Back to Journals » Clinical Ophthalmology » Volume 14
A Review of Topical Cyclosporine A Formulations—A Disease-Modifying Agent for Keratoconjunctivitis Sicca
Authors Jerkins GW, Pattar GR, Kannarr SR
Received 21 August 2019
Accepted for publication 31 January 2020
Published 20 February 2020 Volume 2020:14 Pages 481—489
DOI https://doi.org/10.2147/OPTH.S228070
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Gary W Jerkins,1 Guruprasad R Pattar,2 Shane R Kannarr3
1Advancing Vision Research, Nashville, TN, USA; 2The Eye Care Institute, Louisville, KY, USA; 3Kannarr Eye Care, Pittsburg, KS, USA
Correspondence: Gary W Jerkins
Advancing Vision Associates, 4306 Harding Pike, Nashville, TN 37205, USA
Tel +1 615 502-2871
Email [email protected]
Abstract: Keratoconjunctivitis sicca (KCS) is a multifactorial disease characterized by tear hyperosmolarity, inflammation, and ocular surface damage. Cyclosporine A (CsA) is used as an effective disease-modifying agent to improve the signs and symptoms of KCS by reducing inflammation, which interferes with tear production. This review provides an overview of efficacy, safety, and limitations of currently marketed topical CsA formulations—including CsA ophthalmic emulsion, cationic nanoemulsion, and aqueous nanomicelles—and highlights newer technologies for controlled ocular delivery of CsA and their clinical implications. Long available emulsion formulations of CsA are oil-based and have several limitations, including slow onset of efficacy and low intraocular penetration and bioavailability. Aqueous CsA nanomicelle carriers produce rapid improvement in objective signs of KCS such as corneal and conjunctival staining as early as 4 weeks and have acceptable safety profiles. CsA formulations using semifluorinated alkanes or polyaphrons are currently in clinical development, having recently completed Phase 2 studies. Other carriers for CsA currently in the preclinical phase include microemulsions, polymeric aqueous and lyophilized micelles, and hydrogels; these novel formulations have yet to undergo clinical trials. Formulations that improve tissue availability of CsA may be beneficial in clinical practice by providing faster onset of relief and improving patient adherence.
Keywords: cyclosporine A, keratoconjunctivitis sicca, emulsion, nanomicelles, OTX-101
Introduction
Keratoconjunctivitis sicca (KCS) is a complex ocular surface disorder and represents the most common reason for patients needing medical eye care.1,2 KCS is a multifactorial condition, characterized by a loss of homeostasis of the tear film, inflammation, and ocular symptoms such as discomfort, visual disturbance, and foreign body sensation.1,2 Prevalence varies globally with known risk factors such as advancing age and Asian race, and assessments of prevalence range approximately from 5% to 33% depending on disease definition used and examined populations.1 Notably, prevalence of KCS is rising in the younger population potentially due to increased digital screen time.1 Overall, KCS is associated with reduced work productivity and represents a significant cost burden.1
Pathophysiology of Keratoconjunctivitis Sicca
The central mechanism of KCS is a vicious cycle where inflammation—stimulated by desiccating or hyperosmolar stress—leads to ocular surface damage.3,4 Hyperosmolarity can be caused by a number of factors, including lacrimal gland destruction due to Sjögren syndrome; age-related degeneration of the lacrimal gland, conjunctiva, and meibomian glands; damage to sensory nerves during refractive surgery; reduced tear production due to systemic medications; and meibomian gland disease.3
Irrespective of the etiology of KCS, chronic inflammation—especially T-cell mediated immune response—and tear hyperosmolarity are common perpetuating factors.3–5 At the onset of the inflammatory response, levels of mitogen-activated protein kinases (MAPKs) and nuclear factor κB increase on the ocular surface.6,7 Activation of MAPK cascades triggers secretion of various inflammatory mediators, including interleukin-1β (IL-1β) and tumor necrosis factor α, which mediate activation of resident dendritic cells and T-cell recruitment to the ocular surface.6,7 Additional inflammatory mediators released from recruited T-cells along with tear hyperosmolarity further accentuate cellular damage and loss of epithelial and goblet cells leading to a vicious cycle of tear film instability and chronic inflammation.3,6 The cascade of events results in loss of ocular homeostasis that leads to the ocular symptoms and signs.3
Management of Keratoconjunctivitis Sicca
The treatment guidelines for KCS recommend a step-wise approach with various treatment options based on disease severity.8 Initial steps are patient education, lid hygiene, modification of environmental factors, and ocular lubricants.8 Although ocular lubricants are a mainstay of KCS, they offer only palliative relief and have no disease-modifying potential.8 If these initial strategies are ineffective, next steps include nonpharmacological therapies such as tear conservation through punctal occlusion, pulsed light therapy, and moisture goggles, as well as pharmacological therapies such as macrolide antibiotics (which may have anti–inflammatory potential in dry eye associated with meibomian gland disease or blepharitis), corticosteroids, cyclosporine A (CsA), and lymphocyte function-associated antigen-1 antagonists.8 Surgical approaches such as surgical punctal occlusion, tarsorrhaphy, and salivary gland transplantation may also be considered.8 Management of meibomian gland dysfunction is an important aspect of overall KCS management and may include warm compresses and device-assisted physical heating therapies to remove the blockage from the meibomian glands, as well as ω-3 essential fatty acid supplementation.8 This review discusses the emergence of topical CsA as a disease-modifying treatment for KCS, provides an overview of the currently marketed topical CsA emulsion and its limitations, and highlights novel CsA formulations.
Cyclosporine A and Its Mechanism of Action
Cyclosporine A is a cyclic polypeptide and a widely used immunosuppressant, with inhibitory action of both cell-mediated and humoral immunity.9 Systemic CsA is indicated to prevent graft rejection and treat autoimmune conditions.8,9 Topical CsA was developed to increase tear production in patients with KCS and its disease-modifying and anti–inflammatory action in KCS is well established.8,10 Benefits of using CsA in KCS include improvement of subjective symptoms and objective ocular signs such as improved tear production, tear break-up time, and corneal and conjunctival staining scores.11 Other indications of topical CsA include meibomian gland dysfunction, viral keratitis, punctate and neurotrophic keratopathy, vernal keratoconjunctivitis, and prevention of graft rejection following ocular surgeries.12 Topical administration of CsA allows for lower systemic absorption, and thereby reduces the risk of systemic adverse events (AEs) such as nephrotoxicity, hypertension, anemia, and hyperesthesia.13
Cyclosporine A is a calcineurin inhibitor and acts by blocking T cell infiltration, activation, and the subsequent release of inflammatory cytokines as illustrated in Figure 1.12,14,15 In addition, CsA has an antiapoptotic and protective action on human conjunctival epithelial cells, unlike corticosteroids.8,14 Topical CsA use is associated with fewer complications than steroids, long-term use of which is associated with cataract development, increased intraocular pressure, and opportunistic infections.8,12
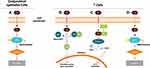 |
Figure 1 Mechanism of action of cyclosporine A. (A) Inhibits intrinsic mitochondrial pathway and caspase activation with an antiapoptotic and protective action on human conjunctival epithelial cells.8,14 (B) Inhibits nuclear factor κB (NFκB) activation and the subsequent release of proinflammatory cytokines through modulating proteasome activity.15,42 (C) Binds with cyclophilin to form a calcineurin complex and inhibits dephosphorylation of nuclear factor of activated T cells and the subsequent release of interleukin 2.12 (D) Induces T cell apoptosis by regulating Fas/Fas ligand expression, caspase activation, and mitochondrial permeability transition pore (MPTP) opening.12 Abbreviations: CsA, cyclosporine A; CyP, cyclophilin; Iĸßα, nuclear factor of ĸ light polypeptide gene enhancer in ß-cell inhibitor, alpha; IL-2, interleukin 2; MPTP, mitochondrial permeability transition pore; NFAT, nuclear factor of activated T cells; NFkB, nuclear factor of kB; P, phosphorylated. |
This review examines the challenges to topical CsA delivery for the treatment of KCS, and presents currently available formulations, both marketed and in development.
Challenges to Topical Delivery of CsA
Ocular targets for drug delivery in KCS include cornea, conjunctiva, tear film, and lacrimal and meibomian glands.3 Amount of drug available for intraocular tissue penetration largely depends on the physiochemical properties of the delivery vehicle.16 Ocular drug delivery and optimal tissue bioavailability have been a continued challenge due to the presence of dynamic and static ocular barriers.17 Static barriers include anatomic structures such as corneal epithelium, stroma, and blood-aqueous barrier. Various dynamic factors, such as tear turnover rate, dilution with tear, blinking, and lacrimation, shorten the residence time of topically administered drugs. Drug permeability into the conjunctiva is further limited due to extensive vascular and lymphatic drainage.18 Loss of drug volume can also occur due to gravity and nasolacrimal drainage.17 Rapid tear film turnover time of 2 to 3 mins washes topically applied drug solutions in just 15 to 30 s after administration.18 These ocular barriers and defense mechanisms limit the bioavailability of topically administered drugs to less than 5%.19
High hydrophobicity and poor aqueous solubility of CsA make it extremely difficult to formulate as conventional topical eye drops.19,20 The release and subsequent intraocular penetration of CsA from a lipophilic carrier, such as an emulsion, are generally low.16 Although emulsions have the advantage of rapid ocular spreading upon application, lower bioavailability of lipophilic drugs from emulsions limits the amount of CsA available in an already challenging aqueous ocular environment.13,16 Clinical results show that the time to onset of efficacy with CsA emulsions is 6 months on average.21,22 Such long lead times to initial efficacy may interfere with patient adherence to treatment in KCS. Given the chronic nature of KCS, repeated and continued usage is important to attain therapeutic levels of CsA in ocular tissues.17
Available Ophthalmic CsA Formulations
An ophthalmic emulsion of 0.05% CsA (Restasis®; Allergan, Inc., Irvine, CA, USA) (Table 1) was the first topical CsA approved by the US Food and Drug Administration (FDA) in 2003 to increase tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with KCS.8,10,13 This conventional CsA 0.05% emulsion is a preservative-free anionic oil-in-water formulation comprising castor oil with polysorbate 80 and carbomer copolymer acting as an emulsifying and stabilizing agent, respectively.13 In 2 multicenter and randomized Phase 3 trials, twice-daily administration of CsA 0.05% emulsion for 6 months significantly improved corneal staining and anesthetized Schirmer’s tear test values from baseline compared with vehicle (P ≤0.05).21 Conjunctival staining in temporal and nasal conjunctival zones improved significantly from baseline in both CsA 0.05% emulsion and vehicle groups; however, there was no significant difference between CsA 0.05% emulsion and vehicle treatment.21 About 80% of enrolled patients completed treatment with CsA 0.05% emulsion and 6.5% patients discontinued treatment due to AEs.21 In a Phase 4 trial, CsA 0.05% emulsion significantly (P <0.001) improved tear film breakup time from baseline in patients with KCS at month 6.22 About 43% of enrolled and treated patients experienced AEs and the most frequent ocular AEs were installation site reactions including burning and pain.22
 |
Table 1 Comparison of Emulsion and Aqueous Formulations of Cyclosporine A |
A cationic nanoemulsion (Ikervis®; Santen SAS, Evry, France) (Table 1) was developed and approved by the European Medical Agency in 2015 for use in severe keratitis that has not improved despite the use of artificial tears.13,23 The cationic nanoemulsion was expected to have improved bioavailability and residence time compared with anionic emulsions, potentially due to the electrostatic interactions between the positively charged oil droplets and negatively charged mucins in the corneal epithelium.13 In a phase 3 study, treatment with cationic emulsion for 6 months significantly improved corneal staining compared with vehicle (P = 0.037).24 There was a higher proportion of patients with a combined result of ≥2 grades improvement in corneal staining and ≥30% improvement in the ocular surface disease index after 6 months of treatment with cationic emulsion (28.6%) vs vehicle (23.1%). However, these improvements were not significant compared with the vehicle.24 Moreover, the percentage of patients (29.2%) that experienced ocular discomfort following instillation was higher than in the vehicle group (8.9%).24 There was a discontinuation rate of about 10% with cationic emulsion treatment.25
OTX-101 0.09% (CEQUA™; cyclosporine A 0.09%; Sun Pharmaceutical Industries, Inc., Princeton, NJ, USA) (Table 1) is a clear aqueous nanomicellar formulation approved by the FDA in 2018 to increase tear production in patients with KCS.17,27 Nanomicelles are amphiphilic molecules that self-assemble into typical supramolecular aggregates above the critical micelle concentration in the aqueous medium.17 Hydrophobic interactions of core-forming units drive the micelle formation with a water-insoluble or hydrophobic core and an outer water-soluble or hydrophilic shell.17 Nanomicellar technology allows the encapsulation of hydrophobic CsA within its hydrophilic core that, in turn, favors better dispersion and solubility of CsA into the precorneal tear film.17,19 When the temperature of the medium rises above dissociation temperature, nanomicelles disassemble into monomers and release the drug into surrounding aqueous tissue.19 Nanomicelles are thermodynamically stable even at higher dilutions and exhibit a reversible association with CsA (Figure 2).17,19 Their small size (10–80 nm; average of 22 nm) may allow nanomicelles to easily diffuse through scleral aqueous pores (20–80 nm in size).19
 |
Figure 2 Suspension of cyclosporine A in emulsion vs nanomicelles. |
A preclinical study compared the ocular distribution and tolerability of OTX-101 and CsA 0.05% emulsion in New Zealand white rabbits.28 Following a single dosing, there was a higher CsA concentration with OTX-101 0.05% compared with CsA 0.05% emulsion in most ocular tissue samples, including the cornea (2.18-fold) and superior bulbar conjunctiva (1.76-fold) with minimal systemic exposure.28 There was a dose-related increase in CsA with repeat dosing of OTX-101 0.05% and this also resulted in higher concentrations of CsA in ocular tissues and aqueous humor than CsA 0.05% emulsion.28
Phase 2b/3 and 3 clinical studies showed that twice-daily administration of OTX-101 0.09% was superior to vehicle in increasing tear production and improving ocular signs from baseline, including conjunctival and corneal staining in patients with KCS.29,30 Importantly, improvement in these objective signs was seen at and after 4 weeks of therapy.29,30 In a pooled analysis of phase 2b/3 and 3 trials, least square mean change from baseline for total corneal staining on day 28 was −0.9 and −0.5 for OTX-101 0.09% and vehicle, respectively (P = 0.0008).31 Similarly, there was a significant reduction in total conjunctival staining from baseline (P = 0.0316) on day 28 compared with the vehicle.32 It is important to note that the degree of conjunctival staining correlates with the severity of KCS.5,33 Moreover, clearing of conjunctival and corneal staining implies an improvement in ocular surface integrity and resolution of underlying pathology.21 On day 84, there was a significantly higher proportion of patients with an increase in Schirmer’s scores from baseline of ≥10 mm in the OTX-101 0.09% group vs vehicle (16.6% vs 9.0%, respectively, P <0.0001).34
About 93% of enrolled patients on OTX-101 0.09% completed phase 2b/3 and 3 studies, and the withdrawal rate due to AEs was less than 4%.29,30,32 Most of the treatment-emergent AEs that occurred with OTX-101 0.09% during the 3-month treatment period were mild or moderate in nature and resolved without any treatment.29,30,32 Instillation site pain was the most frequent AE (21.8% in OTX-101 0.09% group and 4.0% in vehicle group) and there were no serious AEs considered related to treatment.29,30,32
Formulations of Cyclosporine A Under Development
A CsA nonaqueous formulation (CyclASol®, Novaliq, Heidelberg, Germany) (Table 1) uses semifluorinated alkanes as a water-free solvent.35 Liquid semifluorinated alkanes are amphiphilic compounds that are colorless, chemically and physiologically inert and laser-stable.36 Their low surface tension allows them to spread rapidly over the ocular surface, minimizing visual disturbance.35 Efficacy and safety of CsA nonaqueous solution were evaluated in an exploratory phase 2, randomized, vehicle-controlled study.35 Treatment with 0.1% cyclosporine nonaqueous solution significantly improved total corneal staining at 4 and 12 weeks—but not 16 weeks—vs CsA 0.05% emulsion (open-label active comparator; P ≤0.05).35 Improvements in total corneal staining vs vehicle (double-masked comparator) were not statistically significant.35 Adverse events were reported in 31.4% of patients; 1.2% of patients withdrew from the study due to AEs.35
MC2-03 (MC2 Therapeutics, Hørsholm, Denmark) (Table 1) uses polyaphrons dispersed in carbomer hydrogel as carriers for CsA.13 Polyaphrons are lipid-based formulations characterized by a high concentration of the dispersed oil phase and a surfactant-to-oil ratio much lower than emulsions.13 Preliminary results from a phase 2 study in patients with dry eye and moderate to severe keratitis showed 6 months of treatment with MC2-03 0.06% led to a significantly higher percent of patients with a clinically relevant response (defined as 2-grade improvement in corneal staining on the modified Oxford scale) in both eyes vs ocular lubricant (43.1% vs 22.8%, P = 0.0289).37 The most common AEs included eye pain and eye irritation, and no serious AEs were reported to be related to study drugs.37
Other novel carriers under development include Microemulsion Drug Ocular Penetration System (MiDROPS) of CsA (Table 1).20 Droplets in this system are <10 nm in diameter and are thermodynamically stable with longer shelf life.20 Unlike conventional emulsions, MiDROPS are in a single phase and can be easily manufactured at a lower cost. This microemulsion was developed to safely deliver higher amounts of CsA to ocular tissue than existing formulations.20 A preclinical study in Dutch-belted rabbits that compared MiDROPS and conventional CsA 0.05% emulsion showed MiDROPS delivered amounts of CsA into ocular tissue 2 to 3 times greater than CsA 0.05% emulsion.20 Both twice daily 0.05% and once-daily 0.1% CsA-MiDROPS in mice significantly reduced corneal permeability, T cell infiltration, and conjunctival goblet cell loss compared with CsA 0.05% emulsion.20
Another novel formulation under development loads lyophilized CsA into polymeric micelles (Table 1). Methoxy poly (ethylene glycol)-poly (lactide) polymer, the carrier used in this system is biocompatible and biodegradable and can help enhance drug penetration with lesser tolerability issues. Lyophilization of CsA into dried powder form provides the advantage of extended shelf-life potentially by preventing aggregation and leakage of drug.38 Similar to MiDROPS, instillation of lyophilized CsA-loaded micelles resulted in 4.5-fold (P <0.05) higher CsA concentrations in cornea of rabbits than CsA 0.05% emulsion.38 In addition, studies are under way to test a different aqueous micellar formulation containing poloxamer 407 and a water-soluble derivative of vitamin E as excipients to enhance CsA solubility and intraocular penetration.39 These components may provide the advantage of enhanced drug solubility and permeability in addition to beneficial antioxidant effect from vitamin E.39 On examination, these CsA loaded micelles demonstrated stability upon dilution and increased CsA solubility and delivery to ocular tissues.39
Another approach to increase bioavailability and residence time of drug is by using contact lenses with hydrogel-loaded drug particles. A recent study has explored monomeric gels loaded with high concentrations of CsA for in situ formation of drug particles, to bypass tolerability issues associated with excipients.40 However, scattering of light by the drug particle can cause decreased transparency of lenses.40 Further clinical studies are needed to evaluate the clinical efficacy and safety of these novel carrier systems.
Implications for Clinical Practice
The growing prevalence of KCS1 stresses the need for availability of effective and well-tolerated treatments. Clinically, KCS is often described as chronic and progressive,41 and better patient compliance may result in improved long-term outcomes and potentially reduced use of therapy. Currently available CsA emulsions have several limitations including low bioavailability of CsA and slow onset of action,21,22,26 which may interfere with patient adherence. Novel formulations for ophthalmic use, including aqueous nanomicellar technology, may help overcome these challenges by enhancing and maximizing tissue availability of CsA for rapid efficacy, potentially improving patient compliance and clinical outcomes in KCS.
Abbreviations
AE, adverse event; CsA, cyclosporine A; CyP, cyclophilin; FDA, US Food and Drug Administration; Iĸßα, nuclear factor of ĸ light polypeptide gene enhancer in ß-cells inhibitor, alpha; IL-2, interleukin 2; KCS, keratoconjunctivitis sicca; MAPK, mitogen-activated protein kinase; MiDROPS, Microemulsion Drug Ocular Penetration System; MPTP, mitochondrial permeability transition pore; NFAT, nuclear factor of activated T cells; NFkB, nuclear factor of kB; P, phosphorylated; OSDI, Ocular Surface Disease Index.
Acknowledgments
Writing and editorial support for manuscript preparation were provided by Ginny Feltzin, PhD (AlphaBioCom, LLC, King of Prussia, PA) and funded by Sun Pharmaceutical Industries, Inc. (Princeton, NJ). All authors met the International Council of Medical Journal Editors criteria and received neither honoraria nor payment for authorship.
Disclosure
SK reports personal fees from Allergan, Alcon, Bausch & Lomb, Essilor, Johnson & Johnson, Optovue, and Vision Source. The authors report no other conflicts of interest in this work.
References
1. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334–365. doi:10.1016/j.jtos.2017.05.003
2. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II Report Executive Summary. Ocul Surf. 2017;15(4):802–812. doi:10.1016/j.jtos.2017.08.003
3. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. doi:10.1016/j.jtos.2017.05.011
4. Abidi A, Shukla P, Ahmad A. Lifitegrast: a novel drug for treatment of dry eye disease. J Pharmacol Pharmacother. 2016;7(4):194–198. doi:10.4103/0976-500X.195920
5. Rolando M, Barabino S, Mingari C, Moretti S, Giuffrida S, Calabria G. Distribution of conjunctival HLA-DR expression and the pathogenesis of damage in early dry eyes. Cornea. 2005;24(8):951–954. doi:10.1097/01.ico.0000157421.93522.00
6. Zhang X, Qu Y, He X, et al. Dry Eye Management: targeting the Ocular Surface Microenvironment. Int J Mol Sci. 2017;18(7):1398. doi:10.3390/ijms18071398
7. Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32(1):19–41. doi:10.3109/08830185.2012.748052
8. Jones L, Downie LE, Korb D, et al. TFOS DEWS II Management and Therapy Report. Ocul Surf. 2017;15(3):575–628. doi:10.1016/j.jtos.2017.05.006
9. Neoral® (Cyclosporine capsules and oral solution, USP) MODIFIED. Full prescribing information. Novartis. East Hanover, NJ. 2009.
10. RESTASIS® (Cyclosporine ophthalmic emulsion) 0.05% for topical ophthalmic use. Full prescribing information. Allergan. Irvine, CA. 2017.
11. Schultz C. Safety and efficacy of cyclosporine in the treatment of chronic dry eye. Ophthalmol Eye Dis. 2014;6:37–42. doi:10.4137/OED.S16067
12. Ambroziak AM, Szaflik J, Szaflik JP, Ambroziak M, Witkiewicz J, Skopinski P. Immunomodulation on the ocular surface: a review. Cent Eur J Immunol. 2016;41(2):195–208. doi:10.5114/ceji.2016.60995
13. Lallemand F, Schmitt M, Bourges JL, Gurny R, Benita S, Garrigue JS. Cyclosporine A delivery to the eye: a comprehensive review of academic and industrial efforts. Eur J Pharm Biopharm. 2017;117:14–28. doi:10.1016/j.ejpb.2017.03.006
14. Gao J, Sana R, Calder V, et al. Mitochondrial permeability transition pore in inflammatory apoptosis of human conjunctival epithelial cells and T cells: effect of cyclosporin A. Invest Ophthalmol Vis Sci. 2013;54(7):4717–4733. doi:10.1167/iovs.13-11681
15. Meyer S, Kohler NG, Joly A. Cyclosporine A is an uncompetitive inhibitor of proteasome activity and prevents NF-kappaB activation. FEBS Lett. 1997;413(2):354–358. doi:10.1016/S0014-5793(97)00930-7
16. Cholkar K, Patel A, Vadlapudi AD, Mitra AK. Novel Nanomicellar Formulation Approaches for Anterior and Posterior Segment Ocular Drug Delivery. Recent Pat Nanomed. 2012;2(2):82–95. doi:10.2174/1877912311202020082
17. Vaishya RD, Khurana V, Patel S, Mitra AK. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(5):422–437. doi:10.1002/wnan.1272
18. Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348–360. doi:10.1208/s12248-010-9183-3
19. Cholkar K, Gilger BC, Mitra AK. Topical, Aqueous, Clear Cyclosporine Formulation Design for Anterior and Posterior Ocular Delivery. Transl Vis Sci Technol. 2015;4(3):1. doi:10.1167/tvst.4.3.1
20. Coursey TG, Wassel RA, Quiambao AB, Farjo RA. Once-Daily Cyclosporine-A-MiDROPS for Treatment of Dry Eye Disease. Transl Vis Sci Technol. 2018;7(5):24. doi:10.1167/tvst.7.5.24
21. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmol. 2000;107(4):631–639. doi:10.1016/S0161-6420(99)00176-1
22. Stonecipher KG, Torkildsen GL, Ousler GW
23. IKERVIS® (Ciclosporin ophthalmic emulsion) 1 mg/mL, for topical ophthalmic use. Full prescribing information. Santen SAS, Evry, France. 2015.
24. Leonardi A, Van Setten G, Amrane M, et al. Efficacy and safety of 0.1% cyclosporine A cationic emulsion in the treatment of severe dry eye disease: a multicenter randomized trial. Eur J Ophthalmol. 2016;26(4):287–296. doi:10.5301/ejo.5000779
25. Baudouin C, Irkec M, Messmer EM, et al. Clinical impact of inflammation in dry eye disease: proceedings of the ODISSEY group meeting. Acta Ophthalmol. 2018;96(2):111–119. doi:10.1111/aos.2018.96.issue-2
26. Kuwano M, Ibuki H, Morikawa N, Ota A, Kawashima Y. Cyclosporine A formulation affects its ocular distribution in rabbits. Pharm Res. 2002;19(1):108–111. doi:10.1023/A:1013671819604
27. CEQUATM (cyclosporine ophthalmic solution 0.09%). Full prescribing information. Sun Pharmaceutical Industries, Inc., Cranbury, NJ. 2018.
28. Weiss SL, Kramer WG. Ocular Distribution of Cyclosporine Following Topical Administration of OTX-101 in New Zealand White Rabbits. J Ocul Pharmacol Ther. 2019;35:395–402. doi:10.1089/jop.2018.0106
29. Tauber J, Schechter BA, Bacharach J, et al. II/III, randomized, double-masked, vehicle-controlled, dose-ranging study of the safety and efficacy of OTX-101 in the treatment of dry eye disease. Clin Ophthalmol. 2018;12:1921–1929. doi:10.2147/OPTH.S175065
30. Goldberg DF, Malhotra RP, Schechter BA, Justice A, Weiss SL, Sheppard JD. A Phase 3, Randomized, Double-Masked Study of OTX-101 Ophthalmic Solution 0.09% in the Treatment of Dry Eye Disease. Ophthalmol. 2019;126:1230–1237. doi:10.1016/j.ophtha.2019.03.050
31. Malhotra R, Devries DK, Luchs J, et al. Effect of OTX-101, a Novel Nanomicellar Formulation of Cyclosporine A, on Corneal Staining in Patients With Keratoconjunctivitis Sicca: a Pooled Analysis of Phase 2b/3 and Phase 3 Studies. Cornea. 2019;38:1259–1265. doi:10.1097/ICO.0000000000001989
32. Smyth-Medina R, Johnston J, Devries DK, et al. Effect of OTX-101, a Novel Nanomicellar Formulation of Cyclosporine A, on Conjunctival Staining in Patients with Keratoconjunctivitis Sicca: a Pooled Analysis of Phase 2b/3 and 3 Clinical Trials. J Ocul Pharmacol Ther. 2019;35:388–394. doi:10.1089/jop.2018.0154
33. Uchiyama E, Aronowicz JD, Butovich IA, McCulley JP. Pattern of vital staining and its correlation with aqueous tear deficiency and meibomian gland dropout. Eye Contact Lens. 2007;33(4):177–179. doi:10.1097/01.icl.0000253054.10349.2f
34. Sheppard J, Kannarr S, Luchs J, et al. Efficacy and Safety of OTX-101, a Novel Nanomicellar Formulation of Cyclosporine A, for the Treatment of Keratoconjunctivitis Sicca: pooled Analysis of a Phase 2b/3 and Phase 3 Study. Eye Contact Lens. 2020;46: (Supple1) S14–S19.
35. Wirta DL, Torkildsen GL, Moreira HR, et al. A Clinical Phase II Study to Assess Efficacy, Safety, and Tolerability of Waterfree Cyclosporine Formulation for Treatment of Dry Eye Disease. Ophthalmol. 2019;126(6):792–800. doi:10.1016/j.ophtha.2019.01.024
36. Meinert H, Roy T. Semifluorinated alkanes A new class of compounds with outstanding properties for use in ophthalmology. Eur J Ophthalmol. 2000;10(3):189–197. doi:10.1177/112067210001000301
37. Therapeutics M. MC2 Therapeutics A/S announces topline results showing a favorable safety profile and reduction in corneal staining for MC2-03 in dry eye patients with moderate to severe keratitis; 2019. Available from: https://www.mc2therapeutics.com/news/?nid=5bce1c4d05398.
38. Yu Y, Chen D, Li Y, Yang W, Tu J, Shen Y. Improving the topical ocular pharmacokinetics of lyophilized cyclosporine A-loaded micelles: formulation, in vitro and in vivo studies. Drug Deliv. 2018;25(1):888–899. doi:10.1080/10717544.2018.1458923
39. Grimaudo MA, Pescina S, Padula C, et al. Poloxamer 407/TPGS Mixed Micelles as Promising Carriers for Cyclosporine Ocular Delivery. Mol Pharm. 2018;15(2):571–584. doi:10.1021/acs.molpharmaceut.7b00939
40. Kapoor Y, Dixon P, Sekar P, Chauhan A. Incorporation of drug particles for extended release of Cyclosporine A from poly-hydroxyethyl methacrylate hydrogels. Eur J Pharm Biopharm. 2017;120:73–79. doi:10.1016/j.ejpb.2017.08.007
41. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008
42. Du S, Hiramatsu N, Hayakawa K, et al. Suppression of NF-kappaB by cyclosporin a and tacrolimus (FK506) via induction of the C/EBP family: implication for unfolded protein response. J Immunol. 2009;182(11):7201–7211. doi:10.4049/jimmunol.0801772
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
