Back to Journals » Infection and Drug Resistance » Volume 13
A Simple Scoring Algorithm That Predicts Abscesses in Adults with Community-Onset Klebsiella pneumoniae Bacteremia: Hypermucoviscosity Matters
Authors Hong MY , Hsieh CC, Yang CY, Lee CH, Ko WC , Lee CC
Received 2 December 2019
Accepted for publication 25 March 2020
Published 14 April 2020 Volume 2020:13 Pages 1045—1055
DOI https://doi.org/10.2147/IDR.S240809
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Ming-Yuan Hong,1,2 Chih-Chia Hsieh,1,2 Chao-Yung Yang,1 Chung-Hsun Lee,1,2 Wen-Chien Ko,2,3 Ching-Chi Lee1,3– 5
1Department of Emergency Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan; 2Department of Medicine, National Cheng Kung University Medical College, Tainan, Taiwan; 3Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan; 4Department of Adult Critical Care Medicine, Tainan Sin-Lau Hospital, Tainan, Taiwan; 5Graduate Institute of Medical Sciences, College of Health Sciences, Chang Jung Christian University, Tainan, Taiwan
Correspondence: Wen-Chien Ko
Division of Infectious Disease, Department of Internal Medicine, National Cheng Kung University Hospital, No. 138, Sheng Li Road, Tainan 70403, Taiwan
Tel +886-6-2353535 ext. 3596
Fax +886-6-2752038
Email [email protected]
Ching-Chi Lee
Graduate Institute of Medical Sciences, College of Health Sciences, Chang Jung Christian University, No.1, Changda Rd., Gueiren District., Tainan City 71101, Taiwan
Tel +886-6-2785123 Ext 3326
Fax +886-6-2990586
Email [email protected]
Introduction: Klebsiella pneumoniae is a pathogen commonly found in community-onset bacteremia. It causes an invasive syndrome that is frequently presented by metastatic infections and abscesses elsewhere and that is necessary for surgical or drainage intervention. To achieve a scoring algorithm to identify patients with community-onset K. pneumoniae bacteremia (CoKPB) who are at risk for abscess occurrences, a retrospective cohort study consisting of adults with CoKPB was conducted.
Methods: A 6-year cohort study consisting of adults having community-onset monomicrobial K. pneumoniae bacteremia was conducted. In addition to clinical variables collected from medical records, the hypermucoviscosity (HMV)-related gene (rmpA and magA) and an HMV phenotype were integrated into the proposed scoring algorithm.
Results: Of the 258 eligible adults, only 79 (30.6%) had abscesses related to bacteremia. Besides the presence of magA (ie, capsular serotype K1) and the HMV-phenotype, five clinical predictors were significantly associated with abscesses in a multivariate analysis: male gender, comorbidities with diabetes mellitus or neurological disorders, recent chemotherapy, and high c-reactive protein levels. Together, these predictors were used to calculate the CoKPB abscess score. Based on the scoring algorithm, a cut-off value of +2 yielded the high sensitivity (93.7%) and the acceptable specificity (50.8%); the area under the ROC curve was 0.83.
Conclusion: A simple scoring algorithm that has substantial sensitivity and satisfactory specificity was proposed and the importance of the HMV phenotype or capsular K1 serotype was emphasized. The proposed predictive model needs external validation, but this scoring algorithm might be convenient and useful for clinicians.
Keywords: Klebsiella pneumoniae, bacteremia, hepatic abscesses, extrahepatic abscesses
Introduction
Bloodstream infections are serious, life-threatening infections that are associated with significant healthcare costs and poor patient prognoses.1,2 Klebsiella pneumoniae is one of the common Gram-negative bacteria in the community and it causes various types of infections.3 However, an international collaborative study indicated the geographic differences in clinical manifestations.4 Notably in Asia, as a second leading pathogen in community-onset bacteremia,4 a new invasive syndrome consisting of hepatic abscesses, frequently accompanied by metastatic infections with abscess occurrences elsewhere, has globally emerged as a substantial public health problem in the community.5,6
Dissimilar to classical K. pneumoniae, a new variant of hypermucoviscous K. pneumoniae was described due to its predominant ability to develop pyogenic hepatic abscesses and septic metastasis, even in apparently healthy adults.7 Adequate or rapid drainage has been recommended to treat this invasive syndrome and to achieve a better clinical response.6,8 Several patient characteristics, such as the comorbidity of diabetes mellitus or alcoholism, contribute to the invasive syndrome; notably, the strongest predictors of septic metastasis are by far isolates with a hypermucoviscosity (HMV) phenotype and capsular K1 serotype.5,6,9 However, these study populations were all limited to patients with hepatic abscesses and were not representative of all patients infected by K. pneumoniae. Moreover, the effect of HMV on the occurrence of hepatic or extra-hepatic abscesses among patients with bloodstream infections remains unclear. For patients with K. pneumoniae bacteremia, it is clinically beneficial to identify which patients are at increased risk of developing abscesses and need timely imaging studies and further surgical or drainage intervention. Thus, we aimed to integrate HMV-related factors into developing a scoring algorithm that can be simply and accurately applied to aid the clinician for early identifying patients at greater risk for the abscess occurrence and further to analyze the impact of HMV-related factors on patient prognoses.
Methods
Study Design
This 4-year cohort study was retrospectively conducted at an emergency department (ED) of a university-affiliated hospital in southern Taiwan during the period between January 2012 and December 2015. During the study period, adults with monomicrobial K. pneumoniae bacteremia in the ED were studied. Their medical records were retrospectively reviewed for capturing clinical information, in terms of sources of transfer, vital signs, bacteremia severity, comorbidity types, laboratory data, and initial sepsis-related syndrome, immediately following each ED visit. Furthermore, records of prior hospitalization, immunosuppressive agents or chemotherapy received prior to the ED visit, durations and types of antimicrobial administration, imaging studies, bacteremia sources, the length of the hospital stay, and patient outcomes were assessed from the chart records.
The exclusion criteria were patients lacking clinical information prior to the study endpoint when chart reviewing, those experienced the hospital transfer prior to ED visits (ie, uncertain sites of bacteremia onset), those with hospital-onset bloodstream infections, and those lacking the comprehensive imaging studies (ie, chest X-ray plus either abdominal computed tomography [CT] or sonography). Moreover, for patients categorized in the non-abscess group, a follow-up of at least 90 days after the bacteremia onset was conducted to ensure that there was no recurrence of K. pneumoniae bacteremia or abscesses; those not followed during this period were excluded. All medical records were reviewed by random two of the authors, and the records were discussed by the two authors if any discrepancy was discovered. The primary endpoint was Day 28 after the bacteremia onset (ie, ED arrival) for overall patients and the secondary endpoint was Day 90 after bacteremia onset for patients in the non-abscess group.
Microbiological Methods
K. pneumoniae was identified by biochemical tests and then confirmed using a Vitek system (BioMe’rieux, France) by the Gram-negative-identification card. The susceptibility of antibiotics was determined using the disc diffuse method, and the interpretation followed the contemporary breakpoint recommended by the Clinical and Laboratory Standards Institute.10 ESBL production was detected by the phenotypic confirmatory test with the cephalosporin-clavulanate combination disks.11
Detection of HMV Genotype and Phenotype
The HMV phenotype and HMV-related genotype (ie, magA and rmpA) were assessed for K. pneumoniae isolated from eligible bacteremia patients. To assess the HMV phenotype, a bacteriologic loop was used to stretch a mucoviscous string from the colony; the formation of the strings of >5 mm in length was defined as a positive string test result, as previously reported.5 To detect the chromosome-mediated mucoviscosity-associated gene A (magA) and mucoid phenotype A (rmpA) genes, PCR was performed using previously described PCR primers and conditions, with genomic DNA or plasmid DNA as the template, respectively.9
Definitions
Bacteremia was defined as bacterial growth of blood cultures drawn from central or peripheral venipuncture, after exclusion of contaminant sampling. Blood culture sampling with potentially contaminating pathogens, such as coagulase-negative staphylococci, Micrococcus species, Bacillus species, Propionibacterium species, and Gram-positive bacilli, were considered to be contaminated based on previously established criteria.12 Community-onset bacteremia indicates that the place of the bacteremia onset is inside the community and includes community-acquired and long-term healthcare facility-acquired bloodstream infections.13,14 Patients were assigned to the abscess group if the bacteremic episode occurred in conjunction with hepatic and/or extra-hepatic abscesses that were diagnosed by imaging and microbiological studies. As previously described,14,15 the virulence gene magA and the surrounding 33-kb genomic region was demonstrated to be the genetic determinant of capsular serotype K1. As previous description,16 appropriate antimicrobial administration was defined as antimicrobial therapy to which the isolated K. pneumoniae was susceptible in vitro and in which the dosage and route of antimicrobial administration was in accordance with the Sanford Guide.17 Bacteremia severity was graded using a Pitt bacteremia score, which was a validated score based on vital signs, mental status, receipt of mechanical ventilation, vasopressor agent use, and recent cardiac arrest.14,16 Hematological malignancies and solid tumors were grouped as malignancies, while comorbidities were defined as previously described.18 The comorbidity severity was assessed by a delineated McCabe classification, in which comorbidities were categorized as the rapid fatal, ultimately fatal, and non-fatal groups.19 The clinical categorization of the sources of bacteremic episodes followed the published definitions from the Centers for Disease Control and Prevention.20 The death by all causes was regarded as crude mortality.
Statistical Analysis
The Statistical Package for the Social Sciences for Windows (Chicago, Illinois, USA, Version 23.0) was tested for statistical analyses. Continuous and categorical variables were compared using Student’s t and Chi-square (or Fisher’s exact) tests, respectively. To recognized independent predictors of abscess occurrences, all variables with P values of <0.1 recognized by univariate analyses were included for the stepwise, backward logistic regression model. Furthermore, to identify the independent predictor of 28-day mortality and further investigate the impact of HMV factors or abscess occurrences on 28-day mortality, all variables with P values of <0.1, recognized by univariate analyses, and HMV factors were together processing under the model of hierarchical logistic regression. The curve of receiver operating characteristic (ROC) was performed to estimate the accuracy of a prediction tool.21 A P value of <0.05 was considered significant.
Results
Patient Population
The 4-year cohort consisting of 287 bacteremic adults of community-onset monomicrobial K. pneumoniae was conducted. After exclusion of 29 cases, 258 adults were included in the present study (Figure 1). Of the 258 patients, only 79 (30.6%) had abscesses; the distributions of the various abscess types are shown in Figure 1. Of the 179 patients without abscesses, none were excluded due to incomplete follow-up within 90 days of the onset of bacteremia, and none displayed a recurrence of K. pneumoniae bacteremia or abscesses during those 90 days. The major comorbidities of the total 258 patients included diabetes mellitus (109 patients, 42.2%), hypertension (106, 41.1%), malignancies (81, 31.4%), neurological disorders (61, 23.6%), liver cirrhosis (48, 18.8%), chronic kidney diseases (37, 14.3%), coronary artery diseases (26, 10.1%), and chronic obstructive pulmonary diseases (21, 8.1%).
 |
Figure 1 Schematic flow chart. |
Because four patients had multiple foci of infections, 184 portals of entry were disclosed among the 179 patients in the non-abscess group. The leading source of bacteremia was the respiratory tract infection (48 patients, 25.8%), followed by the urinary tract (42, 23.5%), primary bacteremia (30, 16.8%), the biliary tract (29, 16.2%), intra-abdominal (23, 12.8%), vascular catheter-related (6, 3.4%), bone and joint (3, 1.7%), endocarditis (2, 1.1%), the central nervous system (2, 1.1%), and skin and soft-tissue (1, 0.6%). Of 81 abscess forms among 79 patients in the abscess group, the leading was liver abscesses (50 patients, 63.3%), followed by 8 patients (10.1%) with respiratory tract abscesses (6 with lung abscesses, 2 with empyema), 8 (10.1%) with soft-tissue abscesses (4 with pyomyositis, 2 with psoas muscle abscesses, 1 with a perianal abscess, and 1 with a deep neck abscess), 7 (8.9%) with renal abscesses, 3 (3.8%) with epidural abscesses, 2 (2.5%) with cholangeal abscesses, 2 (2.5%) with peritoneal abscesses, and 1 (1.2%) with mycotic aneurysm.
Of the total 258 K. pneumoniae, the susceptibility rate to cefepime was the highest (250 isolates, 96.9%), followed by cefotaxime (240, 93.0%), levofloxacin (237, 91.9%), cefuroxime (228, 88.4%), ampicillin/sulbactam (221, 85.7%), and cefazolin (192, 74.4%). Notably, ESBL-producers only accounted for 5.8% (15 isolates) of K. pneumoniae isolated in the overall cohort.
Predictors of Abscesses
The following predictors were significantly associated with abscesses in univariate analyses (Table 1): old age, recent chemotherapy, severe comorbidities, malignancies, chronic kidney disease, a low platelet count, a high C-reactive protein (CRP) level, or a high serum creatinine level at bacteremia onset. Of note, a strong relationship between the HMV phenotype or genotype (ie, rmpA and magA) and abscesses was discovered (Figure 2A). Additionally, K. pneumoniae with the HMV phenotype or genotype was more frequently observed in patients having liver abscesses, compared to those with extra-hepatic abscesses (Figure 2B).
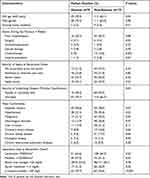 |
Table 1 Clinical Characteristics of Adults with Community-Onset Monomicrobial K. pneumoniae Bacteremia, Categorized by the Presence or Absence of Abscesses |
However, when the variables with P<0.1, recognized by the univariate analysis (Table 1), and HMV phenotype or genotype (as shown in Figure 2A) were together assessed by the multivariate regression model, only seven variables were independently linked to abscess occurrences (Table 2): male gender, the HMV phenotype, the presence of magA (ie, capsular serotype K1), recent chemotherapy, the comorbidity with neurological disorder or diabetes mellitus, and a high CRP level at bacteremia onset. Notably, the presence of the HMV phenotype and capsular K1 serotype was by far the most powerful predictors of abscess formation; the association of abscess occurrences and rmpA was not significant under the multivariate regression model.
 |
Table 2 Multivariate Analysis of Risk Factors for Abscess Formation Among Community-Onset Monomicrobial K. pneumoniae Bacteremia |
Performance of Prediction Rule
According to the odds ratios of these independent predictors recognized in Table 2, the score of each risk predictor was assigned, and three scoring algorithms that corresponded to community-onset K. pneumoniae abscesses score (CoKPAS) models 1, 2, and 3 were developed. The score for model 1 included +1 point for each of five positive predictors (ie, male sex, the HMV phenotype, the capsular K1 serotype, comorbid diabetes mellitus and the high CRP level) and −1 point for each of two negative predictors (ie, recent chemotherapy and neurological disorders). To emphasize the importance of HMV, model 2 was modified from model 1 by assigning 0 points to the capsular serotype K1, and model 3 was furtherly modified by additionally assigning 0 points to the HMV phenotype.
The ROC curve of CoKPAS model 1 (Figure 3) demonstrated a good ability in predicting abscess occurrences, with an area under the curve of 0.83. However, the prediction ability of models 2 and 3 was inferior to that of model 1, with areas under the ROC curve of 0.80 and 0.77, respectively. In CoKPAS model 1, the performance of the prediction model for abscess occurrences in adults with community-onset K. pneumoniae bacteremia, including sensitivity, specificity, predictive values, and likelihood ratio, was shown in Table 3. Various cut-off points, ranging from 2 to 4, were used. With higher cut-off points, the sensitivity rates decreased and specificity rates increased. Maximum sensitivity rate, 93.7%, was observed at the lowest cut-off point (total score = 2); and maximum specificity rate (96.6%) at the highest cut-off point (total score = 4).
 |
Table 3 The Sensitivity, Specificity, Predictive Value, and Likelihood Ratio of the CoKPBAS (Model 1) for Abscess Occurrences in Various Cut-Off Points |
Clinical Characteristics and Outcomes in Bacteremic Patients with and Without Abscesses
A greater proportion of patients in the abscess group had hepatobiliary tract infections, skin and soft-tissue infections, multiple portals of entry, and long lengths of hospital stays. Meanwhile, fewer patients in the abscess group had respiratory tract infections, urinary tract infections, intra-abdominal infections, primary bacteremia, an appropriate antibiotic delay >3 days, and examination by abdominal sonography using univariate analyses (Table 4). Of note, the proportions of chest X-rays and abdominal CT scans as well as the period between ED triage and imaging studies were similar between the two groups. In addition, the 14-day and 28-day mortality rates and the crude mortality rate at discharge were low in patients in the abscess group.
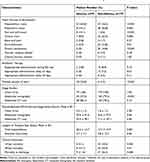 |
Table 4 Infection Sources, Clinical Management, and Outcome of Adults with Community-Onset Monomicrobial K. pneumoniae Bacteremia, Categorized by the Presence or Absence of Abscessesa |
Predictors of 28-Day Mortality
The predictor of 28-day mortality recognized by the univariate analysis included nursing-home residents, the critical illness (Pitt bacteremia score ≥ 4) at bacteremia onset, delayed administration of appropriate antimicrobials ≥3 days, bacteremic pneumonia, bacteremia due to urinary tract infections, fatal comorbidities (McCabe classification), and comorbidities of diabetes mellitus. However, the adverse impact of the HMV phenotype, capsular K1 serotype, and rmpA-harboring isolate on 28-day mortality was not significant. When significant predictors recognized by the univariate analysis and HMV factors were together processing under the model of hierarchical logistic regression (Table 5), independent predictors of 28-day mortality only included the critical illness at bacteremia onset, delayed administration of appropriate antimicrobials ≥3 days, bacteremic pneumonia, and fatal comorbidities. Notably, HMV factors, in terms of the HMV phenotype, capsular K1 serotype, and rmpA-harboring isolate, remained not a significant determinant of 28-day mortality in the multivariate analysis.
 |
Table 5 Hypermucoviscosity and Risk Factors of 28-Day Crude Mortality |
Although the adverse impacts of abscess occurrences on 28-day mortality (odds ratio [OR], 0.36; P = 0.01) was recognized in the univariate analysis (supplemental Table), the association of abscess occurrences and 28-day mortality was not evidenced (Adjusted OR, 0.73; P = 0.53) in the multivariate analysis, after adjusting the following independent determinates of 28-day mortality: the critical illness at bacteremia onset, delayed administration of appropriate antimicrobials ≥3 days, bacteremic pneumonia, and fatal comorbidities.
Discussion
To achieve better prognoses of patients with the invasive syndrome caused by hypermucoviscous K. pneumoniae, adequate and rapid drainage or surgery for the abscess complication is recommended.6 Additionally, timely and aggressive antimicrobial administration has been suggested, eg, a third-generation cephalosporin is preferable to a first-generation cephalosporin.22 Therefore, the scoring algorithm proposed in this study could assist clinicians in providing timely imaging studies, surgical or drainage intervention, and prescriptions of third-generation cephalosporin. The use of this algorithm may also reduce costs by reducing the usage of expensive imaging studies and avoiding unnecessary kidney injuries caused by contrast medium in those who are not truly at the high risk for the abscess occurrence.
In the past, a new, hypervirulent or hypermucoviscous variant of K. pneumoniae has slowly emerged worldwide. However, new evidence has indicated that HMV and hypervirulence are two different phenotypes that should not be used synonymously.7 Hypervirulence genes detected in K. pneumoniae included p-rmpA, iroB, iucA, and iutA.23 On the other hand, the specific genotype, primarily rmpA, and the capsular serotype K1/K2 were proposed to be defining characteristics of hypermucoviscous K. pneumoniae.7,9 More importantly, the K1 serotype was the most prevalent one in the Far Eastern region.7 Accordingly, in addition to clinical information and the HMV phenotype, capsular serotype K1 and rmpA were selected as the eligible component constituted to the simple scoring algorithm in predicting the abscess occurrence herein. Furthermore, all the HMV factors were irrespective of short-term prognoses and thus it is reasonable to believe that these factors were not contributory to predict patient outcomes, as shown in Table 5.
In this study, the importance of the HMV phenotype, magA and rmpA was emphasized in regards to the occurrence of abscesses, especially hepatic abscesses, among adults with community-onset K. pneumoniae bacteremia. Thus, it was reasonable that we integrated these HMV-related variables to propose a scoring algorithm that was accurate for the early identification of patients at high risk for abscesses. In combination with other clinical parameters that were available immediately at the ED arrival, the ROC of the scoring system proposed that using this simple clinical scoring algorithm has substantial sensitivity and satisfactory specificity. Moreover, based on differences in the areas under the ROC curves of models 1, 2, and 3, the importance of the HMV phenotype and the mucoviscosity-associated magA genes (ie, capsular serotype K1) was also demonstrated. Notably, the relationship between abscess formation and the capsular serotype K1 was strengthened in the current study. This finding was in line with previous reports,15 which indicated the capsular K1 serotype had a strong relationship with primary liver abscesses and hepatic metastatic infections that were secondary to hepatic abscesses.5,15 However, CoKPAS model 2, which did not include the capsular K1serotype, retained an acceptable prediction capability for abscess formation. Thus, even in a laboratory lacking routine detection of K1 isolates, this modified model retains an acceptable prediction capability.
There are several limitations inherent to the design of this study. First, despite numerous exclusion criteria in our study design, only little proportions (27/287, 9.4%) of the entire cohort were excluded. Therefore, the selection basis should be trivial. Second, not all patients without abscesses underwent abdominal CT examination herein. To avoid misclassification for the abscess categorization, patients in the non-abscess group were designed to follow patients for a long period after bacteremia onset to confirm that no recurrence of K. pneumoniae bacteremia or abscesses took place. Consequently, only one patient was excluded from our analyses because of incomplete information; therefore, the misclassification basis should be neglected. Third, because the study population was focusing on one-center adults, a lack of an independent validation is one of the study limitations, and the predictability of this algorithm may not be extended to other populations with varied infection sources and bacteremia severity. Finally, because the incidence of abscess occurrences and prevalence of HMV highly varied in different communities, three cut-off values were offered for clinicians. In a region with a high incidence, the cut-off value of +2 is appropriate to achieve the high sensitivity. However, a well-designed, multinational study is warranted to externally validate the clinical significance of this scoring system.
Conclusively, to rapidly recognize the occurrence of abscesses, a simple scoring algorithm that has substantial sensitivity and satisfactory specificity in adults with community-onset K. pneumoniae bacteremia was proposed. For hospitals lacking routine detection of the capsular K1 serotype, the modified model, which only uses the HMV phenotype combined with five clinical predictors, retains an acceptable prediction capability. An imaging study may also be recommended for patients with a CoKPAS score ≥2. However, the cost-effectiveness of this algorithm should be investigated in the future to assess its clinical utility.
Ethics Approval
The study was approved by the Ethics Committee of National Cheng Kung University Hospital following the Declaration of Helsinki (ER-100-182, 4th ed.). The ethics committee waived the need for written informed consent provided by participants due to the retrospective nature of the study. Because all patient data were analyzed in anonymity, no additional informed consent was required.
Acknowledgments
We would like to thank for providing experimental space and facilities by the Diagnostic Microbiology and Antimicrobial Resistance Laboratory, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Funding
This study was partially supported by research grants from the Ministry of Science and Technology (NSC102-2314-B-006-079), Ministry of Health and Welfare (MOHW 109-TDU-B-221-114003), and Sin-Lau Hospital (SLH-M106-01, SLH-M107-02, SLH-M108-01, and SLH-109-04), Taiwan.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Bates DW, Pruess KE, Lee TH. How bad are bacteremia and sepsis? Outcomes in a cohort with suspected bacteremia. Arch Intern Med. 1995;155(6):593–598. doi:10.1001/archinte.1995.00430060050006
2. Haug JB, Harthug S, Kalager T, Digranes A, Solberg CO. Bloodstream infections at a Norwegian university hospital, 1974–1979 and 1988–1989: changing etiology, clinical features, and outcome. Clin Infect Dis. 1994;19(2):246–256. doi:10.1093/clinids/19.2.246
3. Meatherall BL, Gregson D, Ross T, Pitout JD, Laupland KB. Incidence, risk factors, and outcomes of Klebsiella pneumoniae bacteremia. Am J Med. 2009;122(9):866–873. doi:10.1016/j.amjmed.2009.03.034
4. Ko WC, Paterson DL, Sagnimeni AJ, et al. Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg Infect Dis. 2002;8(2):160–166. doi:10.3201/eid0802.010025
5. Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J of Exper Med. 2004;199(5):697–705. doi:10.1084/jem.20030857
6. Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12(11):881–887. doi:10.1016/S1473-3099(12)70205-0
7. Catalán-Nájera JC, Garza-Ramos U, Barrios-Camacho H. Hypervirulence and hypermucoviscosity: two different but complementary Klebsiella spp. phenotypes? Virulence. 2017;8(7):1111–1123. doi:10.1080/21505594.2017.1317412
8. Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis. 2004;39(11):1654–1659. doi:10.1086/425616
9. Yu W-L, Ko W-C, Cheng K-C, et al. Association between rmpA and magA genes and clinical syndromes caused by Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 2006;42(10):1351–1358. doi:10.1086/503420
10. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Approved Standard. Twenty-Ninth Informational Supplement. CLSI Document M100-S29. Wayne, PA: CLSI; 2019.
11. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Approved Standard. Nineteenth Informational Supplement. CLSI Document M100-S19. Wayne, PA: CLSI; 2009.
12. Lee CC, Lin WJ, Shih HI, et al. Clinical significance of potential contaminants in blood cultures among patients in a medical center. J Microbiol Immunol Infect. 2007;40(5):438–444.
13. Hsieh CC, Chen PL, Lee CH, Yang CY, Lee CC, Ko WC. Definitive cefazolin therapy for stabilized adults with community-onset Escherichia coli, Klebsiella species, and proteus mirabilis bacteremia: MIC matters. J Clin Med. 2020;9(1):1. doi:10.3390/jcm9010157
14. Lee CC, Lee CH, Yang CY, Hsieh CC, Tang HJ, Ko WC. Beneficial effects of early empirical administration of appropriate antimicrobials on survival and defervescence in adults with community-onset bacteremia. Crit Care. 2019;23(1):363. doi:10.1186/s13054-019-2632-1
15. Chuang YP, Fang CT, Lai SY, Chang SC, Wang JT. Genetic determinants of capsular serotype K1 of Klebsiella pneumoniae causing primary pyogenic liver abscess. J Infect Dis. 2006;193(5):645–654. doi:10.1086/499968
16. Lee CC, Lee CH, Hong MY, Tang HJ, Ko WC. Timing of appropriate empirical antimicrobial administration and outcome of adults with community-onset bacteremia. Crit Care. 2017;21(1):119. doi:10.1186/s13054-017-1696-z
17. Gilbert DN, Moellering RC
18. Schellevis FG, van der Velden J, van de Lisdonk E, van Eijk JT, van Weel C. Comorbidity of chronic diseases in general practice. J Clin Epidemiol. 1993;46(5):469–473. doi:10.1016/0895-4356(93)90024-U
19. McCabe WR. Gram-negative bacteremia. Adv Intern Med. 1974;19:135–158.
20. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16(3):128–140. doi:10.1016/0196-6553(88)90053-3
21. Centor RM, Schwartz JS. An evaluation of methods for estimating the area under the receiver operating characteristic (ROC) curve. Med Decis Making. 1985;5(2):149–156. doi:10.1177/0272989X8500500204
22. Cheng H-P, Siu LK, Chang F-Y. Extended-spectrum cephalosporin compared to cefazolin for treatment of Klebsiella pneumoniae-caused liver abscess. Antimicrob Agents Chemother. 2003;47(7):2088–2092. doi:10.1128/AAC.47.7.2088-2092.2003
23. Yan Q, Zhou M, Zou M, Liu W-E. Hypervirulent Klebsiella pneumoniae induced ventilator-associated pneumonia in mechanically ventilated patients in China. Euro J Clin Microbiol Infect Dis. 2016;35(3):387–396. doi:10.1007/s10096-015-2551-2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


