Back to Journals » Clinical Interventions in Aging » Volume 10
A stewardship intervention program for safe medication management and use of antidiabetic drugs
Authors Zhao R, He X, Shan Y, Zhu L, Zhou Q
Received 27 April 2015
Accepted for publication 5 June 2015
Published 23 July 2015 Volume 2015:10 Pages 1201—1212
DOI https://doi.org/10.2147/CIA.S87456
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Rui-yi Zhao,1 Xiao-wen He,1 Yan-min Shan,1 Ling-ling Zhu,2 Quan Zhou3
1Clinical Nurse Specialist Section, Division of Nursing, 2Geriatric VIP Care Ward, Division of Nursing, 3Department of Pharmacy, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, People’s Republic of China
Background: Diabetes patients are complex due to considerations of polypharmacy, multimorbidities, medication adherence, dietary habits, health literacy, socioeconomic status, and cultural factors. Meanwhile, insulin and oral hypoglycemic agents are high-alert medications. Therefore it is necessary to require a multidisciplinary team’s integrated endeavors to enhance safe medication management and use of antidiabetic drugs.
Methods: A 5-year stewardship intervention program, including organizational measures and quality improvement activities in storage, prescription, dispensing, administration, and monitoring, was performed in the Second Affiliated Hospital of Zhejiang University, People’s Republic of China, a 3,200-bed hospital with 3.5 million outpatient visits annually.
Results: The Second Affiliated Hospital of Zhejiang University has obtained a 100% implementation rate of standard storage of antidiabetic drugs in the Pharmacy and wards since August 2012. A zero occurrence of dispensing errors related to highly “look-alike” and “sound-alike” NovoMix 30® (biphasic insulin aspart) and NovoRapid® (insulin aspart) has been achieved since October 2011. Insulin injection accuracy among ward nurses significantly increased from 82% (first quarter 2011) to 96% (fourth quarter 2011) (P<0.05). The number of medication administration errors related to insulin continuously decreased from 20 (2011) to six (2014). The occurrence rate of hypoglycemia in non–endocrinology ward diabetes inpatients during 2011–2013 was significantly less than that in 2010 (5.03%–5.53% versus 8.27%) (P<0.01). Percentage of correct management of hypoglycemia by nurses increased from 41.5% (April 2014) to 67.2% (August 2014) (P<0.01). The percentage of outpatient diabetes patients receiving standard insulin injection education increased from 80% (April 2012) to 95.2% (October 2012) (P<0.05). Insulin injection techniques among diabetes outpatients who started to receive insulin were better than indicated in data from two questionnaire surveys in the literature, including the percentage checking injection sites prior to injection (85.6%), priming before injection (98.1%), rotation of injecting sites (98.1%), remixing before use (94.5%), keeping the pen needle under the skin for >10 seconds (99.4%), and using the pen needle only once (88.7%). On-site inspection indicated of great improvement in the percentage of drug-related problems in the antidiabetes regimen between the first and second quarter of 2014 (1.08% versus 0.28%) (P<0.05).
Conclusion: Quality improvements in safe medication management and use of antidiabetic drugs can be achieved by multidisciplinary collaboration among pharmacists, nurses, physicians, and information engineers.
Keywords: diabetes nursing specialists, injection technique, insulin, medication errors, oral hypoglycemic agents, pharmacy, quality improvements
Introduction
Diabetes patients are usually complex due to consideration of polypharmacy, multimorbidities, medication adherence, dietary habits, health literacy, socioeconomic status, and cultural factors.1 Meanwhile, insulin and oral hypoglycemic agents are included in the Institute for Safe Medication Practices (ISMP) list of high-alert medications that bear a heightened risk of causing significant patient harm when used in error.2 Therefore, safe medication use of antidiabetic drugs should arouse a wide concern.
Physicians need to be aware of the pharmacological mechanism of each class of drugs, contraindications, precautions, drug–drug interactions (DDIs), and adverse effects to formulate a safe and effective management plan for diabetes patients.3 Several studies have described that the situation in medication management and use (MMU) of antidiabetic drugs was not optimistic. Classen et al reported that 10.7% of patients exposed to insulin and hypoglycemic agents experienced associated adverse drug events, from the 2004 Medicare Patient Safety Monitoring System sample’s medical records.4 Geller et al estimated the insulin-related hypoglycemia and errors leading to Emergency Department visits and hospitalizations in the USA – the most commonly identified insulin-related hypoglycemia was attributed to reduced food intake and administration of the wrong insulin product. Severe neurologic sequelae and blood glucose levels of 50 mg/dL or less were documented in an estimated 60.6% and 53.4%, respectively.5 Milligan et al analyzed adverse drug events in older people with diabetes in the care home setting, via incident reports obtained from the National Reporting and Learning Service in the UK during 2005–2009. They found 684 reports related to insulin and 84 incidents related to oral hypoglycemic drugs. The most common error category with both types of drug therapy was wrong or unclear dose.6 A study in 2012 showed that three-fourths of the insulin pens users did not follow the manufacturer’s instructions for proper administration and storage of insulin pens.6 Therefore, it is necessary for clinicians and patients to coordinate and participate in rational use of antidiabetic drugs. Mitchell et al observed that correct usage scores were significantly higher if initial education on insulin pens was performed by a pharmacist or nurse.7 Cohen discussed pharmacists’ role in ensuring safe and effective hospital use of insulin in the inpatient setting by minimizing the likelihood of medication errors related to prescribing, transcription, dispensing, administration, storage, and communication.8 However, there is little literature on multidisciplinary teams’ integrated endeavors to continuously enhance safe MMU of antidiabetic drugs in large-scale hospitals.
The Second Affiliated Hospital of Zhejiang University (SAHZU), a comprehensive academic medical center hospital in the People’s Republic of China, successfully passed Joint Commission International (JCI) accreditation on February 24 of 2013.9 SAHZU performed continuous quality improvements in safe MMU of high-alert medications during the journey to JCI accreditation and in the post–JCI accreditation era. The aim of this article was to discuss the effectiveness of stewardship intervention in MMU of antidiabetic drugs and provide some reference for international counterparts.
Methods
Data collection
A 5-year intervention program, covering the period from 2010 to 2014, focused on MMU of insulin/insulin analogs and hypoglycemic drugs in SAHZU, a 3,200-bed hospital with 3.5 million outpatient visits annually (data in 2013) in Zhejiang Province, which has a population of approximately 54.4 million.
The implementation rate of standard storage of antidiabetic drugs in the Pharmacy as well as in the wards was calculated from on-site inspection results. The appropriateness of antidiabetic regimens for inpatients was evaluated by diabetes specialist nurses and auditing pharmacists. Data on insulin injection accuracy among ward nurses, coverage percentage of standard insulin injection education for diabetes outpatients, and insulin injection techniques among diabetes outpatients who started to receive insulin therapy were obtained from on-site inspection, record forms, and follow-up. Adverse drug reactions (ADRs) and medication errors related to antidiabetic drugs were retrospectively analyzed by retrieving data from an online no-fault reporting system for all staff. All hypoglycemia events were derived from a special online electronic platform for diabetes nursing, and the occurrence rate of hypoglycemia in diabetes patients was then calculated. The data presented in the study is available in the archives of the Drug and Therapeutics Committee of SAHZU. Access and use of these data need permission from the SAHZU Drug and Therapeutics Committee. The study was approved by The Ethics Committee at SAHZU and it was in compliance with the Helsinki Declaration.
Comprehensive intervention measures
Organizational measures
SAHZU established a team of diabetes nursing specialists in September 2009. After 2-year endeavors, the team had ten head nurses as core members, three full-time diabetes specialist nurses, and 61 part-time diabetes specialist nurses, on the basis of “one part-time diabetes specialist nurse per ward”. A three-level diabetic nursing management system was thus formed: (1) primary nurses were responsible for providing basic nursing and patient education (first level); (2) part-time diabetes specialist ward nurses should further strengthen diabetic patient education and contact full-time diabetes specialist nurses for consultation, if necessary (second level); and (3) full-time diabetes specialist nurses provided consultation service and regular on-site inspection (third level). Academic salons were held quarterly by a team of diabetes nursing specialists. Physicians and diabetes nurses worked together to treat outpatients in the Diabetes Center. Key indicators of the diabetes specialist nursing service are listed in Table 1.
  | Table 1 Key indicators of the diabetes specialist nursing service |
According to JCI accreditation standards, a working group, named “MMU”, was established in 2011 and played a pivotal role in quality and patient safety associated with medications. Information regarding ADRs, medication errors, and hypoglycemia events were reported to the Division of Medical Management, Division of Nursing, Pharmacy and Office of Quality Management. Targeted quality improvement activities were then carried out.
Standardized storage
Standard storage of antidiabetic drugs focuses on the following points: (1) Unopened bottles or pens of insulin should be kept in the refrigerator until needed and may be used until the expiry date on the label. Insulin that is currently in use should be stored at room temperature for no more than 28 days and then discarded. (2) Storing two insulin formulations with similar-sounding names and similar-looking labels in close proximity could easily lead to confusion, therefore insulin must be stored in separate compartments corresponding to each patient, and each product in use should be accurately labeled with the patient name, identification number, start date, and expiry date (Figure 1). (3) Organizational policy defines how medications delivered by the patient are identified and stored. Insulin formulations and oral hypoglycemic drugs should not be stored at the patients bedside. (4) In the Pharmacy, all antidiabetic drugs should be stored in a special location with standard labels indicating high-alert medications. In each ward, a standard label of high-alert medication should be pasted to the place where insulin/insulin analog are stored. (5) Strengthened management was performed regarding look-alike and sound-alike (LASA) antidiabetic drugs. The list and color photos of LASA medications are available in the hospital local area network. LASA medications are placed apart from one another on the Pharmacy shelf, especially when LASAs don’t act alike, eg, Tritace® (ramipril tablets; Sanofi S.A., Paris, France) and Amary® (glimepiride tablets; Sanofi S.A.), and Monopril® (fosinopril sodium tablets; Sino-American Shanghai Squibb Pharmaceutical Co Ltd, Shanghai, People’s Republic of China) and Glucophage® (metformin hydrochloride tablets; Sino-American Shanghai Squibb Pharmaceutical Co Ltd).
Standardized prescribing
Physicians are required to follow the current edition of National Guideline for Prevention and Treatment of Type 2 Diabetes Mellitus published by Chinese Diabetes Society.10 When a physician prescribes a LASA medication via the electronic medical record (EMR), the interface of EMR will display a yellow background for this physician order. For LASA insulin, a special warning will be seen during prescription. For example, a warning (ie, “Please pay attention to the distinction between NovoMix 30® [biphasic insulin aspart] and NovoRapid® [insulin aspart]”) will display when a physician prescribes NovoRapid® (Novo Nordisk A/S, Hellerup, Denmark). Nonstandard abbreviation of medical terminology (eg, “U” and “IU”) is prohibited from use in physician orders of insulin. Insulin infusion speed and measurable goals for blood glucose level must be specified when the physician order is written. Sulfonylurea hypoglycemic drugs (eg, tolbutamide, glipizide, gliclazide, glibenclamide, glibornuride, gliquidone, glyclopyramide, and glimepiride) are contraindicated in patients with history of allergy to sulfonamide derivatives, such as antimicrobial sulfonamides, diuretics (hydrochlorothiazide, amiloride, and indapamide), COX-2 inhibitors (celecoxib and parecoxib), sulfonylureas, and probenecid.11 If an insulin pen brought in by the patient must be continuously used after admission, medication reconciliation should be conducted, and the patients’ written informed consent must be obtained. Physicians should verify whether the insulin pen is pharmacologically incompatible with other medications during the patient’s stay in hospital. Additionally, the physician order for this insulin must be written, via EMR, in the orders, to let the auditing pharmacist know the use of this medication brought by the patient.
Standardized dispensing
SAHZU improved the interface of the Pharmacy management information system for prescription auditing in January 2013. By this sophisticated software, pharmacists could see patient information, including age, diagnosis, allergy history, body weight, pregnancy status, clinical laboratory data (eg, blood glucose levels), and drug information, such as approved drug name, dose, administration route, dosing frequency, and the list of all current medications, all such information visually displays in the same interface.12
Pharmacists should be aware of “near misses” related to inappropriate use of abbreviations, such as “U” and “IU” instead of “units”. The potential consequence, clinical relevance, and risk management of DDIs associated with oral antidiabetic drugs are listed in Table 2.13–27 Auditing pharmacists should check the appropriateness of drug combinations and communicate with physicians if controversial physician orders are identified. The inpatient Pharmacy started to provide centralized intravenous admixture service for insulin infusion preparation in November 2010.
The dosing time for oral hypoglycemic drugs should be checked by pharmacists. Although most oral hypoglycemic agents should be ingested 15–30 minutes before a meal, some have specific requirements, for example, acarbose tablet should be taken at the start of main meals (taken with first bite of meal). Diamicron® (gliclazide modified release; Servier Laboratories, Neuilly-sur-Seine, France) and Glucotrol XL® (glipizide extended release; Pfizer, Inc., New York, NY, USA) should be given once daily with breakfast. Metformin should be taken with meals to help reduce stomach or bowel side effects. SAHZU introduced two unit-dose, automated dispensing machines in January 2011. An oral diabetic drug will be separately packaged into a polymer bag on which special dosing requirement is printed if it has special requirement of dosing time, which greatly helps nurses administer oral diabetic drugs at the right time. LASA insulin/insulin analogs should be dispensed with obvious distinction. Considering that antidiabetic drugs are fall risk–increasing medications due to potential hypoglycemic events,28 SAHZU required that all insulins/insulin analogs and oral hypoglycemic drugs dispensed by the Pharmacy be labeled with increased fall risk warnings beside the identification of high-alert medications. If outpatients and inpatients receive antidiabetic therapy at discharge, they will get special written patent education from the Pharmacy, such as requirements for medication storage, dosing time, DDIs, and awareness of increasing fall risk.
Administration
All kinds of insulin/insulin analog should be administered according to the Chinese edition of “Injection Recommendation for Patients with Diabetes”29 published by the Chinese Diabetes Society in 2011. In April 2012, diabetes specialist nurses initiated a plan-do-check-act (PDCA) cycle to improve the coverage percentage of standard insulin injection education for outpatients with diabetes. The process is as follows: (1) The attending endocrinology physician assures that there is an indication for using insulin and the patient has not ever received insulin therapy. A special seal, reading “please start insulin therapy only after receiving standard injection technique education”, is then affixed on the outpatient’s medical record. The patient will be instructed to go to the Diabetes Center for special education. Only after receiving injection education can the patient be permitted to get insulin syringe needles from the Diabetes Center. (2) A diabetes specialist nurse (ie, teaching staff) prepares two copies of the insulin injection training record sheet and education card for each patient. By repeated teaching and mock injection on the model, patients or authorized family gradually master injection skills. After signature by both teaching staff and the patient, a copy of the training record sheet and education card is given to the patient. (3) The teaching staff signs on the outpatient medical record so as to let the attending physician know that the patient has completed the process of insulin injection education. Meanwhile, diabetes specialist nurses conduct a telephone follow-up 1 week later, using the record sheet of insulin injection education for diabetic outpatients.
Spot checks on insulin injection accuracy among ward nurses are conducted quarterly. Fifty nurses’ activities are examined using mock injections on the model every quarter. Specialized training was then given. Furthermore, SAHZU has required that two licensed health care professionals must perform a “double check” prior to administering intravenous infusions of insulin, by implementing a standardized independent double-check process, since January 2013.
Monitoring
Physicians and nurses should document ADRs following antidiabetes therapy. Diabetic nurse specialists should investigate the occurrence rate and cause of hypoglycemia among diabetes inpatients. Furthermore, the process of diagnosis and treatment of hypoglycemia was standardized hospital-wide in April 2014.
Outcome measures
The outcome measures of the intervention program included implementation rate of standard storage in the Pharmacy as well as in wards, occurrence of dispensing errors related to antidiabetic drugs, insulin injection accuracy among ward nurses, the number of actual medication administration errors (MAEs) related to insulin/insulin analogs and oral hypoglycemic agents, occurrence rates of hypoglycemia in diabetes inpatients who were not hospitalized in the endocrinology ward, occurrence rate of hypoglycemia in diabetes patients in neurology wards, percentage of correctly managed hypoglycemia, the coverage percentage of standardized insulin injection education for diabetes outpatients, insulin injection techniques among diabetes outpatients who start to receive insulin therapy, and percentage of drug-related problems in the antidiabetes regimen.
Statistical analysis
A descriptive analysis was performed. Pearson’s chi-square test was used for testing percentage differences between two groups. A P-value <0.05 was considered to be statistically significant. A P-value <0.01 was considered to be highly significant.
Results and discussion
Implementation rate of standard storage
SAHZU has achieved a 100% implementation rate of standard storage of antidiabetic drugs in the Pharmacy and wards since August 2012. Meanwhile, the phenomenon that a vial of regular insulin was used for multiple inpatients was abolished from then on.
Medication errors
In August 2011, there were six medication errors related to NovoMix 30® and NovoRapid®, which were two products that looked very similar (Figure 2), including four near misses (one prescribing error and three dispensing errors) and two actual MAEs. The inpatient Pharmacy immediately performed quality improvements, emphasizing that NovoMix 30® must be marked with an additional label specifying “Novomix30®” and colored “blue”, which was distinctive from the background color of NovoRapid® (ie, “orange”). Since October 2011, SAHZU has achieved zero occurrence of dispensing errors related to NovoMix 30® and NovoRapid®.
The number of actual MAEs related to insulin/insulin analogs exhibited continuous decrease in number, from 20 (data in 2011) to six (data in 2014) (Figure 3), and the relative percentage of MAE subtype is presented in Figure 4. There were nine MAEs related to oral hypoglycemic agents during 2011–2013, including two case in 2011 (omission [two]), three cases in 2012 (omission [one], duplicate dosing [one], and improper handling of physician orders [one]), and four cases in 2013 (dosing time error [two] and omission [two]). Inspiringly, there was only one MAE related to oral hypoglycemic drug in 2014 (omission [one]).
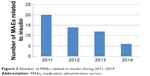  | Figure 3 Number of MAEs related to insulin during 2011–2014. |
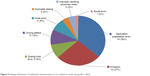  | Figure 4 Subtype distribution of medication administration errors related to insulin during 2011–2014. |
With the aid of the new interface with the prescription auditing software, auditing pharmacists prospectively identified and intercepted 13 potential adverse DDIs, including clarithromycin–repaglinide (n=6), fluvastatin–sulfonylurea (n=3), fluvoxamine–gliclazide (n=1), and metformin–contrast agents (n=3), in 2013. Alternative metabolizing enzyme inhibitors (clarithromycin, fluvastatin, and fluvoxamine) and cessation of metformin before contrast-enhanced examination were suggested by pharmacists, and these suggestions were accepted by physicians. Zero occurrence of abbreviations such as “IU” and “U” and of physician orders without noting infusion rate has been achieved since October 2012. From the beginning of February 2011, physician orders with inappropriate dosing time of oral hypoglycemic agents were abolished. Moreover, the appropriateness of insulin-glucose combination in total parenteral nutrition (TPN) admixture aroused pharmacist’s special concern. The amount of regular insulin given (added directly to the TPN solution) depends on the plasma glucose level; if the level is normal and the final solution contains 25% dextrose, the usual starting dose is 5 to 10 units of regular insulin/L of TPN fluid. Anecdotally, in October 2013, a diabetic patient who just had thoracic surgery was prescribed with TPN therapy. Insulin, 28 units, was included in this TPN order; however, his physician mistakenly prescribed 5% glucose injection instead of 50% glucose injection as the type of carbohydrate. An auditing pharmacist successfully intercepted this prescribing-related near miss with potential severe hypoglycemia.
On-site inspection by diabetes nurse specialists in the first and second quarter of 2014 indicated of great improvement in the appropriateness of antidiabetic drug use in diabetes inpatients (Table S1). In the first quarter, antidiabetes regimens for 1,200 diabetes patients were checked, and 13 cases (1.08%) were identified as having drug-related problems, including inappropriate choice of insulin (n=2), inappropriate drug combination (n=1), inappropriate dosing frequency (n=3), inappropriate dosing route (n=1), and poor awareness of medication reconciliation (n=6). Targeted lectures were then provided by a senior endocrinology physician, a diabetes nurse specialist, and a clinical pharmacist. In the second quarter, antidiabetes regimens for 1,400 diabetes patients were checked, and only four cases (0.28%) were observed with drug-related problems, ie, inappropriate choice of insulin (n=4). There was statistically significant difference in the percentage of drug-related problems in antidiabetes regimen between the two quarters (1.08% versus 0.28%) (P<0.05 [chi-square test]). Medication reconciliation was effectively strengthened in the second quarter of 2014.
Insulin injection technique
The coverage percentage of standard insulin injection education for outpatients with diabetes successfully increased from 80% (April 2012) to 95.2% (October 2012) (P<0.05 [chi-square test]). After interventions, in October 2012 insulin injection techniques were improved among diabetic outpatients who started to receive insulin therapy, including the percentage of checking injection sites prior to injection (85.6%), percentage of priming before injection (98.1%), percentage of rotation of injecting sites (98.1%), percentage of remixing before use (94.5%), percentage of keeping the pen needle under the skin for >10 seconds (99.4%), and percentage of using the pen needle only once (88.7%). The corresponding data seemed more optimistic than those from an insulin injection technique questionnaire survey in 16 countries and 20 centers in mainland People’s Republic of China (Table 3).30,31 Insulin injection accuracy among ward nurses significantly increased from 82% (first quarter 2011) to 96% (fourth quarter 2011) (P<0.05 [chi-square test]).
  | Table 3 Comparison of insulin injection technique in SAHZU program with data from other surveys |
ADRs
The ADRs reporting system showed that there were seventeen cases of ADRs induced by antidiabetic drugs during 2011–2014. Oral hypoglycemic agents accounted for ten ADRs (metformin [four], acarbose [three], gliclazide sustained release [two], and glimepiride [one]). Insulin and insulin analogs accounted for seven ADRs. The clinical manifestation of ADRs included hypoglycemia (n=7), diarrhea (n=4), rash (n=4), abdominal distension (n=2), chills (n=1), vomiting (n=1), increased anal aerofluxus (n=1), itchiness (n=1), and dizziness (n=1). All 17 ADRs were mild and cured with supportive treatment.
Hypoglycemia
Table 4 lists the occurrence rates of hypoglycemia in hospitalized diabetes patients who were not from the endocrinology ward, during 2010–2013. The situation during 2011–2013 was significantly more optimistic than that in 2010 (5.03%–5.53% versus 8.27%) (P<0.01 [chi-square test]). The Department of Neurology was observed to have a high occurrence rate of hypoglycemia in diabetes patients in 2010 (30.5%). Therefore, this department was selected as a place for performing quality improvements. Significant improvements in the occurrence rate of hypoglycemia were achieved with diabetes patients in three wards of Department of Neurology during 2010–2013 (30.5% versus 11.3%–13.9%) (P<0.01 [chi-square test]) (Figure 5). Furthermore, the process of diagnosis and treatment of hypoglycemia was standardized hospital-wide, and continuous quality improvement was achieved regarding the percentage of correctly managed hypoglycemia during April–August 2014 (Figure 6).
  | Table 4 Occurrence rates of hypoglycemia in hospitalized diabetes patients who were not from Department of Endocrinology during 2010–2013 |
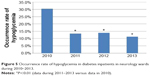  | Figure 5 Occurrence rate of hypoglycemia in diabetes inpatients in neurology wards during 2010–2013. |
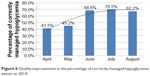  | Figure 6 Quality improvement in the percentage of correctly managed hypoglycemia events in 2014. |
Limitations
Although our program may be of interest to health care professionals elsewhere, it has several limitations. Firstly, the paper is largely descriptive. Ideally, it would have been even better if we had controls (another hospital without the program). Nevertheless, it includes a longitudinal follow-up, and one can appreciate the gradual improvement in outcome year by year. Secondly, the program seems simplistic, and it is obvious that if we undertake “strong measures”, we may expect “strong results”. We did not evaluate the pharmacoeconomic issue (ie, the cost/benefit ratio, the “human cost”, antidiabetes efficacy, and satisfaction from patients and medical staff) or the applicability of these measures over time. Thirdly, the number of ADRs seemed too few, indicating further opportunity of improvements in ADR surveillance.
Conclusion
In this article, we introduced a 5-year continuous intervention program focusing on safe MMU of “high-alert” antidiabetic drugs, and summarized related risk-management measures and quality improvement activities in medication storage, prescribing, dispensing, administration, and monitoring that were implemented at SAHZU during the journey to JCI accreditation and post-JCI accreditation. The goal of intervention program has been achieved through multidisciplinary collaboration among pharmacists, nurses, physicians, and information engineers.
Acknowledgments
This work was supported by Zhejiang Provincial Bureau of Education (grant numbers 201225325 and N20140209), the National Natural Science Foundation of China (grant number 81373488), the National Major Projects of China (grant number 2012ZX09506001–004), and the National Health and Family Planning Commission of the People’s Republic of China (National Key Clinical Discipline Construction: Clinical Nursing Specialist).
Disclosure
The authors report no conflicts of interest in this work.
References
Morello CM, Hirsch JD, Lee KC. Navigating complex patients using an innovative tool: the MTM Spider Web. J Am Pharm Assoc (2003). 2013;53(5):530–538. | ||
ismp.org [homepage on the Internet]. ISMP High-alert medications. Institute for Safe Medication Practices; 2015 [cited October 10, 2014]. Available from: http://www.ismp.org/Tools/highAlertMedicationLists.asp. Accessed June 27, 2015. | ||
Kopecky C. Use of noninsulin antidiabetic medications in hospitalized patients. Crit Care Nurs Clin North Am. 2013;25(1):39–53. | ||
Classen DC, Jaser L, Budnitz DS. Adverse drug events among hospitalized Medicare patients: epidemiology and national estimates from a new approach to surveillance. Jt Comm J Qual Patient Saf. 2010;36(1):12–21. | ||
Geller AI, Shehab N, Lovegrove MC, et al. National estimates of insulin-related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med. 2014;174(5):678–686. | ||
Milligan FJ, Krentz AJ, Sinclair AJ. Diabetes medication patient safety incident reports to the National Reporting and Learning Service: the care home setting. Diabet Med. 2011;28(12):1537–1540. | ||
Mitchell VD, Porter K, Beatty SJ. Administration technique and storage of disposable insulin pens reported by patients with diabetes. Diabetes Educ. 2012;38(5):651–658. | ||
Cohen MR. Pharmacists’ role in ensuring safe and effective hospital use of insulin. Am J Health Syst Pharm. 2010;67(16 Suppl 8):S17–S21. | ||
Joint Commission International [homepage on the Internet]. JCI-Accredited Organizations. Available from: http://www.jointcommissioninternational.org/about-jci/jci-accredited-organizations/?c=China&a=Academic%20Medical%20Center%20Hospital%20Program. Accessed July 10, 2015. | ||
Chinese Diabetes Society. [homepage on the Internet]. Available from: http://www.diab.net.cn/guideline.jsp | ||
Li W, Zhu LL, Zhou Q. Safe medication use based on knowledge of information about contraindications concerning cross allergy and comprehensive clinical intervention. Ther Clin Risk Manag. 2013;9:65–72. | ||
Zhu LL, Zhou Q. Intervention for improving the appropriateness of physician orders for oral medications in geriatric VIP patients during the journey to JCI accreditation. Ther Clin Risk Manag. 2013;9:273–275. | ||
Tornio A, Niemi M, Neuvonen PJ, Backman JT. Drug interactions with oral antidiabetic agents: pharmacokinetic mechanisms and clinical implications. Trends Pharmacol Sci. 2012;33(6):312–322. | ||
Niemi M, Backman JT, Neuvonen M, Laitila J, Neuvonen PJ, Kivistö KT. Effects of fluconazole and fluvoxamine on the pharmacokinetics and pharmacodynamics of glimepiride. Clin Pharmacol Ther. 2001;69(4):194–200. | ||
Shobha JC, Muppidi MR. Interaction between voriconazole and glimepiride. J Postgrad Med. 2010;56(1):44–45. | ||
Schelleman H, Bilker WB, Brensinger CM, Wan F, Hennessy S. Anti-infectives and the risk of severe hypoglycemia in users of glipizide or glyburide. Clin Pharmacol Ther. 2010;88(2):214–222. | ||
Park JY, Kim KA, Park PW, Park CW, Shin JG. Effect of rifampin on the pharmacokinetics and pharmacodynamics of gliclazide. Clin Pharmacol Ther. 2003;74(4):334–340. | ||
Xu H, Williams KM, Liauw WS, Murray M, Day RO, McLachlan AJ. Effects of St John’s wort and CYP2C9 genotype on the pharmacokinetics and pharmacodynamics of gliclazide. Br J Pharmacol. 2008;153(7):1579–1586. | ||
Fichtenbaum CJ, Gerber JG. Interactions between antiretroviral drugs and drugs used for the therapy of the metabolic complications encountered during HIV infection. Clin Pharmacokinet. 2002;41(14):1195–1211. | ||
Baerlocher MO, Asch M, Myers A. Five things to know about...metformin and intravenous contrast. CMAJ. 2013;185(1):E78. | ||
Niemi M, Neuvonen M, Juntti-Patinen L, Backman JT, Neuvonen PJ. Effect of fluconazole on the pharmacokinetics and pharmacodynamics of nateglinide. Clin Pharmacol Ther. 2003;74(1):25–31. | ||
Niemi M, Neuvonen PJ, Kivistö KT. The cytochrome P4503A4 inhibitor clarithromycin increases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther. 2001;70(1):58–65. | ||
Kajosaari LI, Niemi M, Neuvonen M, Laitila J, Neuvonen PJ, Backman JT. Cyclosporine markedly raises the plasma concentrations of repaglinide. Clin Pharmacol Ther. 2005;78(4):388–399. | ||
Honkalammi J, Niemi M, Neuvonen PJ, Backman JT. Dose-dependent interaction between gemfibrozil and repaglinide in humans: strong inhibition of CYP2C8 with subtherapeutic gemfibrozil doses. Drug Metab Dispos. 2011;39(10):1977–1986. | ||
Kajosaari LI, Backman JT, Neuvonen M, Laitila J, Neuvonen PJ. Lack of effect of bezafibrate and fenofibrate on the pharmacokinetics and pharmacodynamics of repaglinide. Br J Clin Pharmacol. 2004;58(4):390–396. | ||
Niemi M, Backman JT, Juntti-Patinen L, Neuvonen M, Neuvonen PJ. Coadministration of gemfibrozil and itraconazole has only a minor effect on the pharmacokinetics of the CYP2C9 and CYP3A4 substrate nateglinide. Br J Clin Pharmacol. 2005;60(2):208–217. | ||
Aquilante CL, Kosmiski LA, Bourne DW, et al. Impact of the CYP2C8 *3 polymorphism on the drug-drug interaction between gemfibrozil and pioglitazone. Br J Clin Pharmacol. 2013;75(1):217–226. | ||
Chen Y, Zhu LL, Zhou Q. Effects of drug pharmacokinetic/pharmacodynamic properties, characteristics of medication use, and relevant pharmacological interventions on fall risk in elderly patients. Ther Clin Risk Manag. 2014;10:437–448. | ||
Chinese Diabetes Society. [homepage on the Internet]. Available from: http://diab.net.cn/uploadfile/zhusheguideline.pdf | ||
De Coninck C, Frid A, Gaspar R, et al. Results and analysis of the 2008–2009 Insulin Injection Technique Questionnaire survey. J Diabetes. 2010;2(3):168–179. | ||
Ji J, Lou Q. Insulin pen injection technique survey in patients with type 2 diabetes in mainland China in 2010. Curr Med Res Opin. 2014;30(6):1087–1093. |
Supplementary material
  | Table S1 Inappropriate medication use in diabetes patients during on-site inspection in the first and second quarter of 2014 |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



