Back to Journals » OncoTargets and Therapy » Volume 8
A recurrent ovarian cancer patient with a history of nine prior chemotherapy regimens who was safely treated with weekly paclitaxel plus bevacizumab and achieved a complete response: a case report
Authors Takatori E, Shoji T, Nagasawa T, Takeuchi S, Hosoyachi A, Sugiyama T
Received 1 January 2015
Accepted for publication 3 March 2015
Published 11 August 2015 Volume 2015:8 Pages 2097—2100
DOI https://doi.org/10.2147/OTT.S80143
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Daniele Santini
Eriko Takatori,1 Tadahiro Shoji,1 Takayuki Nagasawa,1 Satoshi Takeuchi,1 Akira Hosoyachi,2 Toru Sugiyama1
1Department of Obstetrics and Gynecology, School of Medicine, Iwate Medical University, Morioka, Japan; 2Department of Obstetrics and Gynecology, Iwate Prefectural Miyako Hospital, Miyako, Japan
Abstract: Herein, we describe our experience with a recurrent ovarian cancer patient who was treated safely with bevacizumab and who achieved a complete response despite receiving nine prior chemotherapy regimens. The patient was a 54-year-old woman with stage IIIC recurrent ovarian serous adenocarcinoma (grade 3). Computed tomography (CT) revealed that no evidence of ascites, multiple intraperitoneal dissemination, or intrapelvic lymph node metastases was present. The absence of bowel obstruction and disseminated lesions involving the intestinal tract was confirmed by CT. Performance status was 0, and a blood test also indicated preservation of major organ function. In our hospital, weekly paclitaxel plus bevacizumab therapy (paclitaxel at 80 mg/m2 on days 1, 8, and 15; bevacizumab at 15/mg/kg on day 1 and every 21 days thereafter) was started. Eight cycles were administered, with no signs of gastrointestinal perforation, and the antitumor effect was evaluated as a complete response. The observed adverse events included grade 1 hyponatremia and grade 1 hypochloremia, and there was one grade 1 sensory peripheral neuropathy. These adverse events neither delayed treatment nor necessitated any dosage reductions. This case suggests that bevacizumab can be safely administered even to patients with recurrent ovarian cancer who have received three or more prior chemotherapy regimens if there are neither symptoms of bowel obstruction nor lesions suggestive of intestinal invasion on diagnostic imaging.
Keywords: recurrent ovarian cancer, gastrointestinal perforation
Introduction
Recently, overseas, four randomized Phase III trials have reported the addition of bevacizumab to either first-line chemotherapy (GOG-0218 and ICON7) or to second-line chemotherapy in platinum-sensitive (OCEANS Trial) and platinum-resistant (AURELIA Trial) recurrent ovarian cancer. Relative to progression-free survival (PFS), the absolute median PFS advantage of bevacizumab added to chemotherapy followed by maintenance compared with chemotherapy alone (and placebo, in GOG-0218) as first-line treatment was 3.8 months (14.1 vs 10.3 months, respectively) in the GOG-0218 trial1 and 1.5 months (21.8 vs 20.3 months, respectively) in ICON7.2 The median PFS benefit as measured in months associated with adding bevacizumab to chemotherapy seems to be similar in recurrent ovarian cancer, being 4 months in platinum-sensitive relapse (lengthening PFS from 8.4 to 12.4 months in the OCEANS Trial)3 and 3.3 months in platinum-resistant disease (from 3.4 to 6.7 months in the AURELIA Trial).4 In Japan, bevacizumab was approved for health insurance coverage in November 2013 for the treatment of ovarian cancer, which allows administration of this drug at 15 mg/kg every 3 weeks for the cancer. Gastrointestinal (GI) perforation is among the serious adverse events of bevacizumab. In recurrent cases especially, sufficient caution is required when administering bevacizumab. One of the eligibility criteria for the AURELIA trial was that patients had received no more than two prior chemotherapy regimens, ie, those who had received three or more regimens were excluded.4 We herein report our experience with a case in which weekly paclitaxel plus bevacizumab therapy was safely administered to a patient with recurrent ovarian cancer who had received nine prior chemotherapy regimens.
Case report
The patient was a 54-year-old woman, gravida 2, para 2, with stage IIIC ovarian cancer (histology: serous cystadenocarcinoma grade 3). She was referred for the treatment of recurrent cancer to the Department of Obstetrics and Gynecology at our hospital in April 2014.
In June 2004, the patient had undergone simple total hysterectomy, bilateral uterine adnexectomy, and pelvic lymphadenectomy at the previous hospital. However, a tumor remained on the surface of the rectal serosa. After surgery, three cycles of paclitaxel plus carboplatin (TC) therapy (paclitaxel at 175 mg/m2 on day 1; carboplatin [area under the curve [AUC] 6 mg/mL per min] on day 1 and every 21 days thereafter) were administered, followed by interval debulking surgery consisting of omentectomy and para-aortic lymphadenectomy. Because the tumor on the surface of the rectal serosa had macroscopically disappeared, the rectum was not resected. However, because rapid cytology of ascites provided a diagnosis of adenocarcinoma, cisplatin was intraperitoneally injected at 50 mg/m2 during surgery. After interval debulking surgery, six cycles of TC therapy were added. Remission was achieved, and the patient was followed up. In August 2007, secondary debulking surgery (SDS) was performed, with a diagnosis of pelvic recurrence, and recurrent tumors over the entire rectum were removed. During the SDS, cisplatin was again intraperitoneally injected at 50 mg/m2. After SDS, six cycles of TC therapy were administered. Remission was again achieved, and the patient was followed up. In September 2010, with a diagnosis of liver and para-aortic lymph node metastases, six cycles of TC therapy were administered again. The metastatic lesions disappeared, and she was again followed up. In May 2011, because new multiple intraperitoneal dissemination and intrapelvic lymph node metastases were detected, two cycles of pegylated liposomal doxorubicin monotherapy (50 mg/m2 every 28 days) were administered, but there was no clinical response. Starting in September 2011, 20 cycles of gemcitabine monotherapy (1,500 mg/m2 on day 1 and every 14 days thereafter) were given. Tumor reduction was not achieved, and the metastatic lesions grew. Thus, starting in July 2013, she received eight cycles of irinotecan hydrochloride (CPT-11) plus etoposide (VP-16) therapy (irinotecan at 60 mg/m2 on days 1 and 15; oral etoposide at 50 mg/body on days 1 to 21 and every 28 days thereafter). However, further growth of the metastatic lesions was observed. At the previous hospital, the patient and her family were informed that there was no further treatment available at that hospital. The patient and her family had then requested treatment with bevacizumab, and she was thus referred to our hospital. The prior chemotherapy regimens is shown in Table 1.
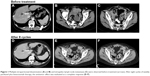
Computed tomography (CT) at our hospital revealed that no evidence of ascites, multiple intraperitoneal dissemination, or intrapelvic lymph node metastases was present (Figure 1). In addition, the absence of bowel obstruction and disseminated lesions involving the intestinal tract was confirmed by CT. Performance status was 0, and a blood test also indicated preservation of major organ functions. After the patient and her family had received a detailed explanation of the possible serious adverse reactions to bevacizumab, such as GI perforation and thrombosis, weekly paclitaxel plus bevacizumab therapy (paclitaxel at 80 mg/m2 on days 1, 8, and 15; bevacizumab at 15 mg/kg on day 1 and every 21 days thereafter) was started in May 2014. To date, eight cycles have been administered. The antitumor effect was evaluated as a complete response (Figure 1). Moreover, the level of carcinoma antigen (CA)125 decreased from 940 U/mL to 49 U/mL. PFS is 7 months, to date, and she remains alive with disease. Regarding adverse events, the observed metabolic/laboratory events were grade 1 hyponatremia and grade 1 hypochloremia, and the only nonmetabolic/laboratory events was grade 1 sensory peripheral neuropathy. These adverse events did not necessitate delay of starting the subsequent treatment cycle or any dosage reductions.
Discussion
The AVF 2949g trial was a clinical study on platinum-resistant recurrent ovarian cancer. Because GI perforation was observed in five (23.8%) of the 21 patients who had received three prior chemotherapy regimens and in 11.4% of patients overall, this trial was terminated early.5 However, the incidence of GI perforation was reevaluated as part of the retrospective analysis of the AVF 2949g trial results in 25 patients, with the following unique exclusion criteria: (a) clinical symptoms of bowel obstruction; (b) evidence of rectosigmoid involvement on pelvic examination; and (c) bowel involvement on CT scan. This subset analysis revealed that although the median number of prior chemotherapy regimens was five (range 2–12), the incidence of GI perforation was zero.6 In the AVF 2949g trial, patients with a high risk of GI perforation, as described above, had not been excluded. It is thus assumed that GI perforation had occurred at a high rate in the high-risk subset. GI perforation caused by administration of bevacizumab appears to occur in patients with intestinal complications rather than in those receiving a certain number of prior chemotherapy regimens. On the other hand, Takano et al reported that GI perforation occurred in the ninth week of weekly paclitaxel plus bevacizumab therapy, which was administered as the fourth-line regimen to patients without these risk factors.7 The GOG-0218 study found the incidence of GI perforation to be significantly increased in patients with a history of inflammatory bowel treatment, large-bowel surgery, and primary small-bowel surgery.8 Meta-analyses have shown the incidence to be significantly increased in patients with a history of bowel surgery, those with symptoms of bowel obstruction, and those with invasive tumors in the area from the rectum to the vagina, indicating that there is no association between the number of prior regimens and GI perforation.9,10 Moreover, in the GOG-0218 study, GI perforation occurred during the sixth cycle or earlier in the majority of the patients, whereas there was only one patient in whom GI perforation occurred during maintenance therapy with bevacizumab during or after the sixth cycle.8 Based on these observations, it is reasonable to assume that in our patient, for whom 7 months have passed to date since the start of treatment, the possibility of developing GI perforation might be low in the absence of a relapse.
At present, there are two ongoing clinical studies on combination bevacizumab therapy for recurrent ovarian cancer: the GOG-0213 study and the Study of Clinical and Biological Prognostic Factors in Patients with Ovarian Cancer Receiving Carboplatin + Paclitaxel with Bevacizumab (MITO16/ManGO), which target patients with platinum-sensitive tumors. According to the eligibility criteria for these studies, the maximum allowable number of prior chemotherapy regimens is one. In the AURELIA trial, which was conducted in patients with platinum-resistant tumors, the maximum allowable number was two regimens.4 With regards to administration of bevacizumab, further studies on the association between the number of prior chemotherapy regimens and GI perforation are needed.
In the AURELIA trial, which was a clinical study targeting patients with platinum-resistant recurrent ovarian cancer, the subanalysis showed that those receiving weekly paclitaxel plus bevacizumab therapy had favorable treatment outcomes, as evidenced by a response rate of 51.3% and the median PFS of 6 months.4 For our patient, weekly paclitaxel plus bevacizumab therapy was selected for the following reasons: pegylated liposomal doxorubicin had been used previously, and topotecan is the recommended drug in Japan when the number of prior chemotherapy regimens is three or less.
In the AURELIA trial, paclitaxel was administered at 80 mg/m2 on days 1, 8, 15, and 22, and bevacizumab at 10 mg/kg on days 1 and 15, with one cycle being defined as 28 days. However, in Japan, administration of bevacizumab at 10 mg/kg every 2 weeks is not approved by the national health insurance scheme. Thus, our patient received a regimen consisting of paclitaxel at 80 mg/m2 on days 1, 8, and 15 and bevacizumab at 15 mg/kg on day 1, with one cycle consisting of 21 days.
In our patient, the possible reasons for responding to this regimen were that 3 years and 3 months had passed since the last dose of paclitaxel, and that weekly administration of paclitaxel at 80 mg/m2 increases the dose intensity. While treatment is ongoing, PFS is 7 months at present, and longer survival is anticipated.
Our patient, who had received nine prior chemotherapy regimens, was safely administered bevacizumab in combination with paclitaxel. Even in patients who have received three or more prior chemotherapy regimens, bevacizumab can be safely administered for recurrent ovarian cancer if neither symptoms of bowel obstruction nor lesions suggestive of intestinal invasion are present on diagnostic imaging. This is the first report in Japan showing that bevacizumab can be concomitantly used for patients with recurrent ovarian cancer regardless of the number of prior chemotherapy regimens. We consider this report to be significant in that the regimen employed herein has potential to contribute to an improved prognosis for such patients.
Disclosure
The authors report no conflicts of interest in this work.
References
Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–2483. | ||
Perren TJ, Swart AM, Pfisterer J, et al; ICON7 Investigators. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–2496. | ||
Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30(17):2039–2045. | ||
Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–1308. | ||
Cannistra SA, Matulonis UA, Penson RT, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25(33):5180–5186. | ||
Simpkins F, Belinson JL, Rose PG. Avoiding bevacizumab related gastrointestinal toxicity for recurrent ovarian cancer by careful patient screening. Gynecol Oncol. 2007;107(1):118–123. | ||
Takano M, Kikuchi Y, Kato M, Yoshikawa T, Kita T. [Bowel perforation associated with bevacizumab therapy in recurrent ovarian cancers without bowel obstruction or bowel involvement]. Gan To Kagaku Ryoho. 2008;35(11):1981–1984. Japanese. | ||
Burger RA, Brady MF, Bookman MA, et al. Risk factors for GI adverse events in a phase III randomized trial of bevacizumab in first-line therapy of advanced ovarian cancer: A Gynecologic Oncology Group Study. J Clin Oncol. 2014;32(12):1210–1217. | ||
Richardson DL, Backes FJ, Hurt JD, et al. Which factors predict bowel complications in patients with recurrent epithelial ovarian cancer being treated with bevacizumab? Gynecol Oncol. 2010;118(1):47–51. | ||
Tanyi JL, McCann G, Hagemann AR, et al. Clinical predictors of bevacizumab-associated gastrointestinal perforation. Gynecol Oncol. 2011;120(3):464–469. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.