Back to Journals » Clinical Ophthalmology » Volume 14
A Real-World Study of Dexamethasone Implant in Treatment-Naïve Patients with Diabetic Macular Edema: Efficacy and Correlation Between Inflammatory Biomarkers and Treatment Outcome
Authors Vadalà M , Sunseri Trapani V , Guarrasi G , Ventura N , Castellucci M , Cillino S
Received 27 April 2020
Accepted for publication 7 August 2020
Published 15 September 2020 Volume 2020:14 Pages 2657—2665
DOI https://doi.org/10.2147/OPTH.S257775
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Maria Vadalà,1,2 Valentina Sunseri Trapani,1 Giulia Guarrasi,1 Nicasio Ventura,1 Massimo Castellucci,1 Salvatore Cillino1
1Biomedicine, Neuroscience and Advanced Diagnostic Department, University of Palermo, Palermo, Italy; 2IEMEST, Euro-Mediterranean Institute of Science and Technology, Palermo, Italy
Correspondence: Maria Vadalà
Biomedicine, Neuroscience and Advanced Diagnostic (BIND) Department, Ophthalmology Institute, University of Palermo, Palermo 90127, Italy
Tel +39 091 6553909
Email [email protected]
Purpose: There has been an increasing clinical interest in specific retinal parameters as non-invasive biomarkers of retinal inflammation in diabetic macular edema (DME) that have been shown to have prognostic value, such as hyperreflective retinal fields (HRFs) and subfoveal neuroretinal detachment (SND).
Methods: We conducted a prospective, non-comparative study of treatment-naïve patients with DME to evaluate the efficacy of a Pro Re Nata (PRN) regimen of intravitreal dexamethasone implant 0.7 mg (DexI, Ozurdex™). After administration, patients underwent subsequent injections according to PRN criteria in case of edema relapse, but not earlier than 4 months after the previous treatment. Patients were evaluated at baseline, within 15 days of injection, and every month thereafter. During all visits, best-corrected visual acuity (BCVA) was recorded; central retinal thickness (CRT), type of edema, presence of SND, and presence and number of HRFs were evaluated using swept-source optical coherence tomography (SS-OCT) 3D. Treatment outcome was defined as changes in BCVA, CRT, SND and HRFs at 12 (T12) and 24 (T24) months compared with baseline (T0).
Results: The study enrolled 24 eyes of 18 patients. The mean duration of follow-up was 18± 6.6 months; for all eyes, T12 data were available, while follow-up reached T24 for 12 eyes. BCVA improved significantly and CRT decreased significantly during treatment; the edema was no longer detectable in 13/24 eyes at T12 and 8/12 eyes at T24. No patient presented SND at T12 and T24, and the mean number of HRFs decreased significantly during treatment. Results with CRT and HRFs correlated with BCVA at 12 and 24 months. No significant adverse events were observed.
Conclusion: In patients with DME, the intravitreal dexamethasone implant was effective and safe in improving both functional and tomographic parameters. This result is consistent with improvement in biomarkers of inflammation.
Keywords: dexamethasone implant, diabetic macular edema, intravitreal implants, Ozurdex, real-world, inflammation
Introduction
The retina and choroid, due to their vascular nature, are the main targets of diabetic microangiopathy. In diabetic patients, vasogenic changes secondary to hyperglycemia induce a breakdown of the blood-retinal barrier with consequent intrastromal exudation, increased levels of inflammatory factors, and activation of the cascade of macular edema formation.1,2
In clinical applications, fluorescein angiography (FA) is the best diagnostic tool to highlight disruption of the blood ocular barrier, but optical coherence tomography (OCT) is today the most sensitive method to identify and quantify macular edema.3,4 Furthermore, its improved technical characteristics, such as swept-source technology, have allowed its sensitivity to increase and led to identification of signs or “biomarkers” of inflammatory activation. OCT can measure the macular thickness (central retinal thickness [CRT]), total volume of the central retina (RTV), and number of hyperreflective retinal fields/foci (HRFs), and can detect subepithelial neurosensory detachment (SND),3,5 interruption of the external limiting membrane (ELM), and inner segment/outer segment (IS/OS). It can also reveal the presence of disorganization of the internal retinal layers (DRIL).6
An emerging biomarker is subfoveal neuroretinal detachment (SND), due to subretinal fluid, which is visible as a hyporeflective area on SD-OCT.5,7 It has a prevalence of 15–30%, is related to a poorer prognosis, and its pathogenesis is possibly linked to the disruption of the limiting membrane, with consequent changes of microglial cells or altered choroidal blood flow.8–11
A relationship has also been observed between morphological and serological or aqueous biomarkers: serum levels of VEGF, soluble intercellular adhesion molecule 1 (ICAM-1), chemotaxis protein, and TNF-α are significantly associated with quantitative and qualitative measurements in spectral-domain OCT (SD-OCT),12,13 supporting the key role of inflammation in DME and in guiding the choice of treatment.
Currently available treatments are aimed at reducing or resolving the edema itself in order to improve or preserve visual function in DME: they are based on the use of laser therapy and intravitreal administration of drugs, such as anti-VEGF therapies (bevacizumab, ranibizumab, aflibercept) or steroids (triamcinolone acetonide, dexamethasone, fluocinolone acetonide).14–16 Unfortunately, local application does not allow effective concentrations of drug to be reached near the macula, due to the limited uptake and penetration.17 Intravitreal injections increase ocular drug delivery, but are invasive and require frequent retreatment, with consequent limitations in their use.17 For this reason, sustained-release drug delivery systems have been developed, which allow high levels of the drug to be reached in target tissues, overcoming the limitations of local administration and short-acting intravitreal injections.17 Sustained-release drug delivery systems can be injected into the vitreous chamber, allowing a gradual and continuous release of drugs, and maintain effective therapeutic levels in the vitreous chamber over a medium (4–6 months) or long (up to 3 years) period of time.15,17–19
Ozurdex™ (Allergan Inc, Irvine, CA, USA) is a sustained release intravitreal rod-shaped (6 mm) implant containing 700 μg of dexamethasone (DexI), releasing 100–1000 μg/mL of the drug per day for the first 2 months.20,21 It was approved by the U. S. Food and Drug Administration (FDA) for the treatment of DME based on the results of a randomized, controlled trial on 1048 patients, which showed a significant improvement in best-corrected visual acuity (BCVA) and a reduction of CRT compared with the sham treatment.22 On the basis of the kinetics of release of the active substance, the DexI was approved with a regimen of administration every 6 months.20 However, pre- and post-marketing studies have shown that after the third month and long before the sixth month the pharmacological efficacy is reduced and macular edema resumes.14,23
DexI has also been associated with a delay in the progression of diabetic retinopathy over 24 months.24 In addition, real-world experience confirmed the results of clinical trials with DexI.25 Many studies have shown that more sustained clinical efficacy is obtained with a regimen of on-demand or Pro Re Nata (PRN) administration after the third month and before the sixth, which counteracts the resumption of disease activity in a timely manner.18,23
The aim of this study was to evaluate the efficacy of a PRN regimen of DexI on DME, and correlate treatment outcomes with inflammatory biomarkers.
Patients and Methods
Study Design and Objective
We conducted a prospective, single institution, non-comparative study to evaluate the efficacy of DexI, administered with a PRN regimen, on 18 treatment-naïve patients (24 eyes) affected by DME. Inclusion criteria were age ≥18 years, type 1 or 2 diabetes mellitus, visual loss due to DME (defined as retinal thickness in the central subfield >350 micron measured with swept-source OCT [SS-OCT] (DRI Triton, Topcon Inc., Japan)), intraretinal or subretinal fluid seen with SS-OCT, contraindication to intravitreal VEGF inhibitor drugs, no other previous therapy for DME and written consent to treatment.
Exclusion criteria were concomitant ocular diseases causing macular edema (ie, neovascular age-related macular degeneration or choroidal neovascularization due to other reasons, retinal vein occlusion, uveitis, and recent intraocular surgery), or compromising visual acuity (except cataract), previous treatment with intraocular corticosteroids, and uncontrolled ocular hypertension or glaucoma.
The study was conducted in conformity with applicable local requirements and regulations regarding the treatment protocol applied according to the product’s label;20 approval from local ethics committee was obtained. All patients gave written informed consent for the study participation and for the collection of data and publication of the results. The study was conducted in accordance with International Conference on Harmonisation (ICH) Harmonized Tripartite Guidelines for Good Clinical Practice and the Declaration of Helsinki Principles.
Treatment and Procedures
The study participants received the DexI via intravitreal injection. After the baseline administration, the patients underwent subsequent injections according to PRN criteria in case of edema relapse (defined as an increase of macular thickness of at least 150 μm) compared to the lowest value recorded as measured with OCT,33–35 but not earlier than 4 months after the previous treatment (according to the pharmacodynamics of the dexamethasone implant).
Hospital-standardized ophthalmic surgical techniques were used to prepare patients and eyes. The DexI was implanted following the manufacturer’s instructions and recommendations.
Assessments
Patients were evaluated at baseline, within 15 days of injection, and every month thereafter. At the baseline visit, demographic and clinical data were collected. During each visit (including the baseline) BCVA (logMAR) was registered and the following assessments were performed using SS-OCT 3D macula 7x7H and macular radial 6.0 protocols: CRT (μm), type of edema (cystoid, diffuse, mixed), and presence of SND. Two operators performed all scans (MV, GG). Poor quality scans (quality index < 40) were rejected and repeated. The scans were read and analyzed by a trained ophthalmologist masked to BCVA (VST).
The presence and number of HRFs within the central 3000 μ around the fovea were manually evaluated as the mean of the count on 6 radial OCT scans at baseline and after 3, 6, 9, and 12 months in a longitudinal fashion, and when possible at 24 months from the first injection.
Hemoglobin A1c (HbA1c) levels were collected at baseline and at 12 and 24 months of follow-up. The number of treatments was also recorded.
Endpoints
The outcome of treatment was defined as changes in BCVA, CRT, SND, and HRFs at 12 (T12) and 24 (T24) months compared to baseline (T0). A comparison between 12-month and 24-month visits was also performed. A correlation between the outcomes of treatment and number of injections was also explored.
Statistical Analysis
All the normally distributed continuous variables were expressed as mean ± standard deviation, whereas variables with skewed distribution were given as median and interquartile IQR range. Categorical variables were expressed as percentage values. Changes during follow-up for continuous variables were evaluated using the Wilcoxon test. Categorical variables were assessed using the McNemar test. The association between changes during follow-up was analyzed by Spearman correlation coefficients. The null hypothesis was rejected at a two-tailed p<0.05. The statistical analyses were performed using the SYSTAT DATA software package, version 13.0 (Systat Software, San Jose, CA, USA).
Results
Demographic and Baseline Data
The study enrolled 24 eyes of 18 patients (18 pseudophakic and 6 phakic). None was affected by glaucoma or ocular hypertension. Baseline data are reported in Table 1.
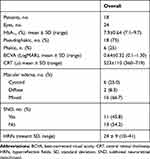 |
Table 1 Baseline Characteristics of the Study Participants |
Follow-Up and Number of Implants
The mean duration of follow-up was 18±6.6 months (range 12–30); for all eyes, T12 data were available, while for 12 eyes, the follow-up reached T24. The mean number of treatments at T12 was 2.6±1.4 (range 1–6). For T12, 5, 5, and 2 patients received 1, 2, and 3 treatments, respectively. Among those with 24 months of follow-up, 1, 1, and 6 patients received 1, 2, and 3 treatments; 2 patients required 4 treatments, while 2 and 1 needed 5 and 6 treatments, respectively. Only one patient was treated with 6 injections. The average retreatment time was 4.3 months. The group who received 24 months of follow-up underwent a mean of 3.4 ±1.4 injections (range 1–6).
Efficacy
The results of treatment are reported in Tables 2–4 and Figure 1. Statistically significant improvement in BCVA and a decrease in CRT were observed at T12; the 12 eyes who with follow-up at T24 presented further improvement.
 |
Table 2 Results of Treatment: Edema |
 |
Table 3 Results of Treatment: Subfoveal Neuroretinal Detachment (SND) |
 |
Table 4 Results of Treatment: Number of Hyperreflective Fields (HRFs) |
 |
Figure 1 Results of treatment: best-corrected visual acuity (BCVA) and central retinal thickness (CRT). |
At baseline, the type of edema was cystoid in 6 (25%) cases, diffuse in 2 (8%), and mixed in 16 (67%) eyes (Table 2), while after 12 months the percentages were 17% cystoid and 29% mixed, and no eye presented diffuse edema. In 13 eyes (54.2%), the edema was no longer detectable. Among the 12 eyes that were followed for 24 months, persistence of edema (mixed) was found in only 4 cases, while it was resolved in the remaining 8 eyes. Figure 1 shows the BCVA and CRT at 12 and 24 months. Overall, the differences between either T12 or T24 compared with T0 were statistically significant (Table 3).
While SND was detectable in 11 eyes at the baseline (46%), it disappeared in all cases after 12 and 24 months of treatment (Table 4). The mean number of HRFs decreased significantly after treatment, from 23.7 at T0 (range 10–41) to 14.3 (range 4–24) at T12 (p<0.0001), to 12.3 (range 2–22) at T24 (p=0.0005) (Table 4).
A correlation between CRT and BCVA was observed at 12 and 24 months (rho=0.6 for both, p=0.004 and p=0.02, respectively); the CRT was not found to be related to the number of injections at T12 and at T24 (rho=0.4 and 0.3, respectively). The number of HRFs at T12 correlated with BCVA at T12 (rho=0.7, p<0.0001) and at T24 (rho=0.7, p=0.04).
HbA1c levels showed decreased from baseline (7.9 ± 0.64%, range 7.1–9.7) to 12 (7.5 ± 0.41%, range 6.2–8.1, p<0.001) and 24 months (7 ± 0.34%, range 6.2–7.9, p<0.001). HbA1c values, however, did not correlate statistically with any clinical or OCT parameters.
Safety
Six eyes were phakic before intravitreal implant (25%). During follow-up, only one eye developed steroid-related cataract (after the second implant) for which an uneventful surgery was performed at 1 month after the third implant. Three eyes (12.5%) presented ocular hypertension after the first implant (<25 mmHg), but all were controlled with topical therapy.
Discussion
In our study, we enrolled a group of naïve patients with DME and evaluated the effect of PRN treatment with DexI on morphological and functional parameters. Although visual function is the key outcome of DME treatment, it is a subjective evaluation and can be influenced by numerous factors, ie, fluctuations in glucose levels or the presence of other ocular disorders. In contrast, morphological parameters such as CRT, presence of HRFs, and qualitative assessment of fluid distribution are more objective and reliable outcome measures for treatment response.26
OCT is the key diagnostic and prognostic tool in the management of DME. It provides high-resolution images and allows objective and precise evaluation of anatomical patterns and pathological retinal changes.27–29 The alterations shown by OCT in DME are significantly related to the severity of the disease and visual impairment;1 this tool, therefore, plays a key role in identifying high-risk patients and in guiding the treatment plan.30 The identification of reliable biomarkers of the development and progression of DME may differentiate various phenotypes, which are possibly related to the main pathogenic mediators and provide valuable information about the choice of therapeutic strategy.5 OCT can predict treatment success or failure of therapy, and its findings are generally accepted in clinical trials as a surrogate marker of efficacy.27
In our study, 12 months after the administration of intravitreal injection of the DexI, we have observed a significant decrease in CRT (−221 µ) and an improvement in BCVA (−0.22 LogMAR); the effect of treatment was maintained in 12 patients who were followed for a longer time, with significant improvement at the 24-month follow up compared with either the baseline or 12-month assessment, showing a sustained effect of the DexI. Concurrently with the decrease in CRT and increase in BCVA, we observed an improvement in inflammatory biomarkers, with a decrease of the mean number of HRFs (−10) and a disappearance of SND in all 11 patients who presented with it at baseline. In the experience of Vujosevic et al, HRFs significantly decreased in number after treatment with both ranibizumab and dexamethasone, without no difference between the two groups, and resolution of HRFs correlated well with CMT reduction.31
In our study, we found a correlation between the reduction in the number of HRFs and the increase in BCVA at T12 and at T24. Other authors have found the same correlation,32,33 while Schreur did not observe significant differences in changes in HRFs between eyes with adequate and insufficient functional (BCVA) and morphological (CRT) response after 3 months, in patients treated with bevacizumab, while a higher number of HRFs at baseline was associated with a better response as far as CRT reduction and BCVA improvement.26 On the other hand, Fonollosa et al did not find any difference in either anatomical (CRT) or functional response (BCVA) between subgroups classified on the basis on the number of HRFs.34
The disappearance of HRFs can be an intriguing biomarker of response to treatment as an effect of the anti-inflammatory mechanism of DexI: whatever the origin of this morphological feature, activation of microglia, migration of retinal pigment epithelium (RPE) cells or degeneration of photoreceptor,35 inflammation is the main pathogenic trigger, supporting the effectiveness of steroid treatment.36 Indeed, corticosteroids have both anti-inflammatory and anti-edematous effects that involve proinflammatory mediators involved in DME and VEGF.37,38 Moreover, CRT thinning and BCVA increase are strictly related to the anti-inflammatory effect on osmotic retinal swelling.
In our study, after 12 months, 54.2% of treated eyes did not present edema; similarly, edema was found in none of the 12 eyes evaluated at 24 months. We observed different responses of the different types of edema found at baseline: all cases of cystoid and diffuse edema resolved, while mixed edema was resolved in most cases at T12 and T24. Analysis for the subtype of edema was not performed because of the limitation of the low number, as well as the interpretation of the data. Some authors have reported that cystoid edema is associated with worse visual acuity at baseline and poorer response to treatment in comparison with diffuse edema,39 but with a higher reduction of CRT after the treatment with anti-VEGF therapy or DEX implant28,40,41 No data are currently available about the pathogenetic implications of the different types of edema. Cystoid edema can be associated with diffuse edema, but it usually resolves earlier after therapy, as an expression of earlier restoration of the central retinal barrier. It is the typical presentation of retinal inflammation seen in uveitis or after cataract surgery, since it is directly related to the ocular level of prostaglandins.42 The earlier and complete resolution of this subtype of edema in our cohort can be associated with a steroid-specific pathogenic effect.
The naïve patients enrolled in our study presented an optimal response to treatment. Many authors have reported a more significant improvement of morphological biomarkers in naïve patients and particularly if compared to refractory patients treated with the DexI.14,43–47
In a comparative study by Choi et al, patients with refractory macular edema showed significantly higher numbers of HRFs compared to the responsive DME group (p<0.001), together with a higher level of IL-1β in aqueous humor (p=0.042).48 A higher number of HRFs at the initial visit was associated with poor responses to three intravitreal injections of bevacizumab and, subsequently, one additional DexI. However, about 50% of non-responders to bevacizumab improved their clinical response after only one DexI. Our data support the indication of the DexI as a first-line treatment in patients with DME with contraindications to anti-VEGF treatment,29 and also reinforce the hypothesis of first-line treatment for the pathogenic target of dexamethasone, especially in naïve patients.25,37
The approved frequency of administration of the dexamethasone implant, identified on the basis of pharmacokinetic data, is 6 months. With this schedule, variable results have been obtained, and in our opinion, a flexible frequency, based on the first signs of recurrence (PRN regimen) is advisable, as confirmed by both clinical trials and in the real-world setting.18,43
We observed a mean of 2.6 treatments over 12 months, with an average retreatment time of 4.3 months. This is in line with that found by other authors.18,23,43,46 A randomized study in patients with DME found that the administration of the DexI with a PRN regimen was associated with better morphological and functional results at 6 months of follow-up than a fixed schedule,49 and Panozzo reported a mean time of the first recurrence of 5.1 months.50 It is noteworthy that, during 24 months of follow-up, the mean number of treatments was lower than in the first year, suggesting a cumulative effect of the steroid implant, optimal effects of early rescheduled injection, or better general compliance of patients.
A systematic review of 21 peer-reviewed publications of real-world data reported that a PRN administration of DexI in diabetic macular edema provided a good long-term efficacy/safety ratio, with a mean number of injections of 1.3 every 6 months (mean retreatment time: 5.3 ± 0.9 months).18 We did not observe an increasing interval between treatments in the few patients who were administered more than three DexI. A larger study of longer duration should be carried out to address this issue. It has been suggested that the mean time of recurrence after treatment with intravitreal steroids is about 4 to 5 months, and this observation, in addition to supporting the validity of the PRN approach, also provides indications for the reduction of the number of clinic visits, and therefore the burden for the patient.14,23
A systematic review of the long-term efficacy and safety of the intravitreal DexI found that longer treatment with DexI may improve the clinical efficacy of therapy, stabilizing DME, and prolonging the effects of treatment, with better visual acuity, and requiring fewer retreatments.18 On the other hand, it should be assumed that, independent of the patient’s clinical condition, longer-term DME requires a longer intervention to stabilize the effects of the therapy, and that the efficacy outcome may not depend on the timing and frequency of injection itself, but rather on early stabilization and maintenance of the clinical condition.18 Timely retreatment at the first signs of recurrence of DME allows partial maintenance of the efficacy already obtained during previous administrations, and optimizes response.
A previous study has documented a correlation between HbA1c and the risk of persistent clinically significant macular edema,51 and good metabolic response has been observed to correlate with better response to anti-VEGF therapy.52 In our study, even if values of HbA1c improved at 12 and 24 months vs baseline, the differences did not significantly correlate with clinical improvement. It is possible that this result is due to the small number of patients in the study: on the other hand, being followed for vision problems could possibly motivate patients towards better control of the systemic disease.
Our study has some strengths, including the long follow-up, enrollment of naïve patients, real-world setting with a PRN administration, and correlation of clinical outcomes with inflammatory biomarkers. The weaknesses of the study relate to the limited number of patients and its monocentric, non-comparative design, which does not allow conclusions to be drawn regarding the efficacy of the DexI compared to other therapeutic strategies.
Conclusions
In diabetic patients with ME, treatment with DexI is effective and safe in improving both functional and tomographic parameters. This result is consistent with the improvement in biomarkers related to inflammation. Further comparative studies, with a larger number of patients, are warranted.
Ethical Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research regulation and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of the University Hospital Policlinico P. Giaccone of Palermo (No. 7/2020).
Acknowledgments
Editorial assistance in preparing the manuscript for submission was provided to the authors by Maria Carla Brunenghi and Ray Hill, independent medical writers, and funded by an unconditioned grant PG-2019-10639 by Allergan plc, Dublin, Ireland, at the request of the investigator. All authors met the ICMJE authorship criteria.
Disclosure
Dr Maria Vadalà reports grants from Allergan SpA, during the conduct of the study; consultant for Novartis SpA, SpA, Abbvie Spa, and Allergan SpA, outside the submitted work. Professor Salvatore Cillino reports grants from Allergan, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Alkuraya H, Kangave D, Abu El-Asrar AM. The correlation between optical coherence tomographic features and severity of retinopathy, macular thickness and visual acuity in diabetic macular edema. Int Ophthalmol. 2005;26:93–99. doi:10.1007/s10792-006-9007-8
2. Romero-Aroca P. Targeting the pathophysiology of diabetic macular edema. Diabetes Care. 2010;33:2484–2485. doi:10.2337/dc10-1580
3. Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54:1–32. doi:10.1016/j.survophthal.2008.10.001
4. Johnson MW. Etiology and treatment of macular edema. Am J Ophthalmol. 2009;147(11–21):e1. doi:10.1016/j.ajo.2008.07.024
5. Vujosevic S, Simo R. Local and systemic inflammatory biomarkers of diabetic retinopathy: an integrative approach. Invest Ophthalmol Vis Sci. 2017;58:BIO68–BIO75. doi:10.1167/iovs.17-21769
6. Midena E, Pilotto E, Bini S. Hyperreflective intraretinal foci as an OCT biomarker of retinal inflammation in diabetic macular edema. Invest Ophthalmol Vis Sci. 2018;59:5366. doi:10.1167/iovs.18-25611
7. Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999;127:688–693. doi:10.1016/S0002-9394(99)00033-1
8. Gaucher D, Sebah C, Erginay A, et al. Optical coherence tomography features during the evolution of serous retinal detachment in patients with diabetic macular edema. Am J Ophthalmol. 2008;145:289–296. doi:10.1016/j.ajo.2007.09.029
9. Nagaoka T, Kitaya N, Sugawara R, et al. Alteration of choroidal circulation in the foveal region in patients with type 2 diabetes. Br J Ophthalmol. 2004;88:1060–1063. doi:10.1136/bjo.2003.035345
10. Seo KH, Yu SY, Kim M, Kwak HW. Visual and morphologic outcomes of intravitreal ranibizumab for diabetic macular edema based on optical coherence tomography patterns. Retina. 2016;36:588–595. doi:10.1097/IAE.0000000000000770
11. Sonoda S, Sakamoto T, Yamashita T, Shirasawa M, Otsuka H, Sonoda Y. Retinal morphologic changes and concentrations of cytokines in eyes with diabetic macular edema. Retina. 2014;34:741–748. doi:10.1097/IAE.0b013e3182a48917
12. Brito P, Costa J, Gomes N, Costa S, Correia-Pinto J, Silva R. Serological inflammatory factors as biomarkers for anatomic response in diabetic macular edema treated with anti-VEGF. J Diabetes Complications. 2018;32:643–649. doi:10.1016/j.jdiacomp.2018.05.006
13. Sun JK, Lin MM, Lammer J, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132:1309–1316. doi:10.1001/jamaophthalmol.2014.2350
14. Garcia-Layana A, Figueroa MS, Arias L, et al. Clinical decision-making when treating diabetic macular edema patients with dexamethasone intravitreal implants. Ophthalmologica. 2018;240:61–72. doi:10.1159/000486800
15. Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–625. doi:10.1016/j.ophtha.2011.01.031
16. Tranos PG, Wickremasinghe SS, Stangos NT, Topouzis F, Tsinopoulos I, Pavesio CE. Macular edema. Surv Ophthalmol. 2004;49:470–490. doi:10.1016/S0039-6257(04)00109-2
17. Augustin AJ. Upcoming therapeutic advances in diabetic macular edema: an intravitreal dexamethasone drug delivery system. Expert Opin Drug Deliv. 2011;8:271–279. doi:10.1517/17425247.2011.548802
18. Bucolo C, Gozzo L, Longo L, Mansueto S, Vitale DC, Drago F. Long-term efficacy and safety profile of multiple injections of intravitreal dexamethasone implant to manage diabetic macular edema: a systematic review of real-world studies. J Pharmacol Sci. 2018;138:219–232. doi:10.1016/j.jphs.2018.11.001
19. Yang Y, Bailey C, Loewenstein A, Massin P. Intravitreal corticosteroids in diabetic macular edema: pharmacokinetic considerations. Retina. 2015;35:2440–2449. doi:10.1097/IAE.0000000000000726
20. European Medicines Agency (EMA) 2015. OZURDEX (dexamethasone) 700 micrograms intravitreal implant in applicator: summary of product characteristics. Available from: https://www.ema.europa.eu/.
21. U. S. Food & Drug Administration (FDA) 2014. OZURDEX (dexamethasone intravitreal implant). Highlights of prescribing information. Available from: https://www.acessdata.fda.gov.
22. Boyer DS, Yoon YH, Belfort R
23. Panozzo G, Gusson E, Panozzo G, Dalla Mura G. Dexamethasone intravitreal implant for diabetic macular edema: indications for a PRN regimen of treatment. Eur J Ophthalmol. 2015;25:347–351. doi:10.5301/ejo.5000563
24. Iglicki M, Zur D, Busch C, Okada M, Loewenstein A. Progression of diabetic retinopathy severity after treatment with dexamethasone implant: a 24-month cohort study the ‘DR-Pro-DEX Study’. Acta Diabetol. 2018;55:541–547. doi:10.1007/s00592-018-1117-z
25. Mello Filho P, Andrade G, Maia A, et al. Effectiveness and safety of intravitreal dexamethasone implant (Ozurdex) in patients with diabetic macular edema: a real-world experience. Ophthalmologica. 2019;241:9–16. doi:10.1159/000492132
26. Schreur V, Altay L, van Asten F, et al. Hyperreflective foci on optical coherence tomography associate with treatment outcome for anti-VEGF in patients with diabetic macular edema. PLoS One. 2018;13:e0206482. doi:10.1371/journal.pone.0206482
27. Murakami T, Yoshimura N. Structural changes in individual retinal layers in diabetic macular edema. J Diabetes Res. 2013;2013:920713. doi:10.1155/2013/920713
28. Wu PC, Lai CH, Chen CL, Kuo CN. Optical coherence tomographic patterns in diabetic macula edema can predict the effects of intravitreal bevacizumab injection as primary treatment. J Ocul Pharmacol Ther. 2012;28:59–64. doi:10.1089/jop.2011.0070
29. Zur D, Iglicki M, Busch C, et al. OCT biomarkers as functional outcome predictors in diabetic macular edema treated with dexamethasone implant. Ophthalmology. 2018;125:267–275. doi:10.1016/j.ophtha.2017.08.031
30. Novais EA, Roisman L, de Oliveira PR, et al. Optical coherence tomography angiography of chorioretinal diseases. Ophthalmic Surg Lasers Imaging Retina. 2016;47:848–861. doi:10.3928/23258160-20160901-09
31. Vujosevic S, Torresin T, Bini S, et al. Imaging retinal inflammatory biomarkers after intravitreal steroid and anti-VEGF treatment in diabetic macular oedema. Acta Ophthalmol. 2016.
32. Coscas G, De Benedetto U, Coscas F, et al. Hyperreflective dots: a new spectral-domain optical coherence tomography entity for follow-up and prognosis in exudative age-related macular degeneration. Ophthalmologica. 2013;229:32–37. doi:10.1159/000342159
33. Liu S, Wang D, Chen F, Zhang X. Hyperreflective foci in OCT image as a biomarker of poor prognosis in diabetic macular edema patients treating with Conbercept in China. BMC Ophthalmol. 2019;19:157. doi:10.1186/s12886-019-1168-0
34. Fonollosa A, Zarranz-Ventura J, Valverde A, et al. Predictive capacity of baseline hyperreflective dots on the intravitreal dexamethasone implant (Ozurdex(R)) outcomes in diabetic macular edema: a multicenter study. Graefes Arch Clin Exp Ophthalmol. 2019;257:2381–2390. doi:10.1007/s00417-019-04446-4
35. Vujosevic S, Berton M, Bini S, Casciano M, Cavarzeran F, Midena E. Hyperreflective retinal spots and visual function after anti-vascular endothelial growth factor treatment in center-involving diabetic macular edema. Retina. 2016;36:1298–1308. doi:10.1097/IAE.0000000000000912
36. Ascaso FJ, Huerva V, Grzybowski A. The role of inflammation in the pathogenesis of macular edema secondary to retinal vascular diseases. Mediators Inflamm. 2014;2014:432685. doi:10.1155/2014/432685
37. Zur D, Iglicki M, Loewenstein A. The role of steroids in the management of diabetic macular edema. Ophthalmic Res. 2019;62:231–236. doi:10.1159/000499540
38. Zur D, Iglicki M, Sala-Puigdollers A, et al. Disorganization of retinal inner layers as a biomarker in patients with diabetic macular oedema treated with dexamethasone implant. Acta Ophthalmol. 2020;98:e217–e23. doi:10.1111/aos.14230
39. Keane PA, Sadda SR. Predicting visual outcomes for macular disease using optical coherence tomography. Saudi J Ophthalmol. 2011;25:145–158. doi:10.1016/j.sjopt.2011.01.003
40. Roh MI, Kim JH, Kwon OW. Features of optical coherence tomography are predictive of visual outcomes after intravitreal bevacizumab injection for diabetic macular edema. Ophthalmologica. 2010;224:374–380. doi:10.1159/000313820
41. Vujosevic S, Torresin T, Berton M, Bini S, Convento E, Midena E. Diabetic macular edema with and without subfoveal neuroretinal detachment: two different morphologic and functional entities. Am J Ophthalmol. 2017;181:149–155. doi:10.1016/j.ajo.2017.06.026
42. Hollo G, Aung T, Cantor LB, Aihara M. Cystoid macular edema related to cataract surgery and topical prostaglandin analogs: mechanism, diagnosis, and management. Surv Ophthalmol. 2020;65:496–512. doi:10.1016/j.survophthal.2020.02.004
43. Escobar-Barranco JJ, Pina-Marin B, Fernandez-Bonet M. Dexamethasone implants in patients with naive or refractory diffuse diabetic macular edema. Ophthalmologica. 2015;233:176–185. doi:10.1159/000371770
44. Guigou S, Hajjar C, Parrat E, et al. [Multicenter Ozurdex(R) assessment for diabetic macular edema: MOZART study]. J Fr Ophtalmol. 2014;37:480–485. doi:10.1016/j.jfo.2014.03.001. French.
45. Iglicki M, Busch C, Zur D, et al. Dexamethasone implant for diabetic macular edema in naive compared with refractory eyes: the international retina group real-life 24-month multicenter study. The IRGREL-DEX Study. Retina. 2019;39:44–51. doi:10.1097/IAE.0000000000002196
46. Malcles A, Dot C, Voirin N, et al. Real-life study in diabetic macular edema treated with dexamethasone implant: the Reldex Study. Retina. 2017;37:753–760. doi:10.1097/IAE.0000000000001234
47. Matonti F, Guigou S, Pommier S, et al. Dexamethasone implants in patients with naive diabetic macular edema. Ophthalmologica. 2016;235:244. doi:10.1159/000446296
48. Choi MY, Jee D, Kwon JW. Characteristics of diabetic macular edema patients refractory to anti-VEGF treatments and a dexamethasone implant. PLoS One. 2019;14:e0222364. doi:10.1371/journal.pone.0222364
49. Sarao V, Veritti D, Furino C, et al. Dexamethasone implant with fixed or individualized regimen in the treatment of diabetic macular oedema: six-month outcomes of the UDBASA study. Acta Ophthalmol. 2017;95:e255–e60. doi:10.1111/aos.13395
50. Panozzo GA, Gusson E, Panozzo G, Dalla Mura G. Dexamethasone intravitreal implant at the time of cataract surgery in eyes with diabetic macular edema. Eur J Ophthalmol. 2017;27:433–437. doi:10.5301/ejo.5000920
51. Do DV, Nguyen QD, Boyer D, et al. One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012;119:1658–1665. doi:10.1016/j.ophtha.2012.02.010
52. Campos A, Campos EJ, do Carmo A, et al. Evaluation of markers of outcome in real-world treatment of diabetic macular edema. Eye Vis (Lond). 2018;5:27. doi:10.1186/s40662-018-0119-9
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
