Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
A Retrospective Analysis of Conversion Therapy with Lenvatinib, Sintilimab, and Arterially-Directed Therapy in Patients with Initially Unresectable Hepatocellular Carcinoma
Authors Gan L , Lang M, Tian X, Ren S , Li G, Liu Y, Han R , Zhu K, Li H, Wu Q, Cui Y, Zhang W, Fang F, Li Q, Song T
Received 25 January 2023
Accepted for publication 30 March 2023
Published 22 April 2023 Volume 2023:10 Pages 673—686
DOI https://doi.org/10.2147/JHC.S404675
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mohamed Shaker
Leijuan Gan,1– 3,* Mengran Lang,1– 4,* Xindi Tian,1– 3 Shaohua Ren,1– 3 Guangtao Li,1– 3 Yayue Liu,1– 3 Ruyu Han,1– 3 Kangwei Zhu,1– 3 Huikai Li,1– 3 Qiang Wu,1– 3 Yunlong Cui,1– 3 Wei Zhang,1– 3 Feng Fang,1– 3 Qiang Li,1– 3 Tianqiang Song1– 3
1Department of Hepatobiliary Cancer, Liver Cancer Center, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin, 300060, People’s Republic of China; 2Key Laboratory of Cancer Prevention and Therapy, Tianjin, People’s Republic of China; 3Tianjin’s Clinical Research Center for Cancer, Tianjin, People’s Republic of China; 4Department of Hepatobiliary Surgery, National Cancer Center/National Clinical Research Center for Cancer/Hebei Cancer Hospital, Chinese Academy of Medical Sciences, Langfang, Hebei, 065001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tianqiang Song, Tel +86-022-23340123, Fax +86 022-23537796, Email [email protected]
Purpose: The purpose of this study was to investigate the triple-combination therapy of lenvatinib plus sintilimab plus arterially-directed therapy as a conversion therapy for initially unresectable hepatocellular carcinoma (HCC).
Patients and Methods: We retrospectively analyzed data from all HCC patients who underwent lenvatinib plus sintilimab plus arterially-directed therapy at Tianjin Medical University Cancer Hospital between December 2018 and October 2020. Of 98 enrolled patients, 37 patients were classified as potentially resectable. We compared the potentially resectable population (PRP) with the non-potentially resectable population (NPRP). The primary study endpoint was conversion rate, and secondary endpoints included progression-free survival (PFS), overall survival (OS), objective response rate (ORR), disease control rate (DCR), and safety.
Results: The baseline characteristics were comparable between populations except for a higher proportion of patients with extrahepatic metastases in the NPRP versus PRP (23/61 [37.7%] vs 3/37 [8.1%], respectively; p=0.003). For PRP, the ORR was 67.6% based on RECIST v1.1 (75.7% based on mRECIST), conversion rate was 40.5% (15/37). Of the 15 patients who underwent surgical resection, three achieved complete pathological remission. The median follow-up for all patients was 28 months (range: 2– 47). For NPRP, the ORR was 22.9% based on RECIST v1.1 (31.1% based on mRECIST), The median PFS for PRP was significantly longer than that of NPRP (25 vs 13 months, p = 0.0025). The median OS for PRP was significantly longer than that of NPRP (not reached VS 21 months, p=0.014). Hypertension was the most common grade ≥ 3 adverse reaction in both PRP and NPRP. No new safety signals were observed for any of the treatments.
Conclusion: The triple-combination therapy of lenvatinib plus sintilimab plus arterially-directed therapy can convert potentially unresectable HCC into resectable disease and improve long-term survival.
Keywords: lenvatinib, sintilimab, conversion therapy, unresectable hepatocellular carcinoma, TACE, HAIC
Introduction
Hepatocellular carcinoma (HCC) is the fifth-most-common malignant cancer and the second leading cause of cancer-related mortality worldwide.1 HCC is a major public health problem in China, where the number of newly diagnosed cases of HCC each year accounts for approximately 47% of the worldwide total.2 Patients with early-stage HCC can often undergo radical treatments such as liver resection, liver transplantation, or radiofrequency ablation.3 However, because the early symptoms of HCC are very unspecific, over 60% of patients in China are diagnosed at intermediate and advanced stages, at which time the opportunity for surgical treatment is already lost.4,5 In addition, hepatitis B virus infection is the cause of the majority of liver cancer cases in China. Such patients often have hepatic insufficiency due to cirrhosis, resulting in less than 30% resection rate.6
The rapid development of immunotherapy and targeted therapy in recent years has greatly improved the prognosis of patients with HCC.7 A 2018 Phase III trial showed that lenvatinib monotherapy improved the objective response rate (ORR) and progression-free survival (PFS) for patients with advanced HCC compared to the previous standard of care, sorafenib.8 Lenvatinib is an oral tyrosine kinase inhibitor (TKI) of vascular endothelial growth factor receptor (VEGFR) 1–3, fibroblast growth factor receptor (FGFR) 1–4, and platelet-derived growth factor receptor (PDGFR) α,9,10 and inhibits tumor angiogenesis and cancer cell growth. In addition, the combination treatment of TKIs with programmed death-1 (PD-1) inhibitors shows synergistic effect that not only modulated the immune microenvironment but also promotes normal function of immunocompetent cells.11,12 TKI and PD-1 inhibitor therapy has also been shown to reprogram the immunosuppressive HCC microenvironment into an immunostimulatory microenvironment.13 Clinically, lenvatinib combined with PD-1 inhibitors results in high ORRs in patients with advanced HCC; Lenvatinib plus pembrolizumab or nivolumab yielded ORRs of 36.0 and 54.3%, respectively, with a PD rate of <10%.14,15 Sintilimab is also a selective anti–PD-1 antibody that specially inhibits interactions between PD-1 and programmed death ligand 1, inhibiting growth of tumors.16
Patients with advanced HCC were routinely treated with arterially-directed therapy such as transcatheter arterial chemoembolization (TACE) or hepatic arterial infusion chemotherapy (HAIC).17 However, the clinical benefit of arterially-directed therapy alone remains limited. Furthermore, TACE and HAIC can cause hypoxia in tumor tissue leading to upregulated expression of hypoxia-inducible factor 1-α and local increases in VEGF, which leads to tumor progression and metastasis.18 While lenvatinib inhibits VEGF, PD-1 inhibitors can also restore the normal function of tumor blood vessels and relieve hypoxia.7 Therefore, the combination of TKIs and PD-1 inhibitors during arterially-directed therapy has synergistic anti-tumor effects. In this regard, evidence suggests the clinical efficacy of arterially-directed therapy can be improved through combination regimens such as lenvatinib with TACE or HAIC or lenvatinib with PD-1 inhibitors and HAIC, with ORRs reported to be 53.1–68.3%,19,20 66.7%,21 and 67.6%.22
Hence, we conducted a study to investigate the effectiveness and safety of triple combination consisting of lenvatinib with sintilimab plus arterially-directed therapy as conversion therapy for patients with initially unresectable intermediate-stage and advanced HCC patients, and explored the clinical outcomes.
Materials and Methods
Study Design and Patients
This is a single-center retrospective study of patients with unresectable intermediate-stage and advanced HCC who received triple combination therapy at Tianjin Medical University Cancer Institute and Hospital between December 2018 and October 2020. The study was conducted in accordance with the ethical standards of the Institutional Review Board of Tianjin Medical University Cancer Institute and Hospital (Ethics approval number: E20210172A) and the recently revised Declaration of Helsinki. Informed consent was obtained from all patients before inclusion.
The inclusion criteria were: 1) age ≥ 18 years; 2) diagnosis of HCC; 3) received treatment of
lenvatinib plus sintilimab plus interventional therapy; 4) Child-Pugh A or B; 5) Barcelona Clinic Liver Cancer (BCLC) stage B or C; 6) Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0–2; 7) measurable target lesions; 8) future liver remnant (FLR) < 40% in patients with cirrhosis, FLR < 30% in patients without cirrhosis; 9) R0 resection is technically difficult; 10) concomitant main portal vein tumor thrombosis or inferior vena cava tumor thrombosis;. Patients who underwent surgical resection were diagnosed with HCC by postoperative pathology, and patients who had not undergone surgery were diagnosed with HCC by imaging data and laboratory tests. All patients were diagnosed with HCC in accordance with the criteria of the European Association for the Study of the Liver23 or the American Association for the Study of Liver Diseases.24 Exclusion criteria included: 1) secondary liver cancer; 2) taking systemic therapy before the triple-combination therapy; 3) Child-Pugh grade C; 4) active hemorrhage; 5) incomplete clinical data. Unresectable HCC can be broadly divided into two categories: surgically unresectable and oncologically unresectable The definition of surgically unresectable is clear, of which including unable to withstand surgical trauma, liver intolerance and insufficient FLR. Oncologically unresectable is defined by technically resectable but with no better expected outcome than non-surgical treatment. In our study, patients meet only one of these conditions were defined as potentially resectable patients (PRP), and patients meet both two conditions were defined as non-potentially resectable patients (NPRP). After triple-combination therapy, conversion surgery will be performed when meet all the following conditions: 1) Patients achieved CR or PR; 2) R0 resection can be performed; 3) FLR>40% in patients with cirrhosis and FLR>30% in patients without cirrhosis after resection; 4) No unresectable extrahepatic lesions and other surgical contraindications; 5) The vascular emboli recede into branches; 6) Child-Pugh A; 7) ECOG PS 0–1.
Procedures
Patients received treatment with lenvatinib (Levima®, Eisai, Tokyo, Japan; 8 mg/d) plus sintilimab (200 mg/3 weeks) on the first day of the treatment cycle plus arterially-directed therapy (TACE or HAIC). Patients underwent TACE once every 4 weeks or HAIC once every 3 weeks. For TACE, after local anesthesia with 5 mL 2% lidocaine, the right femoral artery was punctured and a catheter sheath was introduced. An RH catheter was introduced through the sheath and selective angiography of the common hepatic artery was performed with the guidance of a super-smooth guidewire. Subsequently, a microcatheter was introduced through the RH catheter and super-selectively inserted into the arterial branch supplying the tumor, and embolization was performed by injecting 0.3 g of 300–500 µm microspheres. Approximately 200 mL of 300 mg carboplatin or lobaplatin diluted solution was slowly injected into the RH catheter, and the catheter and sheath were withdrawn. For HAIC after induction of local anesthesia in the operating room, the right femoral artery was punctured, a catheter sheath was introduced, a 5F RH catheter was introduced, and selective angiography of the celiac artery was performed. A microcatheter was then inserted into the target artery and securely fixed in place, and the patient was returned to the ward for infusion of FOLFOX (oxaliplatin, 130 mg/m2; leucovorin, 400 mg/m2; fluorouracil, 400 mg/m2; and 2400 mg/m2 over 46 hours on days 1 and 2). Some patients received TACE and HAIC alternatively depending on their condition. The physicians who performed the interventional procedures had over 10 years of clinical and surgical experience. If the primary lesion was still active or a new lesion was found on re-examination, interventional therapy was repeated on demand.
Evaluation of the Therapeutic Response and Follow-Up
The primary endpoint of the study was conversion rate (the proportion of patients converted from unresectable disease to resectable disease), and secondary endpoints were PFS (the time from the beginning of the treatment to the time of progression or all-cause death), OS (the time from the beginning of the treatment to the time of all-cause death), ORR, DCR, and safety. Tumor responses were evaluated using the Response Evaluation Criteria in Solid Tumors Version 1.1 (RECIST v.1.1)25 and the modified RECIST (mRECIST).26
Patients underwent enhanced CT or DCE-MRI (dynamic-contrast enhanced magnetic resonance imaging) scan every 2–3 circles of treatment to assess the current treatment response. After treatment, liver and kidney function, complete blood count, tumor biomarker, and coagulation tests were performed every month until death or suspension of the study.
Safety Evaluation
Adverse events (AEs) and serious adverse events (SAEs) were monitored and recorded. The category and grade of AEs was assessed using the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE 4.0).27 For patients with any grade ≥3 SAE or unacceptable drug-related AEs of grade 2 or above, the drug was reduced in dose or discontinued until the adverse reaction was resolved or reduced to under grade 2.
Statistical Analysis
Statistical Package for Social Sciences version 23.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Between group differences with a p-value < 0.05 were considered statistically significant. Continuous variables are expressed as mean ± standard deviation or median and quartiles. Normality was assumed for evaluation. For normally distributed continuous data, the t-test for two independent samples was used to evaluate difference between groups, otherwise, the Wilcoxon rank sum test was used. In other cases, the chi-squared test or Fisher’s exact test was used for comparison between groups. The Kaplan-Meier method was used to calculate PFS and OS, and survival curves were compared using the log-rank method.
Results
Patients
98 patients with advanced HCC who received lenvatinib-based triple combination therapy from December 2018 to October 2020 were included in the study. Of them, 37 patients met the criteria for potentially resectable disease (Figure 1). Potentially resectable population (PRP, n=37) and non-potentially resectable population (NPRP, n=61) differed significantly in terms of extrahepatic distant metastases and prior treatment history, with no significant differences in other baseline characteristics (Table 1).
 |
Table 1 Clinical Characteristics of the Patients |
Survival and Disease Progression
The median follow-up time for all patients was 28 months (range: 2–47 months). The median PFS for PRP was significantly longer than that of NPRP (25 VS 13 months, p = 0.0025) (Figure 2A). The median OS for PRP was not reached, but also significantly longer than that of NPRP (not reached VS 21 months, p=0.014) (Figure 2B).
Tumor Response and Conversion Resection
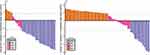
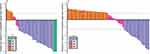
The best tumor responses were listed in Table 2. Waterfall plots were also created to show the size change of intrahepatic target lesion (Figures 3 and 4). For PRP, the ORR was 67.6% based on RECIST v1.1 (Figure 3A) and 75.7% based on mRECIST (Figure 4A), the DCR was 86.5% based on RECIST v1.1 or mRECIST. A total of 15 patients were successfully converted to resectable disease, the conversion rate was 40.5%. For NPRP, the ORR was 22.9% based on RECIST v1.1 (Figure 3B) and 31.1% based on mRECIST (Figure 4B), the DCR was 49.2% based on RECIST v1.1 (50.8% based on mRECIST). No patient in NPRP was successfully converted.
 |
Table 2 Summary of the Best Responses |
Of the 15 successfully converted patients, 13 underwent surgical resection and 2 underwent radiofrequency ablation. The median time to conversion was 4 months (range: 2–15 months). Postoperative pathological assessment indicated three patients with complete remission (pCR). The baseline characteristics of patients who achieved conversion were further analyzed (Supplementary Table 1), and it was found that patients with Child-Pugh class A (good liver function), ECOG PS 0 (good physical condition), no extrahepatic metastases, tumor diameter < 10 cm, and number of tumors < 3 were more likely to be converted successfully.
Postoperative Follow-Up
The median postoperative follow-up time was 15 months, and the 12-month DFS was 86.7%. Among the 15 patients who achieved successful conversion, two of these patients died due to tumor recurrence. Thirteen patients were still alive and cancer-free at the time of this analysis (November 2022). Most of the patients continued treatment with lenvatinib plus a PD-1 inhibitor for 6 months to 1 year after surgery.
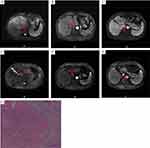
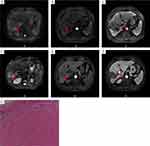
Figures 5 and 6 showed DCE-MRI of two patients who undergoing surgical excision The first patient (Figure 5) was diagnosed with BCLC stage C HCC with tumor thrombosis in the main portal vein and both left and right branches (Figure 5A–C) and treated with lenvatinib plus sintilimab plus HAIC. After three cycles of treatment, MRI images showed the tumor was significantly shrunken, the left lobe of the liver was atrophied, the left portal vein tumor thrombosis was shrunken, and the tumor thrombosis of the main portal vein and right portal vein were disappeared (Figure 5D–F), followed by left hemihepatectomy and cholecystectomy. Pathological examination indicated total necrosis in the mass and vascular tumor thrombi (pCR) (Figure 5G). After the operation, the patient continued to receive lenvatinib 8 mg/d. The second patient (Figure 6) was diagnosed with BCLC stage C HCC with concomitant portal vein tumor thrombosis (Figure 6A–C), after three cycles of lenvatinib, sintilimab and TACE treatment, MRI images showed the tumor and the cancer embolus of right portal vein was shrunken, the main portal vein thrombus was disappeared (Figure 6D–F), followed by right hemihepatectomy and cholecystectomy. Postoperative pathological examination indicated no viable tumor cells in the mass and vascular tumor thrombi (pCR) (Figure 6G). The patient did not undergo postoperative adjuvant treatment. After 18 months’ follow-up the patient was still alive and cancer-free.
Safety
In the potentially resectable population, 89.2% of patients experienced grade ≥ 1 AEs, and 29.7% experienced grade ≥ 3 AEs, of which the most common was hypertension (n = 7 [18.9%]) (Table 3). In the non-potentially resectable population, 89.7% of patients experienced grade ≥ 1 AEs, and 30.6% experienced grade ≥ 3 AEs, of which the most common was hypertension (n = 11 [18.0%]). No new safety signals were observed for any of the treatments.
 |
Table 3 Safety Summary |
Discussion
In this study, patients with initially unresectable advanced HCC were treated with lenvatinib, sintilimab, and arterially-directed therapy. The conversion rate and prognosis of the potentially resectable patient population were better than those of non-potentially resectable patients. In addition, the treatment regimen was well tolerated.
Targeted agents and immunotherapy are developing rapidly. The high ORR generated by combination of two or three treatment modalities provides an opportunity for implementing a conversion therapy strategy in patients with advanced HCC. Previous studies showed that Targeted agents and immunotherapy combination showed good ORR in HCC, ranging from 19% to 54.2%,28 and targeted agents and immunotherapy combination with hepatic artery intervention had an ORR of up to 96% and a conversion resection rate range from 12.7% to 56%.29–31
Although combination therapy can greatly prolong the survival of patients with advanced HCC, surgical resection is still the treatment associated with the best long-term survival in liver cancer patients,32,33 as well as the only radical treatment method. In 1977, Shafer proposed the concept of preoperative conversion, in which preoperative combination therapy leads to a decrease in tumor size and vascularity, thus making surgery feasible.34 Patients who underwent surgery after successful conversion had a radically improved prognosis. The high ORR associated with combination therapy provides a good foundation for the success of conversion therapy. Studies have shown that lenvatinib combined with PD-1 inhibitors can effectively convert initially unresectable HCC into resectable HCC.35 Many researchers have also investigated the outcomes of targeted drugs combined with TACE36 or HAIC37 for conversion of unresectable HCC, showing promising results. However, data supporting the clinical use of these treatment strategies remain limited. According to a Chinese expert consensus on conversion therapy in patients with HCC,28 Unresectable HCC can be broadly divided into two categories; surgically unresectable or oncologically unresectable (technically resectable but with no better expected outcome than non-surgical treatment). Patients with one of these conditions may respond well to initial non-surgical therapy and may subsequently undergo surgery and therefore be classified as “potentially resectable” HCC. However, there is no unified definition of potentially resectable HCC yet.
In our analysis, triple therapy with lenvatinib plus sintilimab plus arterially-directed therapy led to a relatively high ORR in patients with advanced HCC. The ORR in the potentially resectable population in this study was 67.6%, and the conversion surgery rate was 40.5%, which is close to the previously reported results. Responses to this triple therapy regimen were sufficient to allow some patients with initially unresectable HCC to be converted into resectable patients. However, previous studies had a short follow-up period and no long-term survival outcome was observed. In this study, with a median follow-up time of 28 months and a maximum follow-up time of 47 months, median OS of PRP was still not reached, 13 (35.1%) patients of PRP survived longer than 24 months.
Further analysis of the baseline characteristics of the 15 successfully converted patients suggested that patients with Child-Pugh class A, ECOG PS 0, no extrahepatic metastases, tumor size < 10 cm, and tumor number < 3 have a higher possibility of successful conversion, and this is consistent with the criteria for conversion. This suggests that maintaining good liver function before initiating treatment may be beneficial for successful downstaging of patients.38 Combination therapies including arterially-directed therapy may cause liver damage,39 therefore changes in liver function should be carefully monitored during treatment. Patients in good physical condition are better able to tolerate higher blood concentrations of targeted and immunotherapeutic drugs and are more likely to achieve a response to treatment.40 Many guidelines do not recommend primary tumor resection for liver cancer patients with extrahepatic metastasis.32,41 If the primary tumor and metastases are resected simultaneously, the patient may be unable to tolerate the physical trauma caused by such a major surgery. Furthermore, some patients have too large or too many tumors, and it may be difficult to ensure sufficient liver volume after resection while meeting the criteria for resection, even if a treatment response is achieved.
The 5-year survival rate after salvage resection following successful conversion of patients with initially unresectable HCC has been reported to be 24.9–57%, which is equivalent to the 5-year survival rate of patients who directly undergo radical resection.42 In addition, previous studies have shown that the outcomes of patients who meet the criteria for surgical resection but do not undergo surgical resection are worse than those who undergo surgery. In a study by Zhang et al, 831 patients with unresectable liver cancer received TACE and conversion was successfully achieved by a total of 82 patients (9.87%), of whom 43 underwent liver resection. After treatment, the survival rate of the surgery group was significantly higher than that of the non-surgery group.36 A recent report also revealed that after systemic therapy, patients who underwent resection had longer OS than those who did not (median OS not reached vs 15.9 months [95% CI, 7.0–24.7 months]; P <0.001).43 We therefore recommend that patients undergo surgical resection promptly after undergoing downstaging treatment and reaching the criteria for surgery. Firstly, even if the patient is currently responsive to the treatment, drug resistance may develop over time, which can cause tumor progression and loss of the opportunity for surgical resection. Secondly, even if imaging evaluation indicates complete remission, it cannot guarantee that the tumor cells are completely inviable, and surgical resection can maximize the removal of tumor cells that are still viable.44 Finally, immediate surgical treatment can reduce the number of treatment cycles received by the patient,45 which is important as long-term conversion therapy may cause irreversible damage to liver function which can lead to postoperative liver insufficiency. However, in the present study, no patients refused surgery after successful conversion.
Although postoperative adjuvant therapy remains controversial, many studies have suggested that targeted drugs and interventional therapy can reduce the risk of postoperative recurrence and improve outcomes.46,47 In addition, some evidence suggests that postoperative adjuvant treatment should be simplified as much as possible after successful conversion of unresectable liver cancer patients, and a minimal number of effective treatments should be implemented as postoperative maintenance treatments.48 A consensus of Chinese experts on conversion therapy for liver cancer states that if the postoperative pathological evaluation result is pCR or pathological partial response, the original regimen of TKI plus PD-1 inhibitor should be maintained for 6 months or 6–12 months, respectively. However, if the postoperative pathological evaluation result is pathological progressive disease, the treatment plan should be readjusted.49 We also recommend that the original drug regimen be maintained and treatment be continued for 6–12 months after surgery to prevent tumor recurrence and prolong the survival time.
The most common adverse reaction experienced by patients in the present study was hypertension. In general, AEs were mostly mild to moderate and were controlled by discontinuing the drug or reducing the dose. No deaths occurred due to AEs. The triple treatment regimen was well tolerated and no new or unexpected adverse reactions were observed. Among the 15 patients who underwent surgery, all postoperative complications were well controlled and there were no deaths due to complications.
The present study had several limitations. Firstly, its retrospective design and nonrandomized nature made it vulnerable to a variety of potential biases. All patients were from a single hospital, there might be inherent information and selection bias. The findings in this study needed prospective multicenter randomized controlled trials to verify. Secondly, the primary cause of HCC in China is hepatitis B virus infection. Thirdly, it remains to be determined whether our findings are generalizable to Western countries where the primary cause of HCC is hepatitis C virus infection.
Conclusion
In conclusion, lenvatinib combined with sintilimab and arterially-directed therapy can be used to convert patients with initially unresectable intermediate-stage and advanced HCC to resectable disease and improve their long-term survival, with a more obvious benefit for potentially resectable patients. Hence, it’s very important to figure out PRP from moderate-advanced HCC. Besides, patients with Child-Pugh class A, ECOG PS 0, no extrahepatic metastases, tumor size < 10 cm, and tumor number < 3 have a higher possibility of successful conversion. However, these results require confirmation in a large-scale prospective study.
Funding
This work is supported by the grant from the National Natural Science Foundation of China (82173317).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:1. doi:10.3322/caac.21442
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Lau WY, Leung TW, Lai BS, et al. Preoperative systemic chemoimmunotherapy and sequential resection for unresectable hepatocellular carcinoma. Ann Surg. 2001;233(2):236–241. doi:10.1097/00000658-200102000-00013
4. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi:10.1038/s41575-019-0186-y
5. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi:10.1053/j.gastro.2015.12.041
6. Schlachterman A, Craft WW, Hilgenfeldt E, Mitra A, Cabrera R. Current and future treatments for hepatocellular carcinoma. World J Gastroenterol. 2015;21(28):8478–8491. doi:10.3748/wjg.v21.i28.8478
7. Wang Y, Jiang M, Zhu J, et al. The safety and efficacy of lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma. Biom Pharmacother. 2020;132:110797. doi:10.1016/j.biopha.2020.110797
8. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
9. Yamada K, Yamamoto N, Yamada Y, et al. Phase I dose-escalation study and biomarker analysis of E7080 in patients with advanced solid tumors. Clin Cancer Res. 2011;17(8):2528–2537. doi:10.1158/1078-0432.CCR-10-2638
10. Boss DS, Glen H, Beijnen JH, et al. A phase I study of E7080, a multitargeted tyrosine kinase inhibitor, in patients with advanced solid tumours. Br J Cancer. 2012;106(10):1598–1604. doi:10.1038/bjc.2012.154
11. Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7(7):543–555. doi:10.1038/nri2103
12. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3(5):391–400. doi:10.1038/nrd1381
13. Kudo M. Scientific rationale for combined immunotherapy with PD-1/PD-L1 Antibodies and VEGF inhibitors in advanced hepatocellular carcinoma. Cancers. 2020;12:5. doi:10.3390/cancers12051089
14. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/JCO.20.00808
15. Kudo M, Ikeda M, Motomura K, et al. A phase Ib study of lenvatinib (LEN) plus nivolumab (NIV) in patients (pts) with unresectable hepatocellular carcinoma (uHCC): study 117. J Clin Oncol. 2020;38(4_suppl):513. doi:10.1200/JCO.2020.38.4_suppl.513
16. Wang J, Fei K, Jing H, et al. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. mAbs. 2019;11(8):1443–1451. doi:10.1080/19420862.2019.1654303
17. Benson AB, D’Angelica MI, Abbott DE, et al. NCCN Guidelines insights: hepatobiliary cancers, version 1.2017. J Natl Compr Canc Netw. 2017;15(5):563–573. doi:10.6004/jnccn.2017.0059
18. Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49(5):523–529. doi:10.1080/02841850801958890
19. Fu Z, Li X, Zhong J, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021;15:663–675. doi:10.1007/s12072-021-10184-9
20. Ding X, Sun W, Li W, et al. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: a prospective randomized study. Cancer. 2021;127:3782–3793. doi:10.1002/cncr.33677
21. Mai Q, Mo Z, Shi F, Chen X. Lenvatinib plus hepatic arterial infusion of modified FOLFOX regime in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2020;38(15_suppl):e16603–e16603. doi:10.1200/JCO.2020.38.15_suppl.e16603
22. He M-K, Liang R-B, Zhao Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2021;13:175883592110027. doi:10.1177/17588359211002720
23. European Association For The Study Of The Liver. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. doi:10.1016/j.jhep.2011.12.001
24. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
25. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
26. Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72(2):288–306. doi:10.1016/j.jhep.2019.09.026
27. Atkinson TM, Ryan SJ, Bennett AV, et al. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer. 2016;24(8):3669–3676. doi:10.1007/s00520-016-3297-9
28. Sun HC, Zhou J, Wang Z, et al. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr. 2022;11(2):227–252. doi:10.21037/hbsn-21-328
29. Cai M, Huang W, Huang J, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol. 2022;13:848387. doi:10.3389/fimmu.2022.848387
30. Sun HC, Zhu XD. Downstaging conversion therapy in patients with initially unresectable advanced hepatocellular carcinoma: an overview. Front Oncol. 2021;11:772195. doi:10.3389/fonc.2021.772195
31. Tang H, Cao Y, Jian Y, et al. Conversion therapy with an immune checkpoint inhibitor and an antiangiogenic drug for advanced hepatocellular carcinoma: a review. Biosci Trends. 2022;16(2):130–141. doi:10.5582/bst.2022.01019
32. Galle PR, Forner A, Llovet JM, et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
33. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
34. Shafer AD, Selinkoff PM. Preoperative irradiation and chemotherapy for initially unresectable hepatoblastoma. J Pediatr Surg. 1977;12(6):1001–1007. doi:10.1016/0022-3468(77)90612-1
35. Zhu X-D, Huang C, Shen Y-H, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and Anti-PD-1 antibody combinations. Liver Cancer. 2021;2021:1–10.
36. Zhang Y, Huang G, Wang Y, et al. Is salvage liver resection necessary for initially unresectable hepatocellular carcinoma patients downstaged by transarterial chemoembolization? Ten years of experience. Oncologist. 2016;21(12):1442–1449. doi:10.1634/theoncologist.2016-0094
37. He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–960. doi:10.1001/jamaoncol.2019.0250
38. Tomonari T, Sato Y, Tanaka H, et al. Conversion therapy for unresectable hepatocellular carcinoma after lenvatinib: three case reports. Medicine. 2020;99(42):e22782. doi:10.1097/MD.0000000000022782
39. Raoul J-L, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28–36. doi:10.1016/j.ctrv.2018.11.002
40. Hata K, Suetsugu K, Egashira N, et al. Association of lenvatinib plasma concentration with clinical efficacy and adverse events in patients with hepatocellular carcinoma. Cancer Chemother Pharmacol. 2020;86(6):803–813. doi:10.1007/s00280-020-04178-x
41. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
42. Lau WY, Lai ECH. Salvage surgery following downstaging of unresectable hepatocellular carcinoma--a strategy to increase resectability. Ann Surg Oncol. 2007;14(12):3301–3309. doi:10.1245/s10434-007-9549-7
43. Colombo PE, Quenet F, Alric P, et al. Distal pancreatectomy with celiac axis resection (Modified Appleby Procedure) and arterial reconstruction for locally advanced pancreatic adenocarcinoma after FOLFIRINOX chemotherapy and chemoradiation therapy. Ann Surg Oncol. 2021;28(2):1106–1108. doi:10.1245/s10434-020-08740-y
44. Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655–1661.
45. Cheng Y, Wang Q, Li K, et al. Overall survival (OS) update in ALTER 1202: anlotinib as third-line or further-line treatment in relapsed small-cell lung cancer (SCLC). Ann Oncol. 2019;30:v711.
46. Li L, Li B, Zhang M. Postoperative adjuvant transarterial chemoembolization improves the prognosis of hepatocellular carcinoma patients with microvascular invasion: a systematic review and meta-analysis. Acta Radiol. 2020;61(6):723–731. doi:10.1177/0284185119878357
47. Zhang X-P, Chai Z-T, Gao Y-Z, et al. Postoperative adjuvant sorafenib improves survival outcomes in hepatocellular carcinoma patients with microvascular invasion after R0 liver resection: a propensity score matching analysis. HPB. 2019;21(12):1687–1696. doi:10.1016/j.hpb.2019.04.014
48. Zhou H, Song T. Conversion therapy and maintenance therapy for primary hepatocellular carcinoma. Biosci Trends. 2021;15:155–160. doi:10.5582/bst.2021.01091
49. Song T, Lang M, Ren S, Gan L, Lu W. The past, present and future of conversion therapy for liver cancer. Am J Cancer Res. 2021;11(10):4711–4724.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.