Back to Journals » Cancer Management and Research » Volume 12
A Retrospective Study of Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer
Authors Wang Y, He K , Zhou Z, Zhong Y, Li G, Lu J
Received 22 June 2020
Accepted for publication 21 August 2020
Published 15 September 2020 Volume 2020:12 Pages 8491—8496
DOI https://doi.org/10.2147/CMAR.S267330
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Yajing Wang,* Kang He,* Zhaofei Zhou, Yuejiao Zhong, Gang Li, Jianwei Lu
The Affiliated Cancer Hospital of Nanjing Medical University and Jiangsu Cancer Hospital and Jiangsu Institute of Cancer Research, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianwei Lu
The Affiliated Cancer Hospital of Nanjing Medical University and Jiangsu Cancer Hospital and Jiangsu Institute of Cancer Research, Nanjing, People’s Republic of China
Email [email protected]
Objective: To explore the efficacy and safety of neoadjuvant chemotherapy in the doublet and triplet regimens of locally advanced gastric cancer.
Patients and Methods: A retrospective analysis was conducted on 162 patients with gastric cancer who received neoadjuvant chemotherapy, including 74 patients receiving doublet regimen (fluorouracil/platinum) and 88 patients receiving triplet regimen (fluorouracil/platinum/Taxol). Patients in both groups received neoadjuvant chemotherapy for two cycles, and underwent surgical resection 4 weeks after the end of chemotherapy.
Results: The total clinical remission rate was 68.6% (105/153), the phase-down rate was 46.4% (71/153), and the pathological response rate was 59.9% (97/162). In the doublet and triplet regimen, the clinical remission rate was 65.7% (44/67) and 70.9% (61/86) (P = 0.708), the descending period rate was 41.8% (28/67) and 50.0% (43/86) (P = 0.485), and the pathological response rate was 51.4% (38/74) and 67.0% (59/88) (P = 0.190). The median disease-free survival (DFS) and overall survival (OS) of 162 patients were 36.0 and 58.5 months. In the doublet and triplet regimen, the median DFS was 38.0 and 34.0 months (P = 0.377), and the median OS was 59.0 and 56.5 months (P = 0.256). The side effects of the doublet group were significantly lower than those of the triplet group, with leucopenia rate of 45.9% (34/74) and 62.5% (55/88) (P = 0.035); thrombocytopenia rate of 18.9% (14/74) and 35.2% (31/88) (P = 0.021); nausea rate of 45.9% (34/74) and 64.8% (57/88) (P = 0.016), and diarrhea rate of 1.4% (1/74) and 9.1% (8/88) (P = 0.032).
Conclusion: Neoadjuvant chemotherapy is safe and effective for locally advanced gastric cancer. The clinical efficacy of neoadjuvant chemotherapy in the doublet group and the triplet group is equivalent, and the doublet group has better safety and tolerance.
Keywords: locally advanced gastric cancer, neoadjuvant chemotherapy, doublet regimen, triplet regimen, prognosis
Introduction
In 2018, there were 1,033,701 new gastric cancer cases and 782,685 deaths.1 Gastric cancer is one of the cancers with the highest incidence of digestive tract cancer in China. It lacks typical clinical symptoms at the early stage. Nearly two thirds of the patients were diagnosed as in the advanced stage.2 Even with surgery and S-1 combined chemotherapy, the prognosis of locally advanced gastric cancer is still unsatisfactory.3,4 At present, the standard treatment of local advanced gastric cancer is preoperative chemotherapy, surgery, adjuvant chemotherapy and postoperative radiotherapy after surgery.5–7 Complete resection is the key to the treatment of local gastric cancer.8 Preoperative neoadjuvant chemotherapy can kill cancer cells, reduce tumor volume, and create conditions for radical surgical resection.9,10 Neoadjuvant chemotherapy is an effective method to improve the survival rate of resectable gastric cancer. Compared with postoperative chemotherapy, neoadjuvant chemotherapy which can expand surgery has certain theoretical significance.11 Doublet and triplet regimens are promising regimens of neoadjuvant chemotherapy.12–14 Taxol is one of the key drugs for locally advanced gastric cancer. On the basis of these previous studies, it is still controversial as to which of doublet and triplet regimens of neoadjuvant chemotherapy is better. In this study, we retrospectively compared the clinical efficacy, safety and prognosis analysis of locally advanced gastric cancer with doublet and triplet regimens.
Patients and Methods
Specimens Collection
One hundred and sixty-two gastric cancer patients who received preoperative neoadjuvant chemotherapy in Jiangsu cancer hospital from January 2011 to December 2013 were collected. The clinical baseline data are shown in Table 1. The median age was 56.0 years old in 119 males and 43 females. All cases were confirmed as gastric adenocarcinoma by histopathology. After neoadjuvant chemotherapy and preoperative staging performed according to the 8th AJCC stage by CT, B-ultrasound, GI and other imaging examinations, all cases were locally advanced gastric cancer (T3-4aN0-3). All trial participants signed the informed consent agreement before participating in the study. The clinical trial was approved by the clinical research ethics committee of the Jiangsu Cancer Hospital and was conducted in accordance with the Declaration of Helsinki.
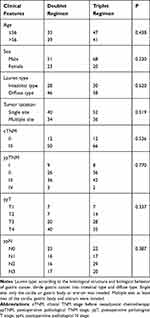 |
Table 1 Characteristics of Patients Between Doublet and Triplet Regimens |
Chemotherapy Regimen
All 162 patients received neoadjuvant chemotherapy. Among them, 74 patients received the doublet regimen with 34 patients receiving tegafur combined oxaliplatin and 40 patients receiving capecitabine combined oxaliplatin; 88 patients received the triplet regimen with 34 patients receiving tegafur and oxaliplatin in combination with docetaxel and 54 patients receiving capecitabine and oxaliplatin in combination with docetaxel.
Efficacy Evaluation
Before and after neoadjuvant chemotherapy, RECIST 1.1 was used to evaluate the efficacy as follows: complete response (CR): all tumor lesions disappeared and remained for 4 weeks; partial response (PR): it was used to reduce the sum of target lesion diameter by at least 30% compared with the baseline level; disease progression (PD): the patient has a new lesion or a lesion with larger diameter after treatment; stable disease (SD): it was between PR and PD.
The Evaluation of the Pathological Response
After radical gastrectomy, according to the relationship between the degree of tumor necrosis or disappearance and the total number of tumors, the chemotherapy response was divided into 0–3 grades. In the case of no degeneration, the surgical specimens were evaluated as grade 0; in the case of intratumoral necrosis, when the degeneration area was less than one third of the tumor, as grade 1a; when the degeneration area was more than one-third and less than two-thirds, as grade 1b; when the degeneration area was more than two-thirds but less than 90%, as grade 2a; when the degenerative area was more than 90% but less than 100%, as grade 2b; in the case of grade 3, there was no residual tumor.
Statistical Analysis
All data were analyzed and processed by SPSS 24.0 software. t-test was used for comparison of measurement data, chi-square test was used for counting data, and Kaplan Meier method was used for survival analysis to draw survival curve. All p values were two-sided. Difference was considered statistically significant (P < 0.05).
Results
Clinical Efficacy
Among the 162 patients with neoadjuvant chemotherapy, 7 cases in the doublet regimen group and 2 cases in the triplet regimen group did not receive CT examination in Jiangsu cancer hospital before chemotherapy, so they were not included in the clinical efficacy evaluation. Among the rest 153 patients, 105 (68.6%), 34 (22.2%) and 14 (9.2%) cases received neoadjuvant chemotherapy, SD and PD, respectively. Among 67 cases in doublet regimen group, 44, 17 and 6 cases, respectively, received PR (65.7%), SD (25.4%) and PD (9.0%) with an objective remission rate of 65.7% and disease control rate of 91.0%. Among 86 cases in triplet regimen group, 61, 17 and 8 cases, respectively, received PR (70.9%), SD (19.8%) and PD (9.3%) with an objective remission rate of 70.9% and disease control rate of 90.7%. There was no statistical difference in objective remission rate and disease control rate between the two groups (P = 0.708).
Descending Period Rate
Compared with the clinical stage before neoadjuvant chemotherapy and the pathological stage after operation, 71 cases (46.4%) got the descending stage. There were 28 patients (41.8%) and 43 patients (50.0%) in the doublet and triplet groups, respectively. There was no statistical difference (P = 0.485).
Pathological Response
According to postoperative histopathology, of all the patients, 35 patients (21.6%) were defined as grade 1, 43 patients (26.5%) grade 2 and 19 patients (11.7%) grade 3. In the doublet group, 13 cases (17.6%) were defined as grade 1, 16 cases (21.6%) grade 2 and 9 cases (12.2%) grade 3. In the triplet group, there were 22 cases (25.0%) defined as grade 1, 27 cases (30.7%) grade 2 and 10 cases (11.4%) grade 3. There was no significant difference between the two groups (P = 0.190).
Survival Analysis
All patients completed the operation. Postoperative follow-up time was until October 31, 2018. Of all patients, 89 cases died (54.9%). The median DFS of all patients was 36.0 months. Of all the patients, the 3-year DFS rate was 50.6%, and the 5-year DFS rate was 42.0%. The median DFS of doublet and triplet groups was 38.0 and 34.0 months (P = 0.377), the 3-year DFS rate 54.1% and 47.7% (P = 0.422) and the 5-year DFS rate 45.9% and 38.6% (P = 0.348), respectively. The median OS of all patients was 58.5 months. Of all the patients, the 3-year OS rate was 62.3%, and the 5-year OS rate was 49.4%. The median OS of doublet and triplet groups was 59.0 and 56.5 months (P = 0.256), the 3-year OS rate 66.2% and 59.1% (P = 0.351) and the 5-year OS rate 50.0% and 48.9% (P = 0.885), respectively. Figure 1 shows the DFS of the two groups. Figure 2 shows the OS of the two groups.
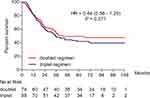 |
Figure 1 Kaplan–Meier estimate of the DFS between doublet and triplet regimens. |
 |
Figure 2 Kaplan–Meier estimate of OS between doublet and triplet regimens. |
Chemotherapy-Related Toxicities
Table 2 shows the details of neoadjuvant chemotherapy-related toxicities. Leucopenia rate was 45.9% in the doublet group and 62.5% in the triplet group (P = 0.035), thrombocytopenia rate was 18.9% in the doublet group and 35.2% in the triplet group (P = 0.021), nausea rate was 45.9% in the doublet group and 64.8% in the triplet group (P = 0.016), and diarrhea rate was 1.4% in the doublet group and 9.1% in the triplet group (P = 0.032). The toxicity effects in the doublet group were significantly lower than those in the triplet group.
 |
Table 2 Neoadjuvant Chemotherapy-Related Toxicities Between Doublet and Triplet Regimens |
Analysis of Prognostic Factors
The results of Cox univariate analysi showed that, cTNM (P = 0.026), ypTNM (P = 0.008), descending period rate (P < 0.001), clinical effect (P < 0.001), CA199 (P < 0.001), histological type (P = 0.048), nerve invasion (P = 0.024), T stage (P = 0.003), N stage (P = 0.043) and pathological response (P = 0.018) are the factors that affect the prognosis of gastric cancer patients (see Table 3). The results of Cox multivariate analysis showed that the descending period rate (P < 0.001) and CA199 (P = 0.038) are independent prognostic factors.
 |
Table 3 Multivariate Analysis of 162 Patients of Gastric Cancer with Neoadjuvant Chemotherapy |
Discussion
In recent years, neoadjuvant chemotherapy for gastric cancer has made remarkable progress and has become one of the comprehensive treatment methods for gastric cancer. The results of MAGIC study showed that the 5-year OS rate of neoadjuvant chemotherapy group (36.3%) was significantly higher than that of the operation alone group (23.0%).7 Similar results were obtained in FNCLCCACCORD07-FFCD9703. Compared with the operation alone group, the neoadjuvant chemotherapy group had a higher 5-year progression-free survival (PFS) rate (34.0% and 19.0%) and OS rate (38.0% and 24.0%).15 The results of JCOG0405 study in Asia show that neoadjuvant chemotherapy can improve the 3-year OS rate (59.0%) and 5-year OS rate (53.0%).16 Ma et al confirmed that neoadjuvant chemotherapy has good efficacy and safety in the treatment of advanced gastric cancer.17 A meta-analysis showed that neoadjuvant chemotherapy combined with surgery significantly reduced mortality in patients with gastric cancer.18 In this study, the PR rate of neoadjuvant chemotherapy was 68.6%, the DFS rates of three and 5 years were 50.6% and 40.2% and the OS rates of three and 5 years were 62.3% and 49.4%, respectively. The results were similar to those reported in the literature.
Neoadjuvant chemotherapy mainly refers to the experience of postoperative and advanced gastric cancer chemotherapy, and there is no unified standard. Doublet and triplet regimens are commonly used neoadjuvant chemotherapy in patients with locally advanced gastric cancer.19–22 The two chemotherapy regimens are both effective in gastric cancer treatment, but which of the two regimens is better is still controversial. In this study, younger men with earlier clinical stage tended to use the triplet regimen while older women with later clinical stage tended to use the doublet regimen. There are also many studies comparing the two regimens, but the results are inconsistent. The clinical study of JACCROGC-01 in Japan confirmed that cisplatin combined with tegio (CS) is safe, feasible and effective in neoadjuvant chemotherapy of locally advanced gastric cancer.23 Wang et al found that in a retrospective study, there was no significant difference in clinical efficacy between the triplet regimen of DOS and the doublet regimen of XELOX, but the median PFS and OS of DOS were significantly better than those of XELOX.24 A Phase II randomized clinical study showed that the pathological response and R0 resection rate of DCS were not better than CS, and the incidence of hematological toxicity was higher.25 Al batran et al reported that in triplet regimen, the incidence of neutropenia and leukopenia in grade III or IV was 52.0% and 28.0%, respectively, higher than that in doublet regimen.26 Lorenzen et al think that although the triplet regimen containing docetaxel can increase the efficacy, the side effects are more obvious, and its efficacy advantage will be offset by the side effects of chemotherapy.27 The results of this study showed that there was no difference in clinical remission rate, descending period rate, pathological response rate, DFS and OS between the doublet regimen and the triplet regimen, but the incidence of toxic effects of the doublet regimen was significantly lower than that of the triplet regimen.
To sum up, neoadjuvant chemotherapy can be used as one of the important means of comprehensive treatment of local advanced gastric cancer. It is still controversial to use neoadjuvant chemotherapy with doublet or triplet regimen, which needs to be further verified by prospective large sample randomized controlled study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Freddie Bray B, Jacques FM, Isabelle SM, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68.
2. Nguyen HV, Nguyen HV, Nguyen LT, et al. A case of advanced gastric cancer with folfiri as a preoperative chemotherapy. Case Rep Oncol Med. 2019;1352173:2019.
3. Yoshikawa T, Sasako M. Gastrointestinal cancer: adjuvant chemotherapy after D2 gastrectomy for gastric cancer. Nat Rev Clin Oncol. 2012;9:192–194. doi:10.1038/nrclinonc.2012.23
4. Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14:97–100. doi:10.1007/s10120-011-0040-6
5. Shinichi Sakuramoto MD, Mitsuru Sasako MD, Toshiharu Yamaguchi MD, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi:10.1056/NEJMoa072252
6. Bang Y-J, Kim Y-W, Yang H-K, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a Phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi:10.1016/S0140-6736(11)61873-4
7. David C, Allum WH, Sally P, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi:10.1056/NEJMoa055531
8. Sasako M. Principles of surgical treatment for curable gastric cancer. J Clin Oncol. 2003;21:274s275s. doi:10.1200/JCO.2003.09.172
9. Fan G-F, Pan -J-J, Fan S, et al. The clinical observation of verapamil in combination with interventional chemotherapy in advanced gastric cancer. Eur Rev Med Pharmacol Sci. 2018;2018:534–543.
10. Newton AD, Datta J, Loaiza-Bonilla A, et al. Neoadjuvant therapy for gastric cancer: current evidence and future directions. J Gastrointest Oncol. 2015;6:534–543. doi:10.3978/j.issn.2078-6891.2015.047
11. Ott K, Lordick F, Herrmann K, et al. The new credo: induction chemotherapy in locally advanced gastric cancer: consequences for surgical strategies. Gastric Cancer. 2008;11:1–9. doi:10.1007/s10120-007-0448-1
12. Katayama H, Tsuburaya A, Mizusawa J, et al. An integrated analysis of two phase II trials (JCOG0001 and JCOG0405) of preoperative chemotherapy followed by D3 gastrectomy for gastric cancer with extensive lymph node metastasis. Gastric Cancer. 2019;22:1301–1307. doi:10.1007/s10120-019-00981-5
13. Yoshikawa T, Tanabe K, Nishikawa K, et al. Induction of a pathological complete response by four courses of neoadjuvant chemotherapy for gastric cancer: early results of the randomized phase II COMPASS trial. Ann Surg Oncol. 2014;21:213–219. doi:10.1245/s10434-013-3055-x
14. Yoshikawa T, Taguri M, Sakuramoto S, et al. A comparison of multimodality treatment: two and four courses of neoadjuvant chemotherapy using S-1/CDDP or S-1/CDDP/docetaxel followed by surgery and S-1 adjuvant chemotherapy for macroscopically resectable serosa-positive gastric cancer: a randomized phase II trial (COMPASS-D trial). Jpn J Clin Oncol. 2012;42:74–77. doi:10.1093/jjco/hyr166
15. Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter Phase III trial. J Clin Oncol. 2011;29:1715–1721. doi:10.1200/JCO.2010.33.0597
16. Tsuburaya A, Mizusawa J, Tanaka Y, et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101:653–660. doi:10.1002/bjs.9484
17. Ma F, Wang B, Xue L, et al. Neoadjuvant chemotherapy improves the survival of patients with neuroendocrine carcinoma and mixed adenoneuroendocrine carcinoma of the stomach. J Cancer Res Clin Oncol. 2020;2020.
18. Coccolini F, Nardi M, Montori G, et al. Neoadjuvant chemotherapy in advanced gastric and esophago-gastric cancer. Meta-analysis of randomized trials. Int J Surg. 2018;51:120–127. doi:10.1016/j.ijsu.2018.01.008
19. Sun G, Wang S, Liu G. Preoperative neoadjuvant chemotherapy on surgical condition and oncogene expression in advanced gastric cancer. Pak J Med Sci. 2020;36:485–489. doi:10.12669/pjms.36.3.1608
20. Al-Batran S-E, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, Phase 2/3 trial. Lancet. 2019;393:1948–1957.
21. Feng D, Leong M, Li T, et al. Surgical outcomes in patients with locally advanced gastric cancer treated with S-1 and oxaliplatin as neoadjuvant chemotherapy. World J Surg Oncol. 2015;13:11. doi:10.1186/s12957-015-0444-6
22. Migita K, Nashimoto A, Yabusaki H, et al. Efficacy of neoadjuvant chemotherapy with docetaxel, cisplatin and S-1 for resectable locally advanced gastric cancer. Int J Clin Oncol. 2015;21:102–109. doi:10.1007/s10147-015-0851-2
23. Yoshikawa T, Omura K, Kobayashi O, et al. A phase II study of preoperative chemotherapy with S-1 plus cisplatin followed by D2/D3 gastrectomy for clinically serosa-positive gastric cancer (JACCRO GC-01 study). Eur J Surg Oncol. 2010;36:546–551. doi:10.1016/j.ejso.2010.04.011
24. Wang Y, Cheng X, Cui Y-H, et al. Efficacy after preoperative capecitabine and oxaliplatin (XELOX) versus docetaxel, oxaliplatin and S1 (DOS) in patients with locally advanced gastric adenocarcinoma: a propensity score matching analysis. BMC Cancer. 2018;18. doi:10.1186/s12885-018-4615-z
25. Aoyama T, Nishikawa K, Fujitani K, et al. Early results of a randomized two-by-two factorial phase II trial comparing neoadjuvant chemotherapy with two and four courses of cisplatin/S-1 and docetaxel/cisplatin/S-1 as neoadjuvant chemotherapy for locally advanced gastric cancer. Ann Oncol. 2017;28:1876–1881. doi:10.1093/annonc/mdx236
26. Al-Batran S-E, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17:1697–1708. doi:10.1016/S1470-2045(16)30531-9
27. Lorenzen S, Pauligk C, Homann N, et al. Feasibility of perioperative chemotherapy with infusional 5-FU, leucovorin, and oxaliplatin with (FLOT) or without (FLO) docetaxel in elderly patients with locally advanced esophagogastric cancer. Br J Cancer. 2013;108:519–526. doi:10.1038/bjc.2012.588
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
