Back to Journals » Clinical Epidemiology » Volume 15
A Review of Major Danish Biobanks: Advantages and Possibilities of Health Research in Denmark
Authors Laugesen K , Mengel-From J, Christensen K , Olsen J, Hougaard DM, Boding L , Olsen A, Erikstrup C , Hetland ML, Høgdall E, Kjaergaard AD, Sørensen E, Brügmann A , Petersen ERB, Brandslund I , Nordestgaard BG , Jensen GB, Skajaa N , Troelsen FS , Fuglsang CH , Svingel LS , Sørensen HT
Received 6 October 2022
Accepted for publication 20 January 2023
Published 21 February 2023 Volume 2023:15 Pages 213—239
DOI https://doi.org/10.2147/CLEP.S392416
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eyal Cohen
Kristina Laugesen,1 Jonas Mengel-From,2,3 Kaare Christensen,2– 4 Jørn Olsen,1 David M Hougaard,5,6 Lasse Boding,7 Anja Olsen,8,9 Christian Erikstrup,10 Merete Lund Hetland,11,12 Estrid Høgdall,12,13 Alisa D Kjaergaard,14 Erik Sørensen,15 Anja Brügmann,16 Eva Rabing Brix Petersen,17 Ivan Brandslund,17,18 Børge G Nordestgaard,19 Gorm B Jensen,20 Nils Skajaa,1 Frederikke Schønfeldt Troelsen,1 Cecilia Hvitfeldt Fuglsang,1 Lise Skovgaard Svingel,1 Henrik T Sørensen1
1Department of Clinical Epidemiology, Aarhus University Hospital and Aarhus University, Aarhus, Denmark; 2Epidemiology, Biostatistics and Biodemography, the Danish Twin Registry, and the Danish Aging Research Center, Department of Public Health, University of Southern Denmark, Odense, Denmark; 3Department of Clinical Genetics, Odense University Hospital, Odense, Denmark; 4Department of Clinical Biochemistry and Pharmacology, Odense University Hospital, Odense, Denmark; 5iPSYCH, Lundbeck Foundation Initiative for Integrative Psychiatric Research, Aarhus, Denmark; 6Center for Neonatal Screening, Department for Congenital Disorders, Statens Serum Institut, Copenhagen, Denmark; 7The Danish National Biobank, Statens Serum Institut, Copenhagen, Denmark; 8Danish Cancer Society Research Center, Danish Cancer Society, Copenhagen, Denmark; 9Department of Public Health, Aarhus University, Aarhus, Denmark; 10Department of Clinical Immunology, Aarhus University Hospital and Department of Clinical Medicine, Aarhus University, Aarhus, Denmark; 11The DANBIO Registry and Copenhagen Center for Arthritis Research (COPECARE), Center for Rheumatology and Spine Diseases, Centre of Head and Orthopaedics, Copenhagen University Hospital Rigshospitalet, Glostrup, Denmark; 12Department of Clinical Medicine, University of Copenhagen, Faculty of Health and Medical Sciences, Copenhagen, Denmark; 13Bio- and GenomeBank Denmark (RBGB), Molecular Unit, Department of Pathology, Herlev Hospital, Herlev, Denmark; 14Steno Diabetes Center Aarhus, Aarhus University Hospital, Aarhus, Denmark; 15Department of Clinical Immunology, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 16Department of Pathology, Aalborg University Hospital, Aalborg, Denmark; 17Department of Clinical Biochemistry and Immunology, Lillebaelt Hospital, Vejle, Denmark; 18Institute of Regional Health Research, University of Southern Denmark, Odense, Denmark; 19The Copenhagen General Population Study, Department of Clinical Biochemistry, Copenhagen University Hospital – Herlev Gentofte, University of Copenhagen, Herlev, Denmark; 20The Copenhagen City Heart Study, Frederiksberg and Bispebjerg Hospital, Frederiksberg, Denmark
Correspondence: Kristina Laugesen, Department of Clinical Epidemiology, Aarhus University Hospital, Olof Palmes Allé 43-45, Aarhus, 8200, Denmark, Tel +45 871 68063, Email [email protected]
Abstract: Biobank research may lead to an improved understanding of disease etiology and advance personalized medicine. Denmark (population ~5.9 million) provides a unique setting for population-based health research. The country is a rich source of biobanks and the universal, tax-funded healthcare system delivers routinely collected data to numerous registries and databases. By virtue of the civil registration number (assigned uniquely to all Danish citizens), biological specimens stored in biobanks can be combined with clinical and demographic data from these population-based health registries and databases. In this review, we aim to provide an understanding of advantages and possibilities of biobank research in Denmark. As knowledge about the Danish setting is needed to grasp the full potential, we first introduce the Danish healthcare system, the Civil Registration System, the population-based registries, and the interface with biobanks. We then describe the biobank infrastructures, comprising the Danish National Biobank Initiative, the Bio- and Genome Bank Denmark, and the Danish National Genome Center. Further, we briefly provide an overview of fourteen selected biobanks, including: The Danish Newborn Screening Biobank; The Danish National Birth Cohort; The Danish Twin Registry Biobank; Diet, Cancer and Health; Diet, Cancer and Health – Next generations; Danish Centre for Strategic Research in Type 2 Diabetes; Vejle Diabetes Biobank; The Copenhagen Hospital Biobank; The Copenhagen City Heart Study; The Copenhagen General Population Study; The Danish Cancer Biobank; The Danish Rheumatological Biobank; The Danish Blood Donor Study; and The Danish Pathology Databank. Last, we inform on practical aspects, such as data access, and discuss future implications.
Keywords: biobank, research, healthcare system, registries, epidemiology, precision medicine
Introduction
Biobank research may lead to an improved understanding of disease etiology and advance personalized medicine with the purpose of targeted prevention and diagnostic efforts and more efficient and safe treatment. Human biological specimens, combined with clinical and demographic data from population-based health registries and databases, play an important role in the advances of health research. Denmark provides a unique setting for such research and offers possibilities for studying biomarkers, epigenetics, genome-wide associations, and gene–environment interactions in large study populations.1,2 Furthermore, data from the Danish biobanks are frequently included in multinational projects or consortia.3–17 Denmark has around 5.9 million inhabitants; a universal, tax-funded healthcare system; and a rich source of hundreds of biobanks, registries, and databases available for research.18,19 The unique civil registration number, assigned to all Danish citizens, enables accurate linkage of such resources at the individual level and ensures virtually no loss to follow-up.20
In this review, we provide an overview of advantages and possibilities of biobank research in Denmark. Knowledge about the setting is a necessity to understand the full potential. Therefore, we first describe the Danish healthcare system, the population-based registries and databases, and the interface with biobanks. We then compile information about key infrastructures related to biobank collection and storage and fourteen selected large biobanks. Last, we inform on practical aspects, including data access, and discuss future implications.
Setting
The Danish Healthcare System
Denmark is a country of 5.9 million inhabitants (87% of Danish origin, 8.5% immigrants or descendants from non-Western countries, and 4.8% immigrants or descendants from Western countries).21 The country has a welfare state model where healthcare is tax-funded and mainly free of user payment for all Danish citizens, including free access to general practitioners and hospitals, and partial reimbursement of prescribed medications.18,19 The Danish healthcare sector is divided into the primary healthcare sector, such as general practitioners and other healthcare professionals in private practice (physiotherapists, chiropractors, psychologists, dentists, and private practicing medical specialists), the secondary healthcare sector (general hospitals), and the tertiary healthcare sector (university hospitals). Despite private practice, all general practitioners and the majority of other private practices are reimbursed by the public healthcare system. The hospital sector provides hospital emergency care, inpatient treatment, hospital outpatient clinic care, including psychiatric hospital care.18,19 Less than 2% of the total hospital capacity is private. Except in emergencies, patients need referral from a general practitioner to access the hospital sector and the majority of private medical specialists. Hence, general practitioners (~20% of the total physician work force) play an essential role as gatekeepers.18,19
From an administrative perspective, the Danish healthcare system has three levels: the national level (state), the regional level (five regions united in the interest organization Danish Regions), and the local level (98 municipalities). The government (state), headed by The Ministry of Health, outlines the framework of the healthcare system by legislation, national guidelines, and healthcare monitoring.18,19 The Ministry of Health consists of the ministry and nine agencies, including the Danish Health Authority, the Danish Medicines Agency, Statens Serum Institute (SSI), the Danish Health Data Authority, the Danish Patient Safety Authority, the Danish Agency for Patient Complaints, the National Center of Ethics, and the Danish National Genome Center. The five regions operate and own the public hospitals. Together, the Danish Ministry of Health and the Danish Regions are further responsible for national strategies for personalized medicine, including improvements in Danish biobank infrastructures.22 The municipalities are responsible for social services, primary disease prevention and health promotion, home care and nursing homes, and rehabilitation outside hospitals.18,19
The Danish healthcare system delivers routinely and prospectively collected data to various population-based registries and databases. The universal access to healthcare diminishes selection of specific patient groups into the registries and ensures presentation from all segments of the population.18,19 A detailed description of the Danish healthcare system and the registries is out of the scope of this review. In Table 1, we provide references for key papers that describe the healthcare system and some frequently used population-based registries. In the next section, we briefly describe how Danish registries based on routinely collected data can enrich biobank research.
 |
Table 1 Overview of Papers About the Danish Healthcare System and Selected Population-Based Registries |
The Danish Civil Registration System, the Population-Based Registries and the Interface with Biobanks
The civil registration number is a unique, personal identity number assigned to all Danish residents at birth or upon immigration. The 10-digit number encodes the date of birth and sex and is part of the Danish Civil Registration System established in 1968.19,20 This system covers the entire Danish population and follows each person from assignment of the civil registration number until date of death or emigration, with virtually no loss to follow-up.20 Denmark is therefore a nationwide cohort with an accumulated source population of approximately nine million people (since 1968).19,20 The civil registration number is used in almost all interfaces with the Danish public sector, including all contacts with the healthcare system. The civil registration number represents the key identifier across all Danish registries, databases, and biobanks and allows accurate and easy linkage of data between these (Figure 1). Denmark has hundreds of linkable population-based registries and databases available for research.18,19,23–30 The combination of access to biological materials in biobanks, the features of the Danish healthcare system, the Civil Registration System, and the many registries and databases makes Denmark a unique setting for research with numerous possibilities as displayed in Box 1. The population-based registries can provide additional information, not stored by the biobanks themselves. In some biobank cohorts, they collect detailed information through questionnaires, interviews and clinical examinations when enrolling or following their participants. Still, researchers may lack certain baseline or outcome information. In such circumstances, additional clinical information may be available in registries, such as the National Patient Registry (1977 -),24 the National Cancer Registry (1943 -),28 the National Prescription Registry (1995 -),26 the Psychiatric Central Research Registry (1969 -),25 the Laboratory information systems,31 the clinical quality databases, and in the Medical Birth Registry (1973 -)23 (Table 1 and Box 1). Sociodemographic information, such as income, education level, and employment status is available in the social and demographic registers (1982 -) (Box 1). Moreover, it is possible to characterize participants according to country of origin by linkage to the Civil Registration System. Further, parents, siblings, and offspring can be identified in the Civil Registration System, as the system holds information on the maternal and paternal civil registration number for each index person (Table 1 and Box 1). Although, the routinely collected data are rich in many ways, data availability and quality need consideration before use. Before using these data resources, we recommend to read more thoroughly about the healthcare system and registries (Table 1) and search for data validation papers.
 |
Box 1 Biobank Research in Denmark: Advantages and Possibilities |
Danish Infrastructures Related to Biobank Research
A biobank is a structured collection of individual-level biological specimens and can be divided into research biobanks, clinical biobanks, or biobanks for other purposes (eg donor biobanks).32 Research biobanks are initiated with the direct purpose of research projects, whereas clinical biobanks are for later diagnostic purposes or treatment decisions on the patient level, but may also contribute to research.32 Before describing some of the large biobanks in Denmark, we here describe some key Danish infrastructures related to biobank research. Denmark has made great efforts to improve biobank research as part of the national strategy for personalized medicine.22 The strategy involves the following aims: 1) to improve biobank infrastructure with the purpose to ensure easy, uniform, and high-quality collection and storage of biological specimens; 2) to facilitate research collaborations on both national and international level and between public and private sectors; and 3) to offer a better overview of available biobank material and application processes. Below we provide an overview of three important infrastructures related to biobank research, including the Danish National Biobank Initiative, the Bio- and Genome Bank Denmark, and the Danish National Genome Center.
The Danish National Biobank Initiative
The Danish National Biobank Initiative consists of three parts:33
- The Danish Biobank Register (online tool) that links samples to register information.
- The Danish National Biobank (physical biobank).
- The Coordinating Centre that handles data applications and secures sample access.
The Danish Biobank Register
The Danish Biobank Register is an online tool that provides an overview of biological samples available for research in some of the Danish biobanks. The Danish Biobank Register combines information on biobank samples with information from administrative and health registries (the Civil Registration System, the National Patient Registry, and the National Pathology Registry) in an online database. Very recently, laboratory test results were added to the search engine, starting with more than 100 million tests in the Copenhagen Primary Care Laboratory Database34 and with the ambition to integrate all laboratory test results performed in Denmark. From a practical point of view, researchers submit a request on the website biobanks.dk. The request may contain information on biobank, sample type, and populations of interest (according to eg sex, age, country of birth, parental country of birth, and diagnosis). Following request submission, an email will return with an aggregated overview of available data.
The Danish National Biobank
The Danish National Biobank (hosted by SSI) is the largest biobank in Denmark containing more than 14 million samples from approximately 5 million individuals (Table 2). This biobank stores samples from different SSI-managed and external biobank-managed data sources.33 Examples of SSI-managed collections are the Danish Newborn Screening Biobank, the Danish National Birth Cohort, the Greenland samples, and various diagnostic samples. Examples of external biobank-managed collections are the two Danish Cancer Society cohorts Diet, Cancer, and Health and “Diet, Cancer and Health – next generations”. Staff employed at the Danish National Biobank is available to advise researchers on study design, sample handling, registration, transportation, analysis, and storage.
 |
Table 2 Overview of Biological Samples in the Danish National Biobank |
More recently, the biobank has played a major role in building the Danish COVID-19 test strategy. High-throughput pipelines, already installed in the biobank, were expanded to a capacity of more than 250,000 RT-PCR tests per day. To date, more than 4.2 million throat swab samples are collected in the biobank, including more than 3.3 million COVID-19 positive swab samples. Further, more than 30,000 COVID-19 positive serum or plasma samples are available.
Bio- and Genome Bank Denmark
The Bio- and Genome Bank Denmark is a nationwide cooperation between Danish public hospitals, funded and operated by the Danish Regions (the entities operating the public hospitals in Denmark).35 The Bio- and Genome Bank Denmark includes following individual biobanks, the majority being clinical biobanks: the Danish Cancer Biobank, the Danish Rheumatologic Biobank,36 the Danish Blood Donor Biobank,37,38 the Danish Diabetes Biobank, the Danish Dermatology Biobank, the Danish Screening Biobank, the Danish Genetic Biobank, the Danish Research Biobank, and the Danish COVID-19 Biobank (Figure 2).35 All these biobanks are using the same data registration system. Furthermore, the Danish Pathology Data Bank and Danish Genetics Data Bank are included in the infrastructure.39 The aim of Bio- and Genome Bank Denmark is to ensure biological material for diagnosis or treatment of the patients and to facilitate the use of the expanding infrastructure for both private and public researchers. For example, drug development and research on personalized medicine benefit from collaborative research between the public healthcare sector and the pharmaceutical industry.
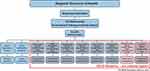 |
Figure 2 Organization of the Bio- and Genome Bank Denmark. Abbreviation: RBGB, The Bio- and Genome Bank Denmark. Notes: Source is with permission from Bio- and Genome Bank Denmark. Available from: https://www.regioner.dk/rbgben/about/organization. The figure shows how the Bio- and Genome Bank Denmark is organized with a Steering Committee, a national secretariat and Scientific Advisory Boards for present and future biobanks. XXX denotes that more and more biobanks are included in the structure. |
The collection and storage of biological samples take place at local hospital departments/centers in each region and are facilitated by well-established infrastructures as part of routine procedures. The secretariat is hosted by Department of Pathology at Herlev Hospital, which has the overview of all samples in Denmark and coordinates samples for diagnostic and research together with regional project leaders. The regional centers are responsible for managing materials from their respective region and for making arrangements with the local hospital departments. Bio-and Genome Bank Denmark is headed by the Regional directors of Health in a structure consisting of a National Steering Committee and a National Secretariat, which serves all the biobanks (Figure 2).35 Information on procedures and organization can be found on the homepage (https://www.regioner.dk/rbgb/biobanker). Table 3 provides an overview of material included in the Bio-and Genome Bank Denmark from 2016 to 2021. Inclusion of material is ongoing and the infrastructure is regularly expanding with new biobanks. All blood samples are fractionated in serum, EDTA plasma, whole blood and buffy coat. All tissue samples are fractionated in dry frozen tissue, optimal cutting temperature compound tissue, RNAlater treated tissue and formalin fixed and paraffin embedded tissue. All tissue samples are verified by pathologists.
 |
Table 3 Number of Unique Biological Samples Included in the Bio- and Genome Bank Denmark from 2016 to 2021 |
The Danish National Genome Center
In 2019, the Danish National Genome Center (an agency under the Ministry of Health) was launched as a part of the national strategy for personalized medicine.40 The goal of the National Genome Center is to advance personalized medicine by performing genome sequencing in patients for clinical and research purposes. The center has national responsibility for processing, analysis, and secure storage of genome data. As well, the center offers guidance in interpretation of data. During the next four years, the plan is to perform whole-genome sequencing in 60,000 selected patients with financial support from the Novo Nordisk Foundation. Currently, 2166 patients have had such sequencing performed. Furthermore, some patients have genetic analyses performed for diagnostic or treatment purposes as part of routine clinical care. Since 2019, it has been mandatory for clinicians to report these analyses to the Danish National Genome Center. Approximately 1940 genomes have been reported to the center in this manner. Before genetic analysis, the responsible clinicians must ensure informed consent.
Overview and Description of Selected Biobanks
Table 4 shows an overview of selected large Danish biobanks. Fourteen biobanks have been chosen for this review and the list is not comprehensive. In the following sections, we provide a short description of each of the biobanks.
 |
Table 4 Overview of Fourteen Biobanks (Population, Period of Enrollment, Data Types, and a Study Example). Detailed Information About Data is Presented in Later Tables |
The Danish Newborn Screening Biobank
The Danish Newborn Screening biobank is a nationwide clinical biobank. Newborn screening for congenital diseases, such as phenylketonuria was implemented nationally in 1975.41 Blood drops from the infant´s heel are collected on filter paper shortly after birth. Following the screening program, the dried blood spot filter paper has been stored in freezers since 1982.41 Annually, an average of 62,000 newborns take part in the screening program. The screening program is voluntary, but more than 99.5% of all parents decide to participate. Hence, the biobank stores blood spot filter paper for almost the entire Danish population born since 1982 (~2,310,680 persons). Because of the virtually complete nationwide coverage of the biobank the distribution of sociodemographic factors, including ethnicity, reflects that of the Danish population at the given time and in the given age span. The Danish Newborn Screening Biobank has, among other projects, contributed with biological samples to the Integrative Psychiatric Research project that aims to investigate genetic and environmental risk factors for mental disorders.42 The Integrative Psychiatric Research project has contributed to a large number of important research projects,43 including genome-wide association studies of psychiatric diseases.14–16 As an example, Wray et al identified 44 genetic loci for major depression.14
The Danish National Birth Cohort
The Danish National Birth Cohort is a nationwide research biobank with data from ~100,000 pregnancies enrolled from 1996 to 2002. Data include biological specimens, primarily blood taken from the mother twice during pregnancy and blood from the umbilical cord taken shortly after birth, and interview and questionnaire data (Table 4). Eligible persons were pregnant women who indicated at their first pregnancy-related visit to their general practitioner that they wished to carry their pregnancy to term and had sufficient Danish language proficiency to complete telephone interviews.44–46 A previous paper has described certain sociodemographic factors in the cohort, including ethnicity.47
Table 5 presents an overview of biological material and Table 6 presents an overview of interview and questionnaire data collected in the Danish National Birth Cohort.46,48–54 The cohort has contributed to more than 600 publications,55 including multinational genome-wide association studies on asthma,3 pubertal timing,4 reproductive behavior,5 and to investigate associations between birth weight and adult disease.6 For instance, Horikoshi et al found that associations between early growth phenotypes and adult cardiometabolic disease were in part the result of shared genetic effects.6
 |
Table 5 Number of Unique Biological Samples in the Danish National Birth Cohort |
 |
Table 6 Overview of Interviews and Questionnaires in the Danish National Birth Cohort |
The Danish Twin Registry and Biobank
The Danish Twin Registry is a nationwide registry established in the 1950s. The registry includes birth cohorts from 1870 to 2009, comprises 175,518 individuals (86,402 pairs of twins, 874 sets of triplets, and 23 sets of quadruplets), and includes questionnaire, interview and clinical examination data (Table 4).56 Ascertainment methods have varied over time (please see Pedersen et al for more details).56 Table 7 provides an overview of questionnaires and interviews conducted from 1994 to 2012.56,57 The Danish Twin Biobank is a nationwide research biobank established in 1997 as part of the second wave of the “Longitudinal Study of Aging Danish Twins” (LSADT) and the first wave of the “Importance of Genes, Familiar and Common Environment for the Development of Insulin Resistance, Abdominal Adiposity and Cardiovascular Risk Factors” (GENIMAKAR) (Table 7). Table 8 shows an overview of biological specimens and molecular data in the Danish Twin Registry and Biobank.56,58–61 The Danish Twin Registry and Biobank has contributed with numerous studies, including studies on the heritability of cancer,7–9 biomarkers for dementia,62 genome-wide association studies of intelligence,10 and epigenetic studies on aging,63,64 cognitive function,65 and mortality.66 For example, Mengel-From et al found new circulating miRNAs linked to dementia.62
 |
Table 7 Overview of Questionnaires, Interviews, or Clinical Examinations in the Danish Twin Registry and Biobank |
 |
Table 8 Overview of Biobank Material and Molecular Data in the Danish Twin Registry and Biobank |
Diet, Cancer, and Health and Diet, Cancer, and Health – Next Generations
Diet, Cancer, and Health is a cohort and research biobank consisting of 57,053 participants, recruited between 1993 and 1997, including questionnaires, clinical examination data, and biological material (Table 4).67 The primary aim of the cohort is to investigate associations of diet and lifestyle with the risk of cancer and other chronic diseases. From 1993 to 1997, 160,725 individuals aged 50 to 64 years from the Copenhagen and Aarhus areas were invited to participate (19% of the total Danish population in that age group). The eligibility criteria were ages 50 to 64 years, birth in Denmark, and no previous diagnosis of cancer (according to the Danish Cancer Registry). Of 160,725 invited individuals, 57,053 (35%) participated. Sociodemographic characteristics of participants and non-participants are described elsewhere.67 Data collected by Diet, Cancer, and Health are shown in Table 9.67–69 Five years after baseline, 54,379 participants were eligible for follow-up questionnaire on diet, lifestyle, social network, and health (Table 9). Among the eligible, 44,904 (83%) responded.
 |
Table 9 Overview of Data in Diet, Cancer, and Health |
Diet, Cancer, and Health – Next Generations is a multigenerational extension of the original Diet, Cancer and Health cohort consisting of 44,869 participants recruited between 2015 and 2019 with questionnaire information, clinical examination data, and collection of biological material.70 Children, their spouses, and grandchildren of the original cohort were identified though the Civil Registration System, and 23,269 (30%) of 78,767 eligible children, 8399 (23%) of 35,977 eligible spouses, and 13,201 (19%) of 69,020 eligible grandchildren participated.70 Sociodemographic characteristics of participants and non-participants are described elsewhere.70 Table 10 provides an overview of data in Diet, Cancer, and Health – Next Generations.70
 |
Table 10 Overview of Data in Diet, Cancer, and Health – Next Generations |
Diet, Cancer, and Health has contributed to more than 1000 publications.71 For instance, Kirkegaard et al showed that adherence to the recommendations by the World Health Organization, World Cancer Research Fund, and the Nordic Nutrition Recommendations for physical activity, waist circumference, smoking, alcohol consumption, and diet reduced colorectal cancer risk considerably.72 Further, the cohort is coordinated with a number of similar international cohorts by the International Agency for Research on Cancer. As example Diet, Cancer, and Health is an associated cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC).12
Danish Centre for Strategic Research in Type 2 Diabetes
The Danish Centre for Strategic Research in Type 2 Diabetes is a nationwide cohort and research biobank with enrollment from 2010.73,74 Currently, the cohort contains interview and questionnaire data, clinical examination data, and biological samples from ~10,000 patients with diagnosed type 2 diabetes (Table 4). The cohort continues to enroll patients from both general practitioners and outpatient hospital clinics. Eligible patients are ≥18 years of age and diagnosed within two years prior to the time of enrollment. Approximately 1000 to 1200 patients are enrolled annually, corresponding to 5% of incident type 2 diabetes patients nationwide.73 Clinical and sociodemographic characteristics of the cohort are described elsewhere.73 Table 11 shows an overview of data in the Danish Centre for Strategic Research in Type 2 Diabetes cohort.73,74 Supplemental individual-level baseline and follow-up data are provided by the Danish Diabetes Database for Adults; a clinical quality database, containing annually or biannually collected data from general practitioners or outpatient clinics from 2005 onwards (Table 11).75 An example of research using this biobank is Gedebjerg et al who showed that serum MBL and MBL expression genotype had a U-shaped association with cardiovascular disease risk in individuals with type 2 diabetes, suggesting that serum MBL is a risk factor for cardiovascular disease in this population.76
 |
Table 11 Overview of Data in the Danish Centre for Strategic Research in Type 2 Diabetes Cohort and the Danish Diabetes Database for Adults |
Vejle Diabetes Biobank
The Vejle Diabetes Biobank is a regional research biobank established between 2007 and 2010.77 The biobank contains questionnaire, clinical examination data and biobank material from 3320 patients with diabetes (N = 2721 with type 2 diabetes and N = 599 with type 1 diabetes) and 4255 non-diabetic individuals from the general population (Table 4).77 As of December 31th 2006, all eligible individuals, were alive, aged between 25 and 75, and living in the former County of Vejle area, and had a Danish first and last name. Potential patients with diabetes were identified through Danish registries and defined as having fulfilled at least one of the following criteria: i) At least one glycated hemoglobin (HbA1c) value ≥48.6 mmol/mol (≥6.6%) in the Clinical Laboratory Information System (period from 1996 to 2006); ii) At least three HbA1c values <48.6 mmol/mol (<6.6%) in the Clinical Laboratory Information System (2002 to 2006); iii) At least one record in the Danish National Prescription Registry for an oral antidiabetic agent or insulin (2006); or iiii) At least one in- or outpatient hospital contact with diabetes recorded in the Danish National Patient Registry (1977 to 2006). Of 14,831 potential diabetes patients, 3345 individuals were excluded due to age (<25 or >75 years) and 1078 were excluded due to non-Danish names. Further, only individuals who acknowledged having diabetes were included. From 10,408 eligible patients, 3320 (32%) were included in the biobank (6234 did not respond and 854 did not acknowledge having diabetes). Non-diabetic individuals were sampled from the general population of the former County of Vejle through the Civil Registration System and matched by sex and age. Following, N = 11,065 received a request to participate and N = 4290 accepted (response of 39%). The final non-diabetic population constituted 4255 individuals (N = 35 acknowledged having diabetes without fulfilling the diabetes inclusion criteria above). Table 12 provides an overview of data in the Vejle Diabetes Biobank.77 Using this biobank, Petersen et al reported that 56% of the T2D and 25% of T1D patients reached target for glycaemic regulation (HbA1c < 53 mmol/mol) at the time of inclusion, 28% of the T2D and 48% of the T1D patients reached target for blood pressure (<140/mmHg), and 34% of the T2D and 55% of the T1D patients reached treatment target for lipids (triglycerides <1.7 mmol/L, LDL <2.6 mmol/L, HDL ≥1.0 mmol/L for men and ≥1.3 mmol/L for women, and total cholesterol <4.5 mmol/L).77
 |
Table 12 Overview of Data in the Vejle Diabetes Biobank |
The Copenhagen Hospital Biobank
The Copenhagen Hospital Biobank is a research biobank, which started in 2009 at Copenhagen University Hospital (Rigshospitalet) and expanded in 2012 to include all hospitals in the Capital Region of Denmark.78 At present, the biobank counts ~425,000 patients (Table 4). Each patient is included only once, ie, the biobank has no repetitive blood sampling. Inclusion continues for as long as funding is available.78 The biobank is based on surplus EDTA whole blood from patients admitted to Danish hospitals in the Copenhagen area for diagnostic or treatment purposes who have samples drawn for blood type testing or red cell antibody screening. This patient group is diverse, no exclusion criteria apply, and the distribution of sociodemographic factors in biobank participants, including ethnicity, reflect that of the underlying patient population.78 The samples are primarily suitable for DNA extraction. For more information on collection procedures, storage, and quality assessment please visit Sørensen et al78 DNA extraction and genetic analyses have been carried out on samples from selected patients and genome-wide genotype data are available for ∼330,000 patients (January 2022).78 Genotyping is performed at deCODE Genetics, Iceland, using an array that examines more than 660,000 SNPs.78 The Copenhagen Hospital Biobank has contributed with research. For instance, Helgadottir et al showed that genetic variation in cholesterol absorption affected levels of circulating non-HDL-C and risk of coronary artery disease.79
The Copenhagen City Heart Study
The Copenhagen City Heart Study (Østerbroundersøgelsen) is a cohort and research biobank consisting of individuals randomly selected to reflect the adult Danish general population. The data collected include questionnaires, clinical examinations, and blood samples (Table 13).
 |
Table 13 Overview of Data in the Copenhagen City Heart Study and the Copenhagen General Population Study |
Originally, the aim of the Copenhagen City Heart Study was to investigate etiology and prevention of cardiovascular disease; the study was designed like the pioneering Framingham Heart Study. However, many other aspects have been added to the study over the years.80 The Copenhagen City Heart Study totals more than 25,000 participants. A random sample of the census population in the central Copenhagen area (Østerbro) was invited to participate in five examination waves: 1976 to 1978, 1981 to 1983, 1991 to 1994, 2001 to 2003, and 2011 to 2014, with a sixth wave for 2022 to 2025. The original cohort (1976 to 1978) comprised a random sample of 19,329 individuals drawn from a population of roughly 90,000 individuals using the Civil Registration System. The random sample was stratified into 5-year age groups ranging from 20 to 93 years, with the emphasis on those aged 35 to 70 years.81 Of those invited, 74% (N = 14,223) participated. For each subsequent examination, all previous participants were re-invited, and the cohort was additionally supplemented with younger individuals (20 to 49 year-olds) due to the otherwise inevitable increase in age and, thus, mortality. Participation declined over time from 74% to 50%. Of the 14,223 individuals participating in the first examination, 22% (3092) participated in all of the first four examinations.80 In the first two examinations, all the blood samples were immediately analyzed for plasma levels of glucose, total cholesterol, high-density lipoprotein cholesterol (HDL) cholesterol, and triglycerides. From the third examination and onwards, all the blood samples were immediately analyzed for additional biochemical parameters (eg, hematology, liver function tests, hormones, etc), and plasma and whole blood samples were stored for later biochemical and DNA analyses. Lange et al used the Copenhagen City Heart Study to describe changes over time in the forced expiratory volume (FEV1) in adults with and without asthma.82
The Copenhagen General Population Study
Modelled on the Copenhagen City Heart Study design, the Copenhagen General Population Study (Herlev/Østerbroundersøgelsen) was initiated in 2003. During 2003 to 2015, a total of almost 110,000 individuals were included (Table 4). All individuals aged 40 years and above, and 25% of individuals aged 20 to 39 years from the greater Copenhagen area (municipalities of Herlev, Ballerup, Gladsaxe, Gentofte, Rødovre, Furesø, Lyngby-Tårbæk, Copenhagen, Egedal, Rudersdal, Allerød, Glostrup, Albertslund, Hørsholm, and Frederikssund) were invited to participate. Of invites, 43% agreed to participate. Originally, only individuals of Danish descent were invited to ensure a homogenous study population for genetic studies. Further, all individuals of Pakistani descent from the capital region of Denmark were also invited, but only 6% agreed to participate. A follow-up examination began in 2014 and is still ongoing. By 2022, more than 50,000 individuals have been re-examined. The re-examination was designed exactly like the first wave in 2003–2015, that is, all individuals aged 40 years and above and 25% of individuals aged 20 to 39 years were invited from the greater Copenhagen area in the same municipalities as listed above. To secure as many reexamined individuals as possible, all those examined in the first wave, but no longer living in the same municipality of Copenhagen were also re-invited.
The combined cohorts (the Copenhagen City Heart Study and the Copenhagen General Population Study) have currently contributed with more than 1000 publications on many subjects,83 covering scientific areas within healthy lifestyle, cardiovascular disease, cancer, chronic lung disease, psychiatry, gastroenterology, hepatology, endocrinology, rheumatology, etc. For instance, Mortensen and Nordestgaard showed that elevated low-density lipoprotein cholesterol was associated with myocardial infarction and other atherosclerotic disease in a primary prevention cohort. People aged 70–100 vs 20–69 years with elevated low-density lipoprotein cholesterol had the highest absolute risks and the lowest estimated number to treat in five years to prevent one event.84
The Danish Cancer Biobank
The Danish Cancer Biobank (part of the Bio- and Genome Bank Denmark) is a clinical biobank, which was established in 2009 and has centers at six hospitals in Denmark.85 The biobank contains samples from patients who have undergone clinical examination and investigation for potential cancer disease (Table 4). Biological material is collected during procedures for diagnostic or treatment purposes and enrollment is ongoing. With approvals, the material can be used for research after ensuring that the patient is not included in the Tissue Utilization Registry (few).86 No exclusion criteria apply and the distribution of sociodemographic factors in biobank participant, including ethnicity, mirror that of the underlying patient population. Table 14 shows an overview of biological material included in the biobank from 2016 to 2020. Using the Danish Cancer Biobank, Reinert et al showed that circulating tumor DNA served as a biomarker for postoperative and post-adjuvant chemotherapy risk stratification, monitoring of adjuvant chemotherapy effectiveness, detection of clinical actionable mutations, and early detection of recurrence in stages I–III colorectal cancer.87
 |
Table 14 Number of Unique Biological Samples Included in the Danish Cancer Biobank from 2016 to 2020 |
The Danish Rheumatologic Biobank
The Danish Rheumatologic Biobank (part of the Bio- and Genome Bank Denmark) is a nationwide collaboration between Danish departments of rheumatology and departments of clinical biochemistry.36,88 This clinical biobank was established in 2015 and contained 8346 patients with inflammatory rheumatic diseases as of September 2021 (rheumatoid arthritis [4455 patients], axial spondyloarthritis [1299 patients], psoriatic arthritis [1223 patients], and others [1369 patients]), and recruitment is ongoing (Table 4). The aim of this biobank is to ensure material for patients own diagnosis and treatment and to support research in personalized medicine of inflammatory rheumatic diseases. Patients are eligible for inclusion if they are followed in routine care for one of the inflammatory diseases above, are ≥ 18 years of age, and are able to give informed consent. No exclusion criteria apply. Patients contribute with one or more of the following samples: i) cross-sectional blood samples when they meet for a scheduled routine clinical visits, ii) longitudinal blood samples collected at initiation of new disease-modifying anti-rheumatic drugs (DMARD) and at specific time points during treatment and at cessation, iii) other biological materials collected when accessible and relevant (ie, during diagnostic or treatment procedures) (Table 15 and Table 16).36 Detailed information on patient demographics, disease characteristics, comorbidities, and lifestyle factors can be obtained from the clinical database DANBIO, which has existed since 2000 and been electronic since 2006 (Table 15).89,90 Several studies use the biobank. A recent study by Saevarsdottir et al performed multiomics analyses of rheumatoid arthritis, and its seropositive and seronegative subsets. The study identified new rheumatoid arthritis risk loci and candidate causal genes. Most sequence variants had impact on the risk of seropositive, but not seronegative disease. Findings pointed to candidate causal genes encoding proteins in the network of interferon alpha/beta and IL-12/IL-23 that signal through the JAK/STAT pathway.91
 |
Table 15 Overview of Data in the Danish Rheumatologic Biobank and DANBIO |
The Danish Blood Donor Study and the Danish Blood Donor Biobank
The Danish Blood Donor Study is a cohort and biobank launched in 2010 with data from 137,574 blood donors (Table 4). The cohort contains questionnaire-based information on health and lifestyle and plasma, and whole blood for DNA extraction.37,38,92 Enrollment is ongoing with inclusion of blood donors in most blood donation facilities nationwide. From 2017, the biobank material has been part of the Bio- and Genome Bank Denmark (named the Danish Blood Donor Biobank).93 Eligible persons are adults aged 18 to 74 years, who donate blood at the Danish blood donation facilities.38,92 Approximately 95% of invited blood donors choose to participate in the biobank. The blood centers are part of the public Danish healthcare system and blood donation is voluntary and unpaid. The Danish Blood Donor Study uses existing blood bank infrastructure for data collection, handling, and storage of samples. Danish blood donors fill out a questionnaire at every donation to assess their health status. Donors must be physically well, weigh more than 50 kilograms, have a civil registration number, and speak and understand Danish. Reasons for permanent exclusion include chronic infections, current or prior cancer, and most autoimmune, neurological, or cardiovascular diseases. Donors are deferred if having an acute infection, or hemoglobin <8.4 mmol/L in male donors and 7.8 mmol/L in female donors. A detailed sociodemographic description of Danish blood donors, including age, sex, education, employment status, and ethnicity, is found elsewhere.94 When giving informed consent, participants grant permission to future contact for acquisition of additional information, to use past and future blood samples for research, and to collect data from Danish registries. Many participants donate blood several times annually. It is therefore possible to collect consecutive biological and questionnaire data.38,92
Table 17 shows an overview of questionnaire-based information on health and lifestyle collected by the Danish Blood Donor Study. The questionnaires have developed over time in content and technology from paper- to a digital-based form.38,92 From March 2010 to July 2015, participants completed a paper-based questionnaire on self-reported physical and mental health and some lifestyle factors. The first follow-up, digital-based questionnaire was used from July 2015 until May 2018. It contained some of the same questions as in the first questionnaire as well as some new additional questions. A new digital-based questionnaire was launched in June 2018 and a smartphone-based questionnaire was introduced in November 2020.
 |
Table 16 Number of Unique Biological Samples Included the Danish Rheumatologic Biobank from 2015 to 2020 |
 |
Table 17 Overview of Questionnaires in the Danish Blood Donor Study |
The majority of participants (~110,000 donors) in the Danish Blood Donor Study have been genotyped for >650,000 SNPs in collaboration with deCODE Genetics, Iceland. For more information on collection procedures, storage, analysis, and quality assessment please see Hansen et al.38 Several studies have been published using the Danish Blood Donor Study and Biobank. For instance, Burgdorf et al found a temporal association between infections with cytomegalovirus or Toxoplasma gondii on human behaviour and mental disease.95
Specimens Recorded in the Danish Pathology Data Bank
The Danish pathology departments provide diagnostic services on tissue and cytological specimens from all hospitals, general practitioners, and specialist clinics. Following immediate clinical use, these specimens are stored in the archives at the local pathology departments. The tissue specimens are paraffin embedded blocks and cytology specimens and are all stored for later clinical use or research purposes. Electronic recording of pathological specimens began in the 1970s and has been nationwide complete since 1997, when all pathology departments started to record national uniform data on all specimens.39 It is mandatory for all pathology departments to report the electronic data. Because of the virtually complete nationwide coverage, data reflect the underlying patient population (who had specimens examined) with respect to sociodemographic characteristics, including ethnicity. Electronic recording paved the way for two national electronic systems; the Danish Pathology Data Bank and the Danish National Pathology Registry, described below. These data sources allow valid and efficient localization of relevant specimens, thus facilitating clinical research.
The Danish National Pathology Registry and the Danish Pathology Data Bank
The Danish National Pathology Registry was established in 1997 and holds nationwide complete records of all pathology diagnoses. In addition, the registry holds historical incomplete records of specimens from some pathology departments dating back to the 1970s.39 The Danish Pathology Data Bank was established in 1999 with the primary purpose to act as a nationwide online registry for pathology diagnoses. All specimens are registered by the examining pathology department and all previous pathology examinations can be retrieved. Further, information on new analyses is automatically sent to update the data bank. Thus, this data bank holds updated information on pathology diagnoses.39 From 2015, the data bank has been part of the Bio- and Genome Bank Denmark. Box 2 shows an overview of variables in the Danish National Pathology Registry.39 A key variable in both the Danish Pathology Data Bank and the Danish National Pathology Registry is the Danish version of Systematized Nomenclature of Medicine (SNOMED) codes. SNOMED codes are structured on six axes: Topography (T), morphology (M), etiology (Æ), function (F), disease (S), and procedure (P). The first two are mandatory in all records and the latter are optional, but generally used in a structured manner. A study example that uses pathological specimens is Erichsen et al, who showed that patients with sessile serrated adenomas/polyps or traditional serrated adenomas were at increased risk of colorectal cancer compared to a cohort matched by sex and age. The risk was similar to or higher than that of conventional adenomas.96
 |
Box 2 Variables in the Danish National Pathology Registry |
Practical Aspects
Biological material can be handed out to Danish public or non-profit researchers or specialists. Private (eg the pharmaceutical industry) or non-Danish investigators must seek such collaboration before initiating a research project. Data transfer to another country might be possible (with appropriate approvals), but the situation depends on type of data and recipient country.
Danish research projects using human biological material typically need the approvals stated below (other types of approvals can be necessary).
- Approval from the Danish Health Research Ethics Committee System.
- Approval from the Danish Data Protection Agency.
- Approval from the respective biobank committees.
The Danish Health Research Ethics Committee System consists of the National Committee and thirteen regional committees (six committees in the Capital Region, two committees in the Region of Southern Denmark, one committee in the Region Zealand, two committees in the Central Denmark Region, one committee in the Northern Denmark Region, and one committee at the Faroe Islands).97 Most projects need approval from one of the regional committees in the region where the responsible investigator works.97 Approval from the Danish Data Protection Agency is obtained by reporting the study to local authorities with data responsibility (universities or regions). Table 18 provides an overview of where to apply for data at the respective biobank committees. Please visit the Supplementary for more detailed information on how to apply. As biobank and registry data are stored at different platforms, linkage of data can be challenging from a practical point of view. To provide guidance for such logistic issues, the Danish Regions and universities have established regional data support centers.98 Clinicians and researchers can request free guidance on practical and logistic issues from these support centers.
 |
Table 18 Where to Apply for Data |
Future Implications
Biobanks will be a cornerstone in future health research. Biobank research can provide the basis for personalized medicine, including targeted prevention, diagnostic efforts, and treatment with the purpose of more efficient, safe, and cost-effective healthcare. In the future, the combination of biomarkers and clinical characteristics may be an integrated part of predicting disease risk, prognosis, or treatment response. Personalized medicine is already applied in the field of oncology, where clinical characteristics and biomarkers are used to guide the most optimal treatment strategy for certain cancers.99 Other medical fields may be less advanced, but have great potential for future research with few examples presented next. Current clinical guidelines for treatment of rheumatic autoimmune and inflammatory diseases revolve around clinical characteristics only. A large number of patients have poor treatment response and/or severe side effects. Consequently, patients must try out several drugs before finding the most ideal, leading to negative patient experience and less cost-effective healthcare. Tailored treatment, including biomarkers, can have great implications for this group of patients.36 Diabetes is another growing field within personalized medicine. For instance, subclassification of type 2 diabetes may associate with individual prognosis and treatment response.100 Moreover, novel biomarkers for prediction of late diabetic complications could be a valuable clinical tool. Prenatal programming is also gaining more attention. Biobanks can play a key role in understanding how prenatal exposures influence later risk of diseases, including how exposures may interact with genetic factors.
In conclusion, the setting in Denmark provides several advantages and possibilities for biobank health research. Biobanks can be linked to population-based registries and databases with the advantage of complete follow-up and the possibility to gain additional clinical and sociodemographic information not stored by the biobanks themselves. Last, Denmark has made great efforts to ensure high-quality biobank infrastructures and offers great expertise in such matters. The future implications of Danish biobank research are promising.
Funding
There is no funding to report.
Disclosure
MLH reports grants from AbbVie, Biogen, BMS, Celltrion, Eli Lilly, Janssen Biologics B.V., Lundbeck Foundation, MSD, Pfizer, Roche, Samsung Bioepis, Sandoz, Novartis; also reports payment/honoraria for lectures, presentations or similar. No personal income but paid to institution from Pfizer, Medac, and Sandoz; participation in a Data Safety Monitoring Board or Advisory Board. No personal income but paid to institution from AbbVie, outside the submitted work. MLH also has chaired the steering committee of the Danish Rheumatology Quality Registry (DANBIO, DRQ), which receives public funding from the hospital owners and funding from pharmaceutical companies. MLH co-chairs EuroSpA, which generates real-world evidence of treatment of psoriatic arthritis and axial spondyloarthritis based on secondary data and is partly funded by Novartis. LSS reports The Department Clinical Epidemiology is involved in studies with funding from various companies as research grants to and administered by Aarhus University. None of these studies are related to the current study. The authors report no other conflicts of interest in this work.
References
1. Frank L. Epidemiology. The epidemiologist’s dream: Denmark. Science. 2003;301(5630):163. doi:10.1126/science.301.5630.163
2. Frank L. Epidemiology. When an entire country is a cohort. Science. 2000;287(5462):2398–2399. doi:10.1126/science.287.5462.2398
3. Demenais F, Margaritte-Jeannin P, Barnes KC, et al. Multiancestry association study identifies new asthma risk loci that colocalize with immune-cell enhancer marks. Nat Genet. 2018;50(1):42–53. doi:10.1038/s41588-017-0014-7
4. Day FR, Thompson DJ, Helgason H, et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat Genet. 2017;49(6):834–841. doi:10.1038/ng.3841
5. Barban N, Jansen R, de Vlaming R, et al. Genome-wide analysis identifies 12 loci influencing human reproductive behavior. Nat Genet. 2016;48(12):1462–1472. doi:10.1038/ng.3698
6. Horikoshi M, Beaumont RN, Day FR, et al. Genome-wide associations for birth weight and correlations with adult disease. Nature. 2016;538(7624):248–252. doi:10.1038/nature19806
7. Hjelmborg JB, Scheike T, Holst K, et al. The heritability of prostate cancer in the Nordic Twin Study of Cancer. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2303–2310. doi:10.1158/1055-9965.EPI-13-0568
8. Möller S, Mucci LA, Harris JR, et al. The heritability of breast cancer among women in the Nordic Twin Study of Cancer. Cancer Epidemiol Biomarkers Prev. 2016;25(1):145–150. doi:10.1158/1055-9965.EPI-15-0913
9. Graff RE, Möller S, Passarelli MN, et al. Familial risk and heritability of colorectal cancer in the Nordic Twin Study of Cancer. Clin Gastroenterol Hepatol. 2017;15(8):1256–1264. doi:10.1016/j.cgh.2016.12.041
10. Savage JE, Jansen PR, Stringer S, et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet. 2018;50(7):912–919. doi:10.1038/s41588-018-0152-6
11. Sigurdsson S, Alexandersson KF, Sulem P, et al. Sequence variants in ARHGAP15, COLQ and FAM155A associate with diverticular disease and diverticulitis. Nat Commun. 2017;8:15789. doi:10.1038/ncomms15789
12. The World Health Organization. EPIC Study. Available from: https://epic.iarc.fr/.
13. DiGuMet Project. Diet x gut microbiome-based metabotypes to determine cardio-metabolic risk and tailor intervention strategies for improved health. Available from: https://www.chalmers.se/en/projects/Pages/Diet-x-gut-microbiome-based-metabotypes-to-determine.aspx.
14. Wray NR, Ripke S, Mattheisen M, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50(5):668–681. doi:10.1038/s41588-018-0090-3
15. Anttila V, Bulik-Sullivan B, Finucane HK, et al. Analysis of shared heritability in common disorders of the brain. Science. 2018;360:6395.
16. Pardiñas AF, Holmans P, Pocklington AJ, et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet. 2018;50(3):381–389. doi:10.1038/s41588-018-0059-2
17. Lotta LA, Sharp SJ, Burgess S, et al. Association between low-density lipoprotein cholesterol-lowering genetic variants and risk of type 2 diabetes: a meta-analysis. JAMA. 2016;316(13):1383–1391. doi:10.1001/jama.2016.14568
18. Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi:10.2147/CLEP.S179083
19. Laugesen K, Ludvigsson JF, Schmidt M, et al. Nordic health registry-based research: a review of health care systems and key registries. Clin Epidemiol. 2021;13:533–554. doi:10.2147/CLEP.S314959
20. Schmidt M, Pedersen L, Sorensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi:10.1007/s10654-014-9930-3
21. Statistic Denmark. Facts about immigrants and descendants in Denmark. Available from: https://www.dst.dk/da/Statistik/nyheder-analyser-publ/bagtal/2019/2019-02-18-fakta-om-indvandrere-og-efterkommere-i-danmark.
22. Healthcare Denmark. New national strategy for personalized medicine. Available from: https://www.healthcaredenmark.dk/news/new-national-strategy-for-personalized-medicine.aspx.
23. Bliddal M, Broe A, Pottegard A, Olsen J, Langhoff-Roos J. The Danish medical birth register. Eur J Epidemiol. 2018;33(1):27–36. doi:10.1007/s10654-018-0356-1
24. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi:10.2147/CLEP.S91125
25. Mors O, Perto GP, Mortensen PB. The Danish psychiatric central research register. Scand J Public Health. 2011;39(7 Suppl):54–57. doi:10.1177/1403494810395825
26. Pottegard A, Schmidt SA, Wallach-Kildemoes H, Sorensen HT, Hallas J, Schmidt M. Data resource profile: the Danish national prescription registry. Int J Epidemiol. 2016. doi:10.1093/ije/dyw213
27. Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health. 2011;39(7 Suppl):26–29. doi:10.1177/1403494811399958
28. Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7 Suppl):42–45. doi:10.1177/1403494810393562
29. Sørensen HT, Pedersen L, Jørgensen J, Ehrenstein V. Danish clinical quality databases - an important and untapped resource for clinical research. Clin Epidemiol. 2016;8:425–427. doi:10.2147/CLEP.S113265
30. Andersen JS, Olivarius Nde F, Krasnik A. The Danish national health service register. Scand J Public Health. 2011;39(7 Suppl):34–37. doi:10.1177/1403494810394718
31. Arendt JFH, Hansen AT, Ladefoged SA, Sørensen HT, Pedersen L, Adelborg K. Existing Data sources in clinical epidemiology: laboratory information system databases in Denmark. Clin Epidemiol. 2020;12:469–475. doi:10.2147/CLEP.S245060
32. Vejledning om biobanker inden for sundhedsområdet: patientrettigheder og myndighedskrav. Available from: https://www.retsinformation.dk/eli/mt/2004/83.
33. Statens Serum Institut. Danmarks Nationale biobank. Available from: https://www.danishnationalbiobank.com/.
34. CopLab. The Copenhagen primary care laboratory database. Available from: https://publichealth.ku.dk/research/databases-for-collaboration/coplab/.
35. Bio and Genome Bank Denmark. Available from: https://www.regioner.dk/rbgben.
36. Kringelbach TM, Glintborg B, Hogdall EV, Johansen JS, Hetland ML. Identification of new biomarkers to promote personalised treatment of patients with inflammatory rheumatic disease: protocol for an open cohort study. BMJ Open. 2018;8(2):e019325. doi:10.1136/bmjopen-2017-019325
37. Pedersen OB, Erikstrup C, Kotzé SR, et al. The Danish Blood donor study: a large, prospective cohort and biobank for medical research. Vox Sang. 2012;102(3):271. doi:10.1111/j.1423-0410.2011.01553.x
38. Hansen TF, Banasik K, Erikstrup C, et al. DBDS Genomic Cohort, a prospective and comprehensive resource for integrative and temporal analysis of genetic, environmental and lifestyle factors affecting health of blood donors. BMJ Open. 2019;9(6):e028401. doi:10.1136/bmjopen-2018-028401
39. Erichsen R, Lash TL, Hamilton-Dutoit SJ, Bjerregaard B, Vyberg M, Pedersen L. Existing data sources for clinical epidemiology: the Danish national pathology registry and data bank. Clin Epidemiol. 2010;2:51–56. doi:10.2147/CLEP.S9908
40. Danish National Genom Center. Available from: https://eng.ngc.dk/.
41. Nordfalk F, Ekstrøm CT. Newborn dried blood spot samples in Denmark: the hidden figures of secondary use and research participation. Eur J Hum Genet. 2019;27(2):203–210. doi:10.1038/s41431-018-0276-2
42. Pedersen CB, Bybjerg-Grauholm J, Pedersen MG, et al. The iPSYCH2012 case-cohort sample: new directions for unravelling genetic and environmental architectures of severe mental disorders. Mol Psychiatry. 2018;23(1):6–14. doi:10.1038/mp.2017.196
43. iPSYCH. Publications. Available from: https://ipsych.dk/en/research/publications/.
44. Olsen J, Melbye M, Olsen SF, et al. The Danish National Birth Cohort--its background, structure and AIM. Scand J Public Health. 2001;29(4):300–307. doi:10.1177/14034948010290040201
45. The Danish National Birth Cohort. About the DNBC. Available at: https://www.dnbc.dk/about-The-dnbc.
46. Olsen SF, Mikkelsen TB, Knudsen VK, et al. Data collected on maternal dietary exposures in the Danish national birth cohort. Paediatr Perinat Epidemiol. 2007;21(1):76–86. doi:10.1111/j.1365-3016.2007.00777.x
47. Mortensen LH, Diderichsen F, Smith GD, Andersen AM. The social gradient in birthweight at term: quantification of the mediating role of maternal smoking and body mass index. Hum Reprod. 2009;24(10):2629–2635. doi:10.1093/humrep/dep211
48. The Danish National Birth Cohort. DNBC Interviews 1–4. Available from: https://www.dnbc.dk/data-available/interviews-1---4.
49. The Danish National Birth Cohort. Food Frequency Questionnaire. Available from: https://www.dnbc.dk/data-available/food-frequency-questionnaire.
50. The Danish National Birth Cohort. 7-year follow-up. Available from: https://www.dnbc.dk/data-available/7-year-follow-up.
51. The Danish National Birth Cohort. 11 year Follow-up. Available from: https://www.dnbc.dk/data-available/11-year-follow-up.
52. The Danish National Birth Cohort. Follow-up among mothers. Avaialbale from: https://www.dnbc.dk/data-available/follow-up-among-mothers.
53. Ernst A, Brix N, Lauridsen LLB, et al. Cohort profile: the puberty cohort in the Danish National Birth Cohort (DNBC). Int J Epidemiol. 2020;49(2):373–374g. doi:10.1093/ije/dyz222
54. Keglberg Hærvig K, Bonde JP, Ramlau-Hansen CH, et al. Fetal Programming of Semen Quality (FEPOS) cohort - A DNBC male-offspring cohort. Clin Epidemiol. 2020;12:757–770. doi:10.2147/CLEP.S242631
55. The Danish National Birth Cohort. Publications. Available from: https://www.dnbc.dk/dnbc-publications.
56. Pedersen DA, Larsen LA, Nygaard M, et al. The Danish Twin registry: an updated overview. Twin Res Hum Genet. 2019;22(6):499–507. doi:10.1017/thg.2019.72
57. Data availability. Available from: https://www.sdu.dk/en/om_sdu/institutter_centre/ist_sundhedstjenesteforsk/centre/dtr/researcher/data.
58. Leboeuf-Yde C, Kyvik KO. At what age does low back pain become a common problem? A study of 29,424 individuals aged 12–41 years. Spine. 1998;23(2):228–234. doi:10.1097/00007632-199801150-00015
59. Skytthe A, Kyvik K, Bathum L, Holm N, Vaupel JW, Christensen K. The Danish Twin Registry in the new millennium. Twin Res Hum Genet. 2006;9(6):763–771. doi:10.1375/twin.9.6.763
60. McGue M, Christensen K. Social activity and healthy aging: a study of aging Danish twins. Twin Res Hum Genet. 2007;10(2):255–265. doi:10.1375/twin.10.2.255
61. Skytthe A, Christiansen L, Kyvik KO, et al. The Danish twin registry: linking surveys, national registers, and biological information. Twin Res Hum Genet. 2013;16(1):104–111. doi:10.1017/thg.2012.77
62. Mengel-From J, Rønne ME, Carlsen AL, et al. Circulating, cell-free micro-RNA profiles reflect discordant development of dementia in monozygotic twins. J Alzheimers Dis. 2018;63(2):591–601. doi:10.3233/JAD-171163
63. Debrabant B, Soerensen M, Christiansen L, et al. DNA methylation age and perceived age in elderly Danish twins. Mech Ageing Dev. 2018;169:40–44. doi:10.1016/j.mad.2017.09.004
64. Lund JB, Li S, Christensen K, et al. Age-dependent DNA methylation patterns on the Y chromosome in elderly males. Aging Cell. 2020;19(2):e12907. doi:10.1111/acel.12907
65. Starnawska A, Tan Q, McGue M, et al. Epigenome-wide association study of cognitive functioning in middle-aged monozygotic twins. Front Aging Neurosci. 2017;9:413. doi:10.3389/fnagi.2017.00413
66. Svane AM, Soerensen M, Lund J, et al. DNA methylation and all-cause mortality in middle-aged and elderly Danish twins. Genes. 2018;9:2. doi:10.3390/genes9020078
67. Tjønneland A, Olsen A, Boll K, et al. Study design, exposure variables, and socioeconomic determinants of participation in Diet, Cancer and Health: a population-based prospective cohort study of 57,053 men and women in Denmark. Scand J Public Health. 2007;35(4):432–441. doi:10.1080/14034940601047986
68. Overvad K, Tjønneland A, Haraldsdóttir J, Ewertz M, Jensen OM. Development of a semiquantitative food frequency questionnaire to assess food, energy and nutrient intake in Denmark. Int J Epidemiol. 1991;20(4):900–905. doi:10.1093/ije/20.4.900
69. Tjønneland A, Overvad K, Haraldsdóttir J, Bang S, Ewertz M, Jensen OM. Validation of a semiquantitative food frequency questionnaire developed in Denmark. Int J Epidemiol. 1991;20(4):906–912. doi:10.1093/ije/20.4.906
70. Petersen KEN, Halkjær J, Loft S, Tjønneland A, Olsen A. Cohort profile and representativeness of participants in the Diet, Cancer and Health-Next Generations cohort study. Eur J Epidemiol. 2022;37(1):117–127. doi:10.1007/s10654-021-00832-7
71. Danish Cancer Society. Diet, cancer and health. Available from: https://www.cancer.dk/research/dcrc-research/diet-cancer-and-health/.
72. Kirkegaard H, Johnsen NF, Christensen J, Frederiksen K, Overvad K, Tjønneland A. Association of adherence to lifestyle recommendations and risk of colorectal cancer: a prospective Danish cohort study. BMJ. 2010;341:c5504. doi:10.1136/bmj.c5504
73. Christensen DH, Nicolaisen SK, Berencsi K, et al. Danish Centre for Strategic Research in Type 2 Diabetes (DD2) project cohort of newly diagnosed patients with type 2 diabetes: a cohort profile. BMJ Open. 2018;8(4):e017273. doi:10.1136/bmjopen-2017-017273
74. Christensen H, Nielsen JS, Sørensen KM, Melbye M, Brandslund I. New national biobank of the Danish center for strategic research on type 2 diabetes (DD2). Clin Epidemiol. 2012;4:37–42. doi:10.2147/CLEP.S33042
75. Thomsen RW, Friborg S, Nielsen JS, Schroll H, Johnsen SP. The Danish centre for strategic research in type 2 diabetes (DD2): organization of diabetes care in Denmark and supplementary data sources for data collection among DD2 study participants. Clin Epidemiol. 2012;4(Suppl 1):15–19. doi:10.2147/CLEP.S30082
76. Gedebjerg A, Bjerre M, Kjaergaard AD, et al. Mannose-binding lectin and risk of cardiovascular events and mortality in type 2 diabetes: a Danish cohort study. Diabetes Care. 2020;43(9):2190–2198. doi:10.2337/dc20-0345
77. Petersen ER, Nielsen AA, Christensen H, et al. Vejle Diabetes Biobank - a resource for studies of the etiologies of diabetes and its comorbidities. Clin Epidemiol. 2016;8:393–413. doi:10.2147/CLEP.S113419
78. Sørensen E, Christiansen L, Wilkowski B, et al. Data resource profile: the Copenhagen Hospital Biobank (CHB). Int J Epidemiol. 2020;2020:1.
79. Helgadottir A, Thorleifsson G, Alexandersson KF, et al. Genetic variability in the absorption of dietary sterols affects the risk of coronary artery disease. Eur Heart J. 2020;41(28):2618–2628. doi:10.1093/eurheartj/ehaa531
80. The Copenhagen City Heart Study (CCHS). Available from: https://clinicaltrials.gov/ct2/show/NCT02993172.
81. The Copenhagen City Heart Study, Osterbroundersøgelsen. A book of tables with data from the first examination (1976–78) and a five year follow-up (1981–83). Scand J Soc Med Suppl. 1989;41:1–160.
82. Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339(17):1194–1200. doi:10.1056/NEJM199810223391703
83. Østerbroundersøgelsen. Available from: https://research.regionh.dk/da/organisations/oesterbroundersoegelsen(66510d7d-482a-4a82-adc0-02dceb6e86ef)/publications.html.
84. Mortensen MB, Nordestgaard BG. Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70–100 years: a contemporary primary prevention cohort. Lancet. 2020;396(10263):1644–1652. doi:10.1016/S0140-6736(20)32233-9
85. Regionernes Bio og Genombank. Available from: http://rbgb.dk/cancer/.
86. Sundhedsdatastyrelsen. Vævsanvendelsesregisteret. Available from: https://sundhedsdatastyrelsen.dk/da/registre-og-services/om-de-nationale-sundhedsregistre/testamenter-og-organdonation/vaevsanvendelsesregisteret.
87. Reinert T, Henriksen TV, Christensen E, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol. 2019;5(8):1124–1131. doi:10.1001/jamaoncol.2019.0528
88. Regionernes Bio og Genombank Reuma. Available from: http://rbgb.dk/reuma/.
89. Ibfelt EH, Jensen DV, Hetland ML. The Danish nationwide clinical register for patients with rheumatoid arthritis: DANBIO. Clin Epidemiol. 2016;8:737–742. doi:10.2147/CLEP.S99490
90. Ibfelt EH, Sørensen J, Jensen DV, et al. Validity and completeness of rheumatoid arthritis diagnoses in the nationwide DANBIO clinical register and the Danish national patient registry. Clin Epidemiol. 2017;9:627–632. doi:10.2147/CLEP.S141438
91. Saevarsdottir S, Stefansdottir L, Sulem P, et al. Multiomics analysis of rheumatoid arthritis yields sequence variants that have large effects on risk of the seropositive subset. Ann Rheum Dis. 2022;81(8):1085–1095. doi:10.1136/annrheumdis-2021-221754
92. Burgdorf KS, Felsted N, Mikkelsen S, et al. Digital questionnaire platform in the Danish blood donor study. Comput Methods Programs Biomed. 2016;135:101–104. doi:10.1016/j.cmpb.2016.07.023
93. Regionernes Bio og Genombank bloddonor. Available from: http://rbgb.dk/bloddonor/.
94. Burgdorf KS, Simonsen J, Sundby A, et al. Socio-demographic characteristics of Danish blood donors. PLoS One. 2017;12(2):e0169112. doi:10.1371/journal.pone.0169112
95. Burgdorf KS, Trabjerg BB, Pedersen MG, et al. Large-scale study of Toxoplasma and Cytomegalovirus shows an association between infection and serious psychiatric disorders. Brain Behav Immun. 2019;79:152–158. doi:10.1016/j.bbi.2019.01.026
96. Erichsen R, Baron JA, Hamilton-Dutoit SJ, et al. Increased risk of colorectal cancer development among patients with serrated polyps. Gastroenterology. 2016;150(4):895–902.e895. doi:10.1053/j.gastro.2015.11.046
97. National Committee on Health Research Ethics. Where to submit my notification? Available from: https://en.nvk.dk/how-to-notify/where-to-submit-my-notification.
98. Personalized medicine. Available from: https://www.regioner.dk/sundhed/medicin/personlig-medicin.
99. Gambardella V, Tarazona N, Cejalvo JM, et al. Personalized medicine: recent progress in cancer therapy. Cancers. 2020;12:4. doi:10.3390/cancers12041009
100. Christensen DH, Nicolaisen SK, Ahlqvist E, et al. Type 2 diabetes classification: a data-driven cluster study of the Danish centre for strategic research in type 2 diabetes (DD2) cohort. BMJ Open Diabetes Res Care. 2022;10:2. doi:10.1136/bmjdrc-2021-002731
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.