates was done by conventional methods such as colony morphology (white- to cream-colored pasty colonies), Gram staining (Gram-positive budding yeast cells with or without pseudohyphae), germ tube test, and HiCrome agar (M1297A-100G; HiMedia Laboratories, Mumbai, India) microscopic morphology on corn meal agar (Microexpress; Tulip Diagnostics, Mumbai, India).11 Colonies were identified by their white to cream color pasty appearance, and Gram stain was done to identify and confirm as Candida. It was then subcultured onto chrome agar for speciation and incubated at 37°C for 48 hours. Isolates were then differentiated by the color produced in chrome agar after 48 hours, ie, green – C. albicans, blue – Candida tropicalis, white – Candida glabrata, and pinkish purple – Candida krusei. The isolates that were not identified on the chromogenic medium by the color were further identified by microscopic morphological features obtained through slide cultures on corn meal agar incubated at 37°C for 48 hours.11 Some of the bloodstream isolates were identified to species level using the VITEK 2 yeast identification system.
Biofilm formation
Growth from chocolate agar was taken in 5 mL of sterile brain heart infusion (BHI) broth and incubated overnight. It was followed by a 1:100 dilution in BHI. Then, 100 µL of diluted broth was incubated at 37°C overnight in commercially available presterilized, polystyrene, round-bottomed, 96-well microtiter plate (Cat no. 2101; Thermo Fischer Scientific, Waltham, MA, USA) for biofilm production. The microtiter plates were washed with distilled water.
Crystal violet assay
After washing with distilled water, the microtiter plate was stained with 120 µL of 0.1% aqueous crystal violet solution (crystal violet, Hi-Cert/ACS-GRM114-10G) and incubated at room temperature for 15 minutes. Each well was washed four times with sterile distilled water, blot dried, immediately de-stained with 125 µL of 95% methanol, and incubated for 15 minutes at room temperature. After de-staining, 100 µL of de-staining solution was transferred to a new well, and the de-staining solution was measured spectrophotometrically using the ELISA reader at 570 nm.12 Each sample was run in duplicates.
The following were the values for the biofilm formation (OD cutoff value – ODC, average OD of negative control – Avg NC)
Avg NC=0.194 SD=0.013, ODC =Avg NC+3×0.013 (SD).13
0.194+0.039, ODC=0.233(1)
OD≤ODC=non-adherent
ODC<OD≤2 ODC=weakly adherent
2ODC<OD≤4 ODC=moderately adherent
4ODC<OD=strongly adherent
Antifungal susceptibility test
It was done by the Kirby-Bauer disk diffusion method, mainly for azoles like fluconazole (25 µg, HiMedia Laboratories) and voriconazole (1 µg; HiMedia Laboratories). It was performed on Mueller-Hinton agar supplemented with 2% glucose and 0.5 g of methylene blue per milliliter for enhancing the definition of growth margins,14,15 with zone diameter for fluconazole (25 µg) ≥19 mm –susceptible, 15–18 mm – susceptible and dose dependent, and ≤14 mm – resistant and that for voriconazole (1 µg) ≥17 mm – susceptible, 14–16 mm – susceptible and dose dependent, and ≤13 mm – resistant.
Along with these, C. albicans ATCC 10231 reference strain was also included in this study. Antifungal susceptibility testing was found to be within satisfactory limits.15 The study samples were collected from the samples that were received routinely in the laboratory. It was not collected separately for this project; hence, informed consent from the patients was not required, and Institutional Ethics Committee, Kasturba Medical College, Mangalore, approved to conduct this study.
The data were analyzed using the SPSS system, version 17.0 (SPSS Inc., Chicago, IL, USA), and subjected to the chi-squared test. At 95% CI, P<0.05 was considered as statistically significant.
Results
Of the total 176 clinical isolates of Candida species, 74 (42%) were obtained from the blood cultures of patients with candidemia, which comprised C. tropicalis (27, 36.4%) followed by C. krusei (19, 25.6%), C. albicans (15, 20.2%), Candida parapsilosis (6, 8.10%), Candida haemulonii (4, 5.4%), and C. glabrata (3, 4%). In all, 102 (58%) Candida species were from women of the reproductive age group diagnosed with VVC involving C. albicans (66, 64.7%) followed by C. tropicalis (23, 22.5%), C. glabrata (10, 9.8%), C. parapsilosis (2, 1.9%), and C. krusei (1). Among the various Candida species isolated, C. albicans (64%) was most common in VVC, while C. tropicalis (23%) was most common in BSC.
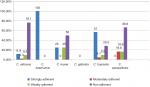
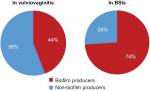
A total of 176 clinical isolates was subjected to biofilm production; among 74 blood culture isolates, 55 (74%) were biofilm producers; strong adherence was seen in C. haemulonii (100%), moderate adherence was seen in C. tropicalis (22%), and weak adherence was seen in C. krusei (21%), equation 1. Among 102 cervical swab isolates, 45 (44.11%) were biofilm producers, whereas C. tropicalis (43%) showed strong adherence (Figure 1 and Table 1). The biofilm production was higher in isolates from BSI than those from VVC.
  | Figure 1 Biofilm formation by Candida species in BSIs and vaginal candidiasis. Abbreviation: BSIs, bloodstream infections. |
Figure 2 describes that C. haemolunii produces strongly adherent biofilm followed by C. tropicalis, while C. albicans and C. parapsilosis produce no biofilm.
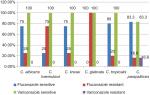
Among the 74 (42%) Candida species isolated from blood, 49 (66.6%) were susceptible to fluconazole while 25 (33.7%) were resistant and 60 (81.1%) isolates were susceptible and 14 (18.9%) were resistant to voriconazole (Figure 3). In our study, C. haemolunii and C. glabrata showed increased resistance to fluconazole, while resistance rates to voriconazole were comparatively very less and seen among some strains of C. parapsilosis (16.6%) and C. albicans (7.1%). Among the species isolated from VVC (102), 81 (79.1%) isolates were susceptible to fluconazole while 21 (20.5%) were resistant and 85 (83.3%) were susceptible and 17 (16.6%) were resistant to voriconazole. C. glabrata showed a high resistance to fluconazole (100%) in VVC (Figure 4).
Discussion
Candida, the opportunistic yeast, has been associated with a wide range of human infections, mortality, and morbidity. The wide spectrum of infections ranges from the superficial skin and its appendages to deep-seated or disseminated Candidiasis.16 During the study period, a total of 176 samples were collected, out of which 74 and 102 isolates were obtained from the BSIs and vulvovaginal infections, respectively.
In the present study, non-albicans Candida (NAC) – C. tropicalis is the most common pathogen to be isolated from patients with BSIs, which is in contrast to the previous studies by Tan et al16 and by Golia et al17 and in line with the study done by Hasan et al18 and Tellapragada et al.19 A higher incidence of C. albicans among vulvovaginal infection was noted in the present study, which is in line with previous studies.2,19 In another study, NAC like C. glabrata and C. tropicalis showed a high incidence in VVC.2 However, next commonly isolated species were NAC indicating their emergence. Hence, NAC cannot be ignored either in BSI or VVC because of their high virulence and tolerance to azoles, making it difficult to treat these infections. Biofilm production is considered as one of the most potent pathogenic traits contributing to treatment failures and recurrent infections. Out of 176 Candida isolates, 100 (56.8%) isolates produced biofilm. In the present study, 55 (74%) blood isolates were biofilm positive; strong biofilm production was seen in C. haemulonii from blood, which is in contrast to study done by Tellapragada et al19 and Deorukhkar et al2 where higher biofilm production was seen in C. albicans. In a study done by Shin et al,5 Kaskatepe et al,20 and Tumbarello et al,21 a higher biofilm production was seen in non-C. albicans.
Among 102 cervical isolates, 45 (44%) were biofilm producers. Strong biofilm production was seen in C. tropicalis, which is in contrast to a study done by Tellapragada et al19 and Harakuni et al,22,23 where a higher biofilm production was noted in C. albicans.
The risk factors for candidemia associated with intensive care unit patients subjected to intravenous and central line catheters, total parenteral nutrition, use of multiple antibiotics, previous steroid therapy, post surgery, and an immunocompromised status.24,25
Out of 176 Candida species, 130 (73.86%) were susceptible to fluconazole, while 46 (26.13%) of them were resistant. In all, 145 (82.38%) of isolates were voriconazole susceptible and 31 (17.6%) were resistant. This shows that there is an increase in resistance to fluconazole, which is in line with the previous study.24 The degree of resistance seen is more common with fluconazole as they are being used more commonly as the initial line of treatment and in empirical therapy. Biofilm formation was prominent in blood isolates when compared to cervical isolates, thus indicating it as one of the virulence markers in BSI; the same was observed in a study done by Harakuni et al.22 Seven of 102 (16.8%) isolates showed biofilm production with resistance to both fluconazole and voriconazole; only one out of 102 isolates showed fluconazole resistance, and among blood samples, 13 of 74 (17.5%) isolates showed biofilm production with resistance to fluconazole and voriconazole and six out of 74 (8.1%) isolates showed biofilm production with resistance to fluconazole alone. We observed a higher degree of resistance to fluconazole in biofilm producers as compared to that in non-producers, which has been seen in NAC and reported in other studies.5,20,25 The limitation of this study is that minimum inhibitory concentration for azoles was not performed.
Conclusion
Biofilm production and antifungal drug resistance were observed more in non-C. albicans, hence indicating its emergence and an issue of crucial importance in the treatment of immunocompromised individuals. Biofilm production could act as one of the factors in reducing the penetrability of the antifungal agents and also increasing the virulence nature of invasive candidiasis. Antifungal drug resistance was more commonly seen with fluconazole. Hence, voriconazole may be used as the initial line of therapy, especially in NAC isolates. These data of Candida-forming biofilms and antifungal susceptibility become necessary to reduce the net effect of the increasing severity of Candida infections, drug resistance, and economic burden.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgment
This study has been supported by Manipal Academy of Higher education, Mangalore, India.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Serrano-Fujarte I, López-Romero E, Reyna-López GE, Martínez-Gámez MA, Vega-González A, Cuéllar-Cruz M. Influence of culture media on biofilm formation by Candida species and response of sessile cells to antifungals and oxidative stress. Biomed Res Int. 2015;2015:1–15. | ||
Deorukhkar SC, Saini S, and Mathew S. Non-albicans Candida Infection: an emerging threat. Interdiscip Perspect Infect Dis. 2014; ID 615958:1-8. | ||
Harriott MM, Lilly EA, Rodriguez TE, Fidel PL, Noverr MC. Candida albicans forms biofilms on the vaginal mucosa. Microbiology. 2010;156(Pt 12):3635–3644. | ||
Chandra J, Mccormick TS, Imamura Y, Mukherjee PK, Ghannoum MA. Interaction of Candida albicans with adherent human peripheral blood mononuclear cells increases C. albicans biofilm formation and results in differential expression of pro- and anti-inflammatory cytokines. Infect Immun. 2007;75(5):2612–2620. | ||
Shin JH, Kee SJ, Shin MG, et al. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: comparison of bloodstream isolates with isolates from other sources. J Clin Microbiol. 2002;40(4):1244–1248. | ||
Deorukhkar SC, Saini S, Mathew S. Virulence factors contributing to pathogenicity of Candida tropicalis and its antifungal susceptibility profile. Int J Microbiol. 2014;2014:1–6. | ||
Tournu H, Dijck PV. Candida biofilms and the host: models and new concepts for eradication. Int J Med. 2011;2012: ID 845352:1–16. | ||
Ramage G, Vandewalle K, Wickes BL, López-Ribot JL. Characteristics of biofilm formation by Candida albicans. Rev Iberoam Micol. 2001;18(4):163–170. | ||
Tumbarello M, Posteraro B, Trecarichi EM, et al. Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J Clin Microbiol. 2007;45(6):1843–1850. | ||
Pahwa N, Kumar R, Nirkhiwale S, Bandi A. Species distribution and drug susceptibility of candida in clinical isolates from a tertiary care centre at Indore. Indian J Med Microbiol. 2014;32(1):44–48. | ||
Agarwal S, Manchanda V, Verma N, Bhalla P. Yeast identification in routine clinical microbiology laboratory and its clinical relevance. Indian J Med Microbiol. 2011;29(2):172–177. | ||
Melek I, Mustafa A, Ayse N. Investigation virulence factors of clinical Candida isolates in relation to atmospheric conditions and genotype. Turk J Med Sci. 2012;42(Supp 2):1476–1483. | ||
Swarna SR, Madhavan R, Gomathi S. A study of biofilm on diabetic foot ulcer. Int J Pharm Biomed Res. 2012;3(4):1809–1810. | ||
Matar MJ, Ostrosky-Zeichner L, Paetznick VL, Rodriguez JR, Chen E, Rex JH. Correlation between E-test, disk diffusion, and microdilution methods for antifungal susceptibility testing of fluconazole and voriconazole. Antimicrob Agents Chemother. 2003;47(5):1647–1651. | ||
Method for antifungal disk diffusion susceptibility testing of yeasts approved guideline. M44-A 2007; Vol. 24; No. 15. | ||
Tan TY, Tan AL, Tee NW, Ng LS. A retrospective analysis of antifungal susceptibilities of Candida bloodstream isolates from Singapore hospitals. Ann Acad Med Singapore. 2008;37(10):835–409. | ||
Golia S, Hittinahalli V, Sangeetha KT et al. Study of biofilm formation as a virulence marker in Candida species isolated from various clinical specimens. JEMDS. 2012;1:1235–1246. | ||
Hasan F, Xess I, Wang X, Jain N, Fries BC. Biofilm formation in clinical Candida isolates and its association with virulence. Microbes Infect. 2009;11(8-9):753–761. | ||
Tellapragada C, Eshwara VK, Johar R, et al. Antifungal susceptibility patterns, in vitro production of virulence factors, and evaluation of diagnostic modalities for the speciation of pathogenic Candida from blood stream infections and vulvovaginal candidiasis. J Pathog. 2014;2014:1–8. | ||
Kaskatepe B, Yildiz S. Investigation of association between slime production by Candida species and susceptibility to fluconazole and voriconazole. Trop J Pharm Res. 2013;12(5):821–825. | ||
Tumbarello M, Posteraro B, Trecarichi EM, et al. Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J Clin Microbiol. 2007;45(6):1843–1850. | ||
Harakuni SU, Karadesai SG, Jamadar N. Biofilm production by Candida: comparison of bloodstream isolates with cervical isolates. Indian J Microbiol. 2012;52(3):504–506. | ||
Paiva LC, Vidigal PG, Donatti L, Svidzinski TI, Consolaro ME. Assessment of in vitro biofilm formation by Candida species isolates from vulvovaginal candidiasis and ultrastructural characteristics. Micron. 2012;43(2-3):497–502. | ||
Bhatt M, Sarangi G, Paty BP, et al. Biofilm as a virulence marker in Candida species in Nosocomial blood stream infection and its correlation with antifungal resistance. Indian J Med Microbiol. 2015;33(Suppl):112–114. | ||
Han SS, Yim JJ, Yoo CG, et al. Clinical characteristics and risk factors for nosocomial candidemia in medical intensive care units: experience in a single hospital in Korea for 6.6 years. J Korean Med Sci. 2010;25(5):671–676. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.